Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Edoxaban (Lixiana) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Apr.
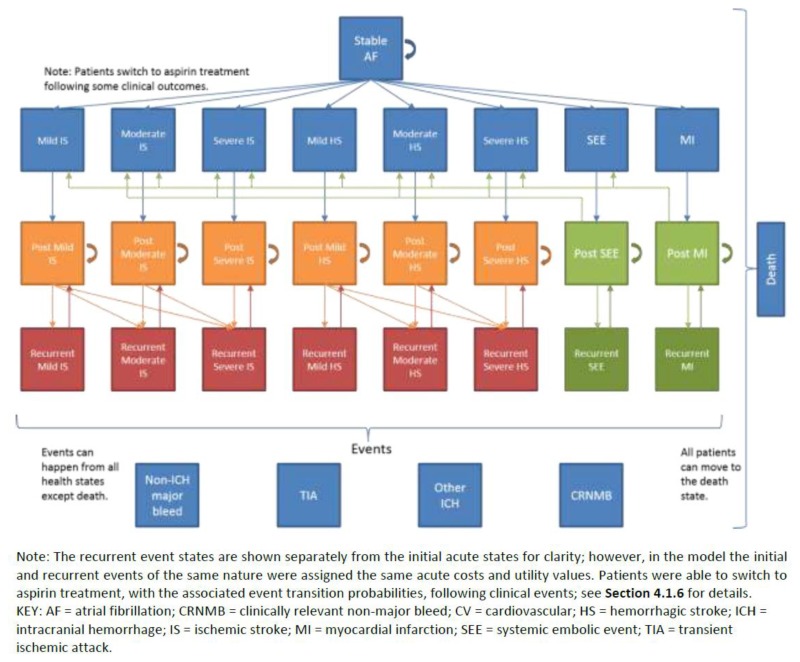
Manufacturer’s Model Structure
The model was validated by a panel of “Canadian Clinical Advisors,”16 and was consistent with previous models in this area, including CADTH Therapeutic Review.15
Table 13Data Sources
| Data Input | Description of Data Source | Comment |
|---|---|---|
| Efficacy | ENGAGE AF-TIMI 48 trial8 | Appropriate |
| Manufacturer-submitted NMA | Appropriate — results consistent with CADTH NMA15 | |
| Natural history | Probability of event on edoxaban, probability of stroke recurrence, case fatality rates: ENGAGE AF-TIMI 48 trial8 | Appropriate |
| Stroke severity distribution, case fatality rates: HETA Group (2014),17 Miller et al. (2016)18 | Appropriate | |
| Stroke risk after MI: Mohan et al. (2009)19 | Appropriate | |
| Increase in events by age: AFI (1994),20 Ariesen et al. (2003),21 Flegel and Hanley (1989),22 Freeman et al. (2011),23 Hylek et al. (2014),24 Bos et al. (2007)25 | Appropriate | |
| Utilities | ENGAGE AF-TIMI 48,8 Luengo-Fernandez et al. (2013),11 Sullivan et al. (2006)10 | Appropriate — similar values to CADTH Therapeutic Review |
| Mortality | Background mortality: Statistics Canada (2011),26,27 Wyse et al. (2001)28 | Appropriate — consistent with CADTH Therapeutic Review |
| Mortality post-event: Fang et al. (2014),29 Berkwelem et al. (2015),30 Harrington et al. (2013)31 | Appropriate | |
| Costs | ||
| Drug | Rivaroxaban, warfarin, apixaban, and
dabigatran: ODB (2016)2 Edoxaban: manufacturer | Appropriate |
| Administration | Warfarin administration: Schulman et al. (2010)14 | Appropriate |
| Event | Sorensen et al. (2011),3 CIHI,4 Cohen et al. (2014),5 Regier et al. (2006)7 | Appropriate — similar values to CADTH Therapeutic Review |
| Post-event health state | Sorensen et al. (2011),3 Cohen et al. (2014),5 Goeree et al. (2005)6 | Appropriate — similar values to CADTH Therapeutic Review |
AFI = Atrial Fibrillation Investigators; CIHI = Canadian Institute of Health Information; HETA = Health Economic and Technology Assessment; MI = myocardial infarction; NMA = network meta-analysis; ODB = Ontario Drug Benefit.
Table 14Manufacturer’s Key Assumptions
| Assumption | Comment |
|---|---|
| Can compare NOACs through NMA | Consistent with CADTH Therapeutic Review |
| Analysis restricted to comparison with warfarin and rivaroxaban | Inappropriate, as all NOACs are potential comparators for edoxaban |
| Population enrolled in ENGAGE AF-TIMI 48 trial is reflective of Canadian patients with NVAF requiring anticoagulation | Likely appropriate, except that patients with CHADS2 = 1 are not reflected in the model |
| Patients could only move to a health state that was as or more severe than their current health state. This restricted the types and severity of subsequent events (e.g., a subsequent stroke could only be as or more severe than the previous stroke). | Likely appropriate, and consistent with previous analyses |
| Transient events (other ICH, non-ICH major bleed, TIA, and CRNMB) assumed to not be associated with long-term costs or resource use | Likely appropriate for most, but not all, patients |
| Risk of TIA assumed equivalent for edoxaban and other NOACs in the absence of data | Appropriate in the absence of data; unlikely to have a major impact on model results |
| Costs and utilities of clinical events assumed to be the same regardless of treatment (higher costs and disutilities for bleeding events assumed for NOACs in sensitivity analysis) | Likely appropriate given lack of specific cost data |
CHADS2 = congestive heart failure, hypertension, age = 75 years, diabetes mellitus, and prior stroke or TIA or thromboembolism; CRNMB = clinically relevant non-major bleed; ICH = intracranial hemorrhage; NMA = network meta-analysis; NOAC = new oral anticoagulant; NVAF = nonvalvular atrial fibrillation; TIA = transient ischemic attack.
- REVIEWER WORKSHEETS - Edoxaban (Lixiana)REVIEWER WORKSHEETS - Edoxaban (Lixiana)
- CONCLUSIONS - Ingenol Mebutate (Picato)CONCLUSIONS - Ingenol Mebutate (Picato)
- LIMITATIONS OF MANUFACTURER’S SUBMISSION - Edoxaban (Lixiana)LIMITATIONS OF MANUFACTURER’S SUBMISSION - Edoxaban (Lixiana)
Your browsing activity is empty.
Activity recording is turned off.
See more...