Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ustekinumab (Stelara) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Apr.
Manufacturer’s Model Structure
The model estimates disease progression through a series of health states, classifying patients with Crohn’s disease (CD) based on Crohn’s Disease Activity Index (CDAI) score as well as the occurrence of surgery and the subsequent loss of response following surgery. The model structure consisted of a decision tree to model the induction-treatment phase and a Markov (cohort) structure to model maintenance treatment for the remainder of the time horizon.
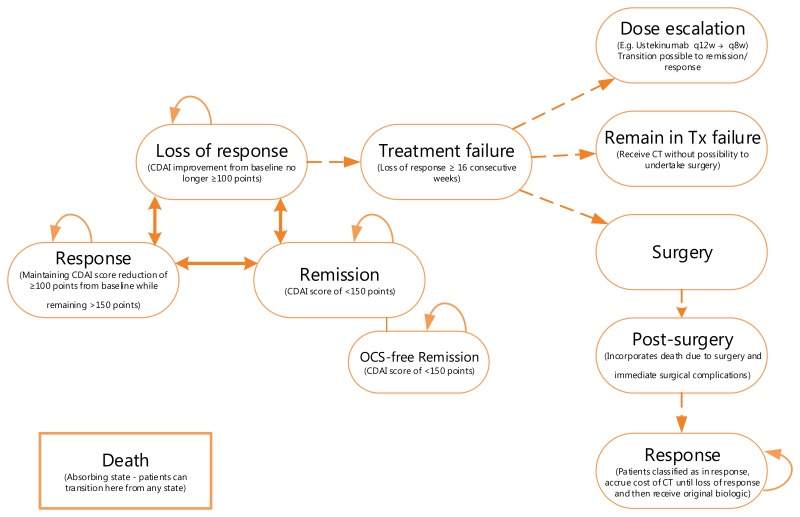
The model structure of the induction phase is based on a decision tree that allocates patients to one of three outcomes following the end of induction treatment: remission, response, and no-response. Patients allocated to the remission or response outcomes move to the maintenance phase based on relative efficacy data for induction (versus conventional therapy) estimated in a network meta-analysis (NMA) conducted by the manufacturer. Patients receiving conventional therapy who do not demonstrate response to the induction treatment are classified as having experienced a treatment failure, and can undertake surgery or remain indefinitely in a state of nonresponse. Patients initiating biologic therapy who do not achieve response after the standard assessment of induction can either receive an additional induction dose, undergo surgery, or remain in a nonresponse state, receiving conventional therapy but not undergoing surgery later (Figure 1).1
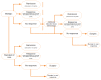
Figure 1
Model Structure — Induction. CDAI = Crohn’s Disease Activity Index. Source: Manufacturer’s pharmacoeconomic submission.
The maintenance phase is modelled using discrete cycles corresponding to the four-week frequency of the assessment of response in the ustekinumab trials. Maintenance is driven by three health states: remission (CDAI < 150), response (CDAI maintained more than 100 points less than baseline CDAI), and loss of response. The base case uses the 100-point definition of CDAI improvement, which was derived from the primary end points of the adalimumab and vedolizumab trials. The infliximab trials used a 70-point definition to assess response, rather than CDAI-100, which requires infliximab results to be interpreted with caution, as its rate of response relative to the comparators would be overestimated.
Oral corticosteroid (CS)-free remission is presented in the diagram as a separate health state, although patients in this health state were assumed to progress based on the same transition probabilities as the remission health state but with lower disease management costs and improved quality of life.1
Figure 2Model Structure — Maintenance
CDAI = Crohn’s Disease Activity Index; CT = conventional therapy; OCS = oral corticosteroid; q8w = every 9 weeks; q12w = every 12 weeks; Tx = therapy.
Source: Manufacturer’s pharmacoeconomic submission.1
Table 8Data Sources
| Data Input | Description of Data Source | Comment |
|---|---|---|
| Efficacy in induction phase | The efficacy of comparators in the induction phase was estimated from an NMA conducted by the manufacturer (2015).12 ORs were estimated from the NMA, analyzing the relative risks of achieving response and remission versus the placebo group from the UNITI-1 and UNITI-2 trials for each treatment of interest (adalimumab, infliximab, vedolizumab) and stratified by treatment experience.12 | The CDR clinical review concluded that there were several limitations with the available indirect comparisons and heterogeneity across studies; as a result, the comparative efficacy of ustekinumab against infliximab, adalimumab, and vedolizumab is uncertain for both the induction and maintenance phases of treatment. The manufacturer mentioned in its submission that it considered the NMA for the maintenance phase to be inappropriate because potential carry-over effects from the induction phase can drive maintenance placebo response rates to vary across trials and influence the relative treatment effects used as inputs for the analysis of the maintenance phase. |
| Efficacy of additional induction doses | Ustekinumab – Based on patient responses in the UNITI-1 and UNITI-2 trials. Adalimumab – From the CHARM study among patients who were not in response at week 4 and who received additional doses into the maintenance phase13 Vedolizumab – Based on the GEMINI II trial patients who failed to demonstrate response at week 6 to doses of vedolizumab 300 mg at week 0 and week 2 and were retained in the study and received additional doses every four weeks14 | |
| Efficacy in maintenance phase | For ustekinumab, the proportions of patient in response, remission, and nonresponse at the beginning and end of maintenance treatment were retrieved from the q.8.w. and q.12.w. arms in IM-UNITI trial.15 Patients who did not receive biologic treatment at any time during the UNITI trials were considered as a proxy for patients on conventional therapy to week 52.16,17 For adalimumab, vedolizumab, and infliximab, the proportions of patients in response, remission, and nonresponse at 52 weeks were obtained from an NMA considering the CHARM, GEMINI-II and ACCENT I (at 56 weeks) trials, respectively.13,14,18 | |
| Patient baseline characteristics | The cohort was assumed to be 36 years old and to weigh 69 kg, based on baseline data from the active treatment arms of the ustekinumab induction and maintenance clinical trials (UNITI-1, UNITI-2, and IM-UNITI).15–17 | Acceptable |
| Utilities | The manufacturer indicated that utility values were obtained from a published Canadian study by Gregor et al.(1997) for the remission, response, nonresponse, surgery, and post-surgery health states.8 The study estimated utility values based on the responses of 180 Canadian patients with CD to the time trade-off, visual analogue scale, and standard gamble methods of health state valuation. A separate publication was used to derive the utility benefit of being in the steroid-free remission health state (Greenberg et al.).19 | The utility value used by the manufacturer for the remission health state (0.88) was higher than the value cited in Gregor et al.29 for the remission health state (0.82). This biases the results in favour of ustekinumab. |
| Resource use | ||
| Adverse events | Adverse events associated with conventional or biologics treatments were not included (except serious infections). | Acceptable |
| Mortality | Background mortality is based on the reported Canadian mortality risk by age and sex.1 | Acceptable |
| Costs | ||
| Drug | Costs of medications were obtained from the Ontario Ministry of Health and Long-Term Care Exceptional Access Program formulary7 and from the Ontario Drug Benefit formulary.10 | Drug wastage and vial-sharing were integrated in the model. |
| Administration | Not included in the base case | Provided a value of $367 per IV administration for use in sensitivity analyses; this was based on a published study on administration costs of IV biologics for rheumatoid arthritis in Finland.20 These cost data are very limited for use in a Canadian perspective, but the results of the analysis are not sensitive to varying this data. |
| Routine management | Costs of management by disease stage were calculated based on health care resource use estimated from an unpublished Delphi panel conducted by Janssen Inc.1 and using costs obtained from the Ontario Schedule of Benefits Physician Services 21 and the Ontario Schedule of Laboratory Fees.22 | The manufacturer’s Delphi panel is associated with uncertainty, especially as it involves a small sample of physicians. This raises uncertainty concerning the included management costs in the model and ultimately the results of the analysis. |
| Serious infections | Costs of serious infections while patients are receiving biologic treatment were included, based on data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) registry on rates of serious infections in patients with IBD who were treated for psoriasis with ustekinumab and other biologic therapies23 and using costs of treatment of infections obtained from the Ontario Case Costing Initiative Database.1 | Acceptable |
| Surgery | Based on the published Canadian study on hospitalizations and operations for Crohn’s disease by Bernstein et al. (2012).24 Health care resource use 6 months before and following surgery was based on the Delphi panel (see Routine management) | The costs of complications of surgery have not been included, given that the management of these short-term complications are assumed to be covered in the cost of surgery. |
CD = Crohn’s disease; CDR = CADTH Common Drug Review; IBD = inflammatory bowel disease; IV = intravenous; NMA = network meta-analysis; OR = odds ratio; q.8.w. = every 8 weeks; q.12.w. = every 12 weeks.
Table 9Manufacturer’s Key Assumptions
| Assumption | Comment |
|---|---|
| The efficacy of infliximab was assumed to be equivalent to that of adalimumab in the population experiencing failure with anti-TNF therapy | Appropriate, as infliximab was the first anti-TNF treatment introduced. |
| Drug-administration costs were not included in the base-case analysis, as treatments were subcutaneous and therefore self-administered | Although ustekinumab requires IV administration in the induction phase, a patient may self-administer in the maintenance phase if a physician determines that it is appropriate after proper training in subcutaneous injection technique. The manufacturer did not mention providing patient management for IV administration at the induction phase. Infliximab and vedolizumab are administered intravenously in both the induction and the maintenance phases, and the manufacturer of these therapies provides patient management for IV administration. |
| Time horizon set at 25 years | Although Crohn’s disease is a chronic and lifelong condition, efficacy of the treatments is expected to wane over a 25-year period. The manufacturer’s model included the waning effects based on real-world evidence. |
| Beyond 1 year, the maintenance transition matrices based on 1-year clinical trial data were adjusted to align with the results of the Chaparro et al. study evaluating the long-term response of adalimumab in patients withCD.25 | This approach favours the results for ustekinumab. |
| For patients in the “loss of response” health state for 16 consecutive weeks, 30% were assumed to undergo immediate surgery. | Based on expert opinion, the percentage of patients undergoing surgery depends on their treatment history: in those who have had resistance to conventional therapies, the percentage will be lower, whereas in those with resistance to previous anti-TNF therapy, the percentage will be higher. However, when the two populations are pooled, the 30% assumption by the manufacturer seems appropriate. |
| Patients maintain post-surgery response based on a median time to loss of response of 24 months. | Despite the lack of long-term evidence to support this assumption, the feedback from the clinical expert suggests it may be appropriate. |
| 94% of patients respond to re-initiation of biologic treatment post-surgery, of which 55% are assumed to be in remission. This was based on the results of a prospective study of patients with IBD that evaluated the rate of response to retreatment after discontinuation of anti-TNF treatments in patients with IBD in deep remission.26 | The manufacturer stated that the included study was not comparable to the target population in the model, given that it assessed IBD patients in deep remission who discontinued biologic treatment and then re-started it. |
| Upon a secondary loss of response after surgery, patients were re-treated with their index biologic drug and re-entered the model in either the “response” or “loss of response” health state. An optional oral corticosteroid-sparing remission health state was included to reflect the improvement in quality of life and decrease in costs from the subset of patients in remission who do not require management with oral corticosteroids. | Although the submitted model included a steroid-free remission health state, the manufacturer did not use it in the base-case analysis. The manufacturer justified this on the basis of the challenge of quantifying the benefit of steroid-free remission with a utility, due to the lack of data. Also, the manufacturer acknowledged the difficulty in estimating the proportion of patients who could achieve steroid-free remission for all of the comparators. This leads to uncertainty of the analysis. |
| The manufacturer assumed that patients remain in a state of response following second surgery, accruing costs and QALYs corresponding to the response health state. | This assumption is limited by the lack of long-term data, including long-term efficacy of biologic drugs post-surgery, as well as disease progression following two total operations. |
CD = Crohn’s disease; IBD = inflammatory bowel disease; IV = intravenous; QALY = quality-adjusted life-year; TNF = tumour necrosis factor.
Manufacturer’s Results
For patients experiencing a failure of conventional therapy only (FCTO), ustekinumab at both doses was associated with the highest number of quality-adjusted life-years (QALYs) versus all other comparators. Compared with conventional therapy, ustekinumab every eight weeks had an incremental cost-utility ratio (ICUR) of $86,424 per QALY, after which ustekinumab every 12 weeks had an ICUR of $50,912 per QALY, followed by the mixed every 12 weeks/every eight weeks ustekinumab group with an ICUR of $69,575 per QALY. Biosimilar infliximab every two weeks had the lowest ICUR compared with conventional therapy, at $32,032 per QALY (Table 10).
Table 10
Manufacturer Base-Case Results Compared With Conventional Therapy.
In the anti-TNF failure subpopulation, biosimilar infliximab every eight weeks had the lowest ICUR of $8,727 per QALY compared with conventional treatments, after which ustekinumab every 12 weeks resulted in an ICUR of $38,764 per QALY, followed by the mixed ustekinumab every eight weeks/every 12 weeks with an ICUR of $74,192 per QALY, and ustekinumab every eight weeks with an ICUR of $83,535 per QALY (Table 10).
The results of the analyses comparing ustekinumab and other biologics with each other are presented in Table 11.
Table 11
Manufacturer Base-Case Results Compared With Other Biologics.
Manufacturer’s Sensitivity Analyses
The manufacturer conducted several deterministic sensitivity analyses varying model parameters in the following manner (with results presented in tornado diagrams):
- Baseline characteristics (age, sex, body weight) were varied by ± 10%.
- Proportion undergoing surgery was varied based on low/high rates reported in the literature.1
- Time to loss of response post-surgery was based on low/high estimates reported in the literature.
- Response to induction treatment was varied using the 95% credible intervals per treatment and by population versus conventional therapy estimated in the NMA.1
- Probabilities of response and remission associated with conventional therapy, to which odds ratios were applied to obtain probabilities of response and remission for biologic drugs, were varied by ± 10%, as were the probabilities of response and remission to additional induction doses following initial nonresponse to induction.1
- For the proportion of patients in CS-free remission, low/high values of 10%/30% were chosen.
- Proportions of patients in whom the dosage was escalated, per treatment, were set to 100% in a deterministic sensitivity analysis to assess the impact of systematic escalation of patients’ dosage, which was also a means to vary the definition of treatment failure, as patients need to demonstrate nonresponse for an additional 16 weeks before their dosage is escalated.1
- All cost inputs other than medication costs were varied by ± 10%, and the management costs increase factor applied to anti-TNF failure patients was set between 1.0 (i.e., no increase) and 1.5, corresponding to the highest estimate of increased costs for these patients obtained through the Delphi panel.
- Utility values were varied by ± 10% to prevent health-state utility values in more severe health states from exceeding the utility values in less severe health states.
- Discount rates were set to 3% and 6%.
Results of the deterministic sensitivity analyses for ustekinumab every eight weeks for the FCTO showed that the base-case results were most sensitive to the proportion undergoing surgery as well as to remission and nonresponse utility values, followed by the efficacy of an additional induction dose. In the population experiencing failure with anti-TNF therapy, the most sensitive parameters were the remission utility value, the efficacy of an additional induction dose, the probability of surgery after 16 weeks in nonresponse, and the cost increase factor for patients experiencing failure with anti-TNF therapy.
Manufacturer’s Probabilistic Sensitivity Analysis
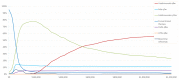
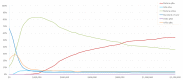
The manufacturer conducted a probabilistic sensitivity analysis (PSA) based on a Monte Carlo simulation in which the model was run for 500 simulations. In each simulation, parameter values were randomly selected based on statistical distributions, simultaneously for all varied parameters. In results using the population experiencing an FCTO, and across all willingness-to-pay thresholds (WTP) up to $1,200,000, ustekinumab every eight weeks and every 12 weeks had the highest net monetary benefit (NMB) in 58.5% and 23.2% of the 500 PSA simulations, respectively. At a WTP of $120,000, ustekinumab every 12 weeks generated the highest NMB in 75.0% of the 500 simulations, followed by adalimumab every two weeks, which generated the highest NMB in 13.2% of the 500 simulations (Figure 3). In the population experiencing failure with anti-TNF therapy, ustekinumab every eight weeks and every 12 weeks had the highest NMB, in 54.1% and 36.3% of the 500 PSA simulations, respectively. Adalimumab every two weeks had the highest NMB in 4.2% of the simulations across all WTP values. At a WTP of $120,000, ustekinumab every 12 weeks generated the highest NMB in 76.2% of the 500 simulations, followed by infliximab every eight weeks, which generated the highest NMB in 11.0% of the 500 simulations (Figure 4).
Manufacturer’s Alternative Costing Scenarios
The manufacturer conducted scenario analyses to assess alternative costing scenarios that varied the assumptions related to ustekinumab medication costs (Table 12):
Table 12
Results of Manufacturer’s Scenario Analysis With Alternative Costing.
- Offering the IV induction dose for free
- Including rebates of varying amounts (5%, 10%, 15%, 20%) on ustekinumab
- Implementing a number of annual per-patient expenditure caps ($20,000, $18,000, $16,000, $14,000; capping ustekinumab every eight weeks at $20,000 annually effectively assumes the cost of every 12 weeks dosage for the every eight weeks regimen)
- Providing ustekinumab at no cost after re-initiating therapy after surgery.
The manufacturer conducted a PSA using 500 simulations. In results using the patient population experiencing FCTO, and across all WTP thresholds up to $1,200,000, ustekinumab every eight weeks and every 12 weeks had generally the highest NMB. It had the highest NMB in 58.5% and 23.2% of the 500 PSA simulations, respectively, for the WTP of $1,200,000. At a WTP of $120,000, ustekinumab every 12 weeks generated the highest NMB in 75.0% of the 500 simulations, followed by adalimumab every two weeks, which generated the highest NMB in 13.2% of the 500 simulations.
In the patient population experiencing failure with anti-TNF therapy, ustekinumab every eight weeks and every 12 weeks had the highest NMB in 54.1% and 36.3% of the 500 PSA simulations, respectively, for the WTP of $1,200,000. Adalimumab every two weeks had the highest NMB in 4.2% of the simulations across all WTP values. At a WTP of $120,000, ustekinumab every 12 weeks generated the highest NMB in 76.2% of the 500 simulations, followed by infliximab every eight weeks, which generated the highest NMB in 11.0% of the 500 simulations.
CADTH Common Drug Review Reanalyses
Utility values for model health states: The manufacturer assumed utility values from a Canadian study (Gregor et al. [1997]) that asked a cohort of patients with CD to rate three hypothetical disease states representing mild (0.82), moderate (0.73), and severe disease (0.54) using a standard gamble approach.8 The study also reported utility values for remission (0.88), chronically active therapy–responsive (0.86), and therapy-resistant (0.74). The manufacturer indicated that utility values from the study were used in published models as part of CADTH technical reports.27 For the manufacturer’s model, the remission, response, and no-response health states were assigned utilities of 0.88, 0.73, and 0.54, respectively. CDR conducted scenario analyses that applied utility values exclusively from each publication (Table 13).
Table 13
Summary of CDR Utility Values for the Reanalyses.
Results of the scenario analyses show the ICUR for ustekinumab compared with conventional therapy and other biologics in FCTO, failure with anti-TNF therapy, and mixed FCTO/failure with anti-TNF therapy (Table 14 and Table 15).
Table 14
CDR Reanalyses Using Published Health State Utility Values by Gregor et al. (1997).
Table 15
CDR Reanalyses Using Published Health State Utility Values Used in CADTH Models.
Excluding real-world evidence to adjust transition probabilities: The manufacturer acknowledged that adjusting the transition probabilities to real data was challenging because of lack of data available to make the calculations. The manufacturer’s approach, associated with significant uncertainty, favours ustekinumab. CDR conducted a reanalysis that excluded the impact of real-world evidence on the transition probabilities. The results for ustekinumab compared with conventional therapy and other biologics are presented in Table 16.
Table 16
CDR Reanalysis Excluding Real-World Evidence From Transition Probabilities.
Model time horizon reduced: The manufacturer’s base-case analysis used a 25-year time horizon, based on a conservative assumption that CD is a chronic and lifelong disease and that patients initiating a biologic therapy would survive at least 25 more years. However, there is a lack of long-term data to support the assumption that the effects of biologic therapy would be sustained without waning over time. CDR conducted an exploratory analysis using a time horizon of 10 years (Table 17).
Table 17
CDR Reanalysis Using a Time Horizon of 10 Years.
Multi-way CDR reanalyses: CDR conducted multi-way scenario reanalyses that varied the health-state utility values and were based on a 25-year time horizon that excluded real-world evidence (Table 18 and Table 19).
Table 18
CDR Multi-way Reanalyses Using Health State Utility Values From CADTH Analyses.
Table 19
CDR Multi-way Reanalyses Using Health State Utility Values From Published Study.
Multi-way CDR reanalyses excluding induction costs: Based on correspondence from the manufacturer indicating that the drug costs for the induction dose of ustekinumab would be reimbursed by the manufacturer, CDR ran the multi-way scenario reanalysis excluding costs of the induction dose (Table 18).
Table 20Results of CDR Multi-way Analysis Using Health State Utility From CADTH Models Excluding Drug Costs for the Induction Dose
| ICUR ($/QALY) | ICUR ($/QALY) Excluding Cost of Induction Dose | ||||
|---|---|---|---|---|---|
| Versus Conventional Therapy | Sequential Analysis | Versus Conventional Therapy | Sequential Analysis | ||
| Population experiencing FCTO | |||||
| Ustekinumab q.12.w. | $115,474 | $115,474 | Ustekinumab q.12.w. | $95,442 | $95,442 |
| Ustekinumab mixed q.8.w./q.12.w. | $147,517 | $623,571 | Ustekinumab q.8.w. | $151,633 | $641,045 |
| Ustekinumab q.8.w. | $169,543 | $658,533 | Ustekinumab mixed q.8.w./q.12.w. | $128,955 | Subject to extended dominancef |
| Biosimilar infliximab q.8.w. | $143,062 | Subject to extended dominancea | Biosimilar infliximab q.8.w. | $143,909 | Subject to extended dominanceg |
| Adalimumab q.2.w. | $164,583 | Subject to extended dominanceb | Adalimumab q.2.w. | $165,251 | Subject to extended dominanceh |
| Vedolizumab q.8.w. | $271,363 | Dominated by adalimumab q.2.w. | Vedolizumab q.8.w. | $271,689 | Dominated by adalimumab q.2.w. |
| Infliximab q.8.w. | $342,856 | Dominated by biosimilar infliximab q.8.w., vedolizumab q.8.w., adalimumab q.2.w., ustekinumab q.12.w. | Infliximab q.8.w. | $344,875 | Dominated by biosimilar infliximab q.8.w., vedolizumab q.8.w., adalimumab q.2.w., ustekinumab q.12.w. |
| Population experiencing failure with anti-TNF therapy | |||||
| Biosimilar infliximab q.8.w. | $90,277 | $90,277 | Ustekinumab q.12.w. | $77,840 | $77,840 |
| Ustekinumab q.12.w. | $131,297 | $228,571 | Ustekinumab mixed q.8.w./q.12.w. | $139,081 | $1,559,521 |
| Ustekinumab mixed q.8.w./q.12.w. | $189,403 | $1,332,167 | Ustekinumab q.8.w. | $153,608 | $1,559,521 |
| Ustekinumab q.8.w. | $203,880 | $1,999,000 | Biosimilar infliximab q.8.w. | $89,469 | Subject to extended dominancei |
| Adalimumab q.2.w. | $134,373 | Dominated by biosimilar infliximab q.8.w. | Adalimumab q.2.w. | $133,183 | Dominated by biosimilar infliximab q.8.w., ustekinumab q.12.w. |
| Infliximab q.8.w. | $284,904 | Dominated by adalimumab q.2.w., biosimilar infliximab q.8.w., ustekinumab q.12.w., ustekinumab mixed | Infliximab q.8.w. | $282,365 | Dominated by adalimumab q.2.w., biosimilar infliximab q.8.w., ustekinumab q.12.w., ustekinumab mixed, ustekinumab q.8.w. |
| Vedolizumab q.8.w. | $500,920 | Dominated by adalimumab q.2.w., biosimilar infliximab q.8.w. | Vedolizumab q.8.w. | $499,971 | Dominated by adalimumab q.2.w., biosimilar infliximab q.8.w., ustekinumab q.12.w. |
| IM-UNITI (mixed) population | |||||
| Ustekinumab q.12.w. | $119,058 | $119,058 | Ustekinumab q.12.w. | $91,260 | $91,260 |
| Ustekinumab q.8.w. | $177,093 | $744,826 | Ustekinumab q.8.w. | $152,083 | $758,251 |
| Biosimilar infliximab q.8.w. | $120,923 | Subject to extended dominancec | Biosimilar infliximab q.8.w. | $121,295 | Subject to extended dominancej |
| Adalimumab q.2.w. | $154,194 | Subject to extended dominanced | Ustekinumab mixed q.8.w./q.12.w. | $131,322 | Subject to extended dominancek |
| Ustekinumab mixed q.8.w./q.12.w. | $157,268 | Subject to extended dominancee | Adalimumab q.2.w. | $153,566 | Subject to extended dominancel |
| Vedolizumab q.8.w. | $311,328 | Dominated by biosimilar infliximab q.8.w., adalimumab q.2.w. | Vedolizumab q.8.w. | $309,918 | Dominated by biosimilar infliximab q.8.w., adalimumab q.2.w., ustekinumab q.12.w. |
| Infliximab q.8.w. | $317,945 | Dominated by biosimilar infliximab q.8.w., adalimumab q.2.w., ustekinumab q.12.w. | Infliximab q.8.w. | $318,909 | Dominated by biosimilar infliximab q.8.w., adalimumab q.2.w., ustekinumab q.12.w. |
FCTO = failure with conventional therapy only; ICUR = incremental cost-utility ratio; QALY = quality-adjusted life-years; q.2.w. = every 2 weeks; q.8.w. = every 8 weeks; q.12.w. = every 12 weeks.
- a
Subject to extended dominance through conventional therapy and ustekinumab q.12.w.
- b
Subject to extended dominance through conventional therapy and ustekinumab q.12.w., biosimilar infliximab q.8.w. and ustekinumab q.12.w., conventional therapy and ustekinumab mixed, biosimilar infliximab q.8.w. and ustekinumab mixed, biosimilar infliximab q.8.w. and ustekinumab q.8.w.
- c
Subject to extended dominance through conventional therapy and ustekinumab q.12.w.
- d
Subject to extended dominance through conventional therapy and ustekinumab q.12.w., biosimilar infliximab q.8.w. and ustekinumab q.12.w., biosimilar infliximab q.8.w. and ustekinumab mixed, biosimilar infliximab q.8.w. and ustekinumab q.8.w.
- e
Subject to extended dominance through ustekinumab q.12.w. and ustekinumab q.8.w.
- f
Subject to extended dominance through ustekinumab q.12.w. and ustekinumab q.8.w.
- g
Subject to extended dominance through conventional therapy and ustekinumab q.12.w., conventional therapy and ustekinumab mixed.
- h
Subject to extended dominance through conventional therapy and ustekinumab q.12.w., biosimilar infliximab q.8.w. and ustekinumab q.12.w., conventional therapy and ustekinumab mixed, biosimilar infliximab q.8.w. and ustekinumab mixed, conventional therapy and ustekinumab q.8.w., biosimilar infliximab q.8.w. and ustekinumab q.8.w.
- i
Subject to extended dominance through conventional therapy and ustekinumab q.12.w.
- j
Subject to extended dominance through conventional therapy and ustekinumab q.12.w.
- k
Subject to extended dominance through ustekinumab q.12.w. and ustekinumab q.8.w.
- l
Subject to extended dominance through conventional therapy and ustekinumab q.12.w., biosimilar infliximab q.8.w. and ustekinumab q.12.w., conventional therapy and ustekinumab mixed, biosimilar infliximab q.8.w. and ustekinumab mixed, conventional therapy and ustekinumab q.8.w., biosimilar infliximab q.8.w. and ustekinumab q.8.w.
- REVIEWER WORKSHEETS - Ustekinumab (Stelara)REVIEWER WORKSHEETS - Ustekinumab (Stelara)
- Discussion - Clinical and Economic Review Report: Vedolizumab (ENTYVIO SC)Discussion - Clinical and Economic Review Report: Vedolizumab (ENTYVIO SC)
- SUMMARY - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)SUMMARY - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)
- Stakeholder Engagement - Clinical Review Report: Icosapent Ethyl (Vascepa)Stakeholder Engagement - Clinical Review Report: Icosapent Ethyl (Vascepa)
- Introduction - Clinical and Economic Review Report: Vedolizumab (ENTYVIO SC)Introduction - Clinical and Economic Review Report: Vedolizumab (ENTYVIO SC)
Your browsing activity is empty.
Activity recording is turned off.
See more...