Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ustekinumab (Stelara) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Apr.
3.1. Findings from the Literature
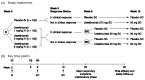
A total of four studies were identified from the literature for inclusion in the systematic review (Figure 1). The included studies are summarized in Table 6 and described in Section 3.2. A list of excluded studies is presented in 0.
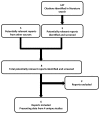
Figure 1
Flow Diagram for Inclusion and Exclusion of Studies.
Table 6
Details of Included Induction Studies.
3.2. Included Studies
3.2.1. Description of Studies
The CDR review included four, multi-centre, multinational, double-blind, randomized, placebo-controlled trials: two phase III induction-treatment studies, UNITI-1 (N = 769) and UNITI-2 (N = 640); one phase III maintenance-treatment study, IM-UNITI (N = 397); and one phase II induction and maintenance study, CERTIFI (N = 526). The phase III studies were designed as superiority studies, whereas the phase II study was a dose-ranging study. All four studies were submitted to CDR by the manufacturer as pivotal studies.
a. Induction Studies
UNITI-1 and UNITI-2 were designed — with identical protocols — to evaluate the efficacy and safety of IV induction regimens of ustekinumab in inducing clinical response in patients with moderately to severely active Crohn’s disease. However, the studies differed with respect to patients’ previous Crohn’s disease treatment experience: UNITI-1 included patients who had had an inadequate response or were intolerant to one or more TNF antagonist therapies, whereas UNITI-2 included patients who had had an inadequate response or were intolerant to conventional therapy only (i.e., corticosteroids or immunomodulators such as 6-MP, AZA, and MTX). Patients in UNITI-2 could have previously received TNF antagonists but could not have failed treatment. Detailed descriptions of the populations are located in Section 3.2.2 of this review.
Patients were randomized in a 1:1:1 ratio to receive a single IV administration of either placebo or one of two induction doses of ustekinumab at week 0, as shown in Figure 2. Patients were allocated to a treatment group using a permuted-block randomization with study region (Asia, Eastern Europe, or rest of world) and Crohn’s Disease Activity Index (CDAI) score (≤ 300 or > 300 points) as the stratification variables. In UNITI-1, randomization was further stratified by initial response to TNF antagonist therapy (yes or no). For patients who had previously received multiple TNF antagonist therapies, their initial response status (yes or no) was determined by whether they had initially responded to the first TNF antagonist therapy received. Allocation to treatment group was done using a central randomization centre by means of an interactive voice response system (IVRS) and/or interactive web response system (IWRS).

Figure 2
Study Design Schematic for Induction Studies UNITI-1 and UNITI-2. IV = intravenous; PE = primary end point; R = randomization. Note: The triangle indicates study drug administration.
Patients randomized to ustekinumab induction therapy who achieved clinical response were eligible to enter the maintenance-treatment study, IM-UNITI, at week 8. Patients who did not enter the maintenance study continued to be followed as part of the induction studies and had a safety follow-up visit at week 20.
After 28 (UNITI-1) and 12 (UNITI-2) patients had been randomized to treatments in the induction studies, the manufacturer temporarily suspended drug administration to patients because of a stability issue with the IV formulation of ustekinumab (130 mg ustekinumab in 26 mL [5 mg/mL; 27 mL fill of liquid]) used in the studies. The manufacturer substituted the 90 mg ustekinumab formulation (which is already approved for SC injection for other indications) for the protocol-specified IV induction administrations. The protocols for the induction (and maintenance) studies were amended to incorporate the use of the 90 mg formulation. Data from the 28 (UNITI-1) and 12 (UNITI-2) patients who were randomized before the study was temporarily stopped were not used in the planned analyses because knowledge of the stability issue could potentially bias the assessments. The manufacturer restarted the randomization with new blocks for each stratum when the study was restarted.
The induction studies ended on the date either the last patient who entered the maintenance study completed the week 8 visit or the last patient who did not enter the maintenance study completed his or her final safety visit at week 20, whichever occurred later. Once the study ended and the database was locked, selected manufacturer personnel were unblinded to induction-treatment assignment, although exactly which personnel this referred to was not specified. The clinical study reports indicate that, in order to protect the integrity of the maintenance study, treatment assignment blinding in the induction studies was maintained for sites, site monitors, and patients until the week 44 analyses for the maintenance study were completed.
b. Maintenance Study
The IM-UNITI study was designed to evaluate the efficacy (clinical remission) and safety of two SC maintenance regimens of ustekinumab (90 mg every eight weeks or every 12 weeks) in patients with moderately to severely active Crohn’s disease who had a clinical response with ustekinumab in the induction studies, UNITI-1 and UNITI-2. Figure 3 shows the study design.

Figure 3
Study Design Schematic of Maintenance Study IM-UNITI. Source: Clinical Study Report for IM-UNITI.
Patients who had a clinical response to ustekinumab induction at week 8 in either UNITI-1 or UNITI-2 were randomly assigned in a 1:1:1 ratio to one of three treatment groups (placebo, ustekinumab 90 mg SC every 12 weeks, or ustekinumab 90 mg SC every eight weeks) based on computer-generated randomization schedule defined a priori. Permuted-block randomization with stratification factors of clinical remission at week 0 (yes or no) and ustekinumab induction dose (130 mg or tiered dose approximating 6 mg/kg ustekinumab) were used. Patients who did not achieve clinical response with ustekinumab induction therapy, as well as all patients who were randomized to placebo, irrespective of whether they achieved clinical response in the UNITI studies, were also eligible to enter IM-UNITI at week 8; however, these patients were not randomized to the primary efficacy population. An IVRS/IWRS dictated the treatment assignment for each patient.
As mentioned, the induction studies were temporarily suspended because of a stability issue with the IV formulation of ustekinumab. Consequently, the maintenance study was also temporarily suspended. A total of 40 patients had been randomized in the induction studies before the studies were temporarily suspended. Because knowledge of the stability issue could bias the assessments, data from nine randomized patients enrolled in IM-UNITI were excluded from the efficacy analyses.
The duration of the double-blind maintenance treatment period of IM-UNITI was 44 weeks, meaning that patients randomized to treatment in the induction studies and treated to the end of IM-UNITI had a total of 52 weeks of treatment. Eligible patients at week 44 could continue into the long-term extension phase and be followed to week 220 (see Appendix 6 for preliminary details of this study).
c. Phase II Study
CERTIFI was a dose-ranging study that evaluated the efficacy and safety of ustekinumab induction and maintenance regimens versus placebo in patients with moderately to severely active Crohn’s disease who had received treatment with one or more TNF antagonists and who had not responded initially to therapy, who had responded and then lost response to therapy, or who were intolerant to therapy at a dose approved for Crohn’s disease.
The study design is depicted in Figure 4. Patients were randomized 1:1:1:1 to placebo or one of three IV ustekinumab induction doses: 1 mg/kg, 3 mg/kg, or 6 mg/kg. Randomizations were performed using a central randomization centre and IVRS. Patients were randomized to a treatment regimen using the adaptive randomization procedure of Pocock and Simon,27,28 with study site and initial response to TNF antagonist therapy (yes or no) as the stratification variables. For patients who had multiple TNF antagonist therapies, their initial response status to TNF antagonist therapy (yes or no) was determined by whether they initially responded to the first TNF antagonist therapy they had received.
CDR considered CERTIFI a supportive study because of several serious limitations, including the adaptive and dose-finding design, and the mix of patients who received various induction regimens in the maintenance phase (Section 3.5 Critical Appraisal). Therefore, only clinical response and remission outcomes from the induction phase of CERTIFI (using the Health Canada–approved induction regimen, ustekinumab 6 mg/kg IV) are presented and interpreted in this review.
3.2.2. Populations
a. Inclusion and ExclusionCriteria
Induction Studies
Inclusion and exclusion criteria for the induction studies are summarized in Table 6. As previously mentioned, the main difference between the patient populations in the two induction studies was their experience with previous treatments for Crohn’s disease. UNITI-1 included patients who had received TNF antagonist therapies (specifically infliximab, adalimumab, or certolizumab pegol at a dose approved for the treatment of Crohn’s disease) and who did not respond initially (primary nonresponse), who responded initially but then lost response (secondary nonresponse), or who were intolerant to the medication. Conversely, UNITI-2 enrolled patients who had failed conventional therapy, and had not previously demonstrated inadequate response or intolerance to one or more TNF antagonist therapies (i.e., infliximab, adalimumab, or certolizumab pegol). Table 9 provides definitions used to identify eligible patients for the UNITI studies based on TNF antagonist failure and/or intolerance.
Table 9
Definitions of Inadequate Initial Response, Loss of Response, or Intolerance to Tumour Necrosis Factor Antagonist Therapies in the Clinical Trials.
Key exclusion criteria for both studies were complications of Crohn’s disease that required surgery or precluded use of CDAI to assess response; history of bowel resection or diversion or any other intra-abdominal surgery within specified time periods; previous treatment with an IL-12 or IL-23 inhibitor, received IV corticosteroids, immunomodulators other than AZA, 6-MP, or MTX, biologics, or total parenteral nutrition within specified time periods; or active or latent tuberculosis (TB); opportunistic infection; HIV; or hepatitis B or C infection.
Maintenance Study
The randomized population consisted of adult patients with a history of moderately to severely active Crohn’s disease who had a clinical response to IV ustekinumab induction therapy (at week 8 of the induction studies, UNITI-1, and UNITI-2).
Patients were excluded from IM-UNITI if there had been specific changes to their concomitant medications due to Crohn’s disease (i.e., lack of efficacy) since week 0 of the induction studies (see footnote a, Table 7 for definition), or if they initiated protocol-prohibited medication or underwent Crohn’s disease–related surgery since week 0 of induction studies, or had signs or symptoms, or diagnosis of any medical condition that would have precluded enrolment in the induction studies.
Table 7
Details of Included Maintenance Study.
Phase II Study
Patients were eligible for inclusion in CERTIFI if they were adults aged 18 years or older with active Crohn’s disease (defined as a baseline CDAI score of ≥ 220 and ≤ 450) or fistulizing Crohn’s disease of at least three months’ duration, with colitis, ileitis, or ileocolitis, confirmed by radiography and/or endoscopy (Table 8). As in UNITI-1, patients had to have received infliximab, adalimumab, or certolizumab pegol at a dose approved for the treatment of Crohn’s disease and they did not respond initially, they responded initially but then lost response with continued therapy, or they were intolerant to the medication (Table 9).
Table 8
Details of Included Phase II Study.
Patients were excluded for similar reasons and criteria as in the induction studies.
b. Baseline Characteristics
Of note, the manufacturer defined baseline as the time of randomization in the UNITI and CERTIFI studies. For IM-UNITI, baseline for most efficacy analyses was defined as the time of randomization (IM-UNITI week 0/week 8 of the UNITI studies); however, for the presentation of baseline characteristics and concomitant medication use (and certain efficacy analyses; Section 3.2.5 Statistical Analysis), baseline was defined as the baseline in the UNITI studies.
Induction Studies
Key baseline demographic and Crohn’s disease characteristics from the induction phase studies are summarized in Table 10. ▬ There were more women than men enrolled in the two studies (range: 52% to 59% women); higher proportions of women were randomized to ustekinumab treatment groups than to placebo. The mean age was lower in UNITI-1 (37.3 years in both groups) than in UNITI-2 (placebo: 38.4 years; ustekinumab: 40.2 years), ▬
Table 10
Summary of Baseline Characteristics From the Induction Studies.
The mean duration of disease was approximately two to four years longer in UNITI-1 than in UNITI-2. Mean baseline CDAI scores were lower in UNITI-2 (302.2 points in both groups) compared with UNITI-1 (placebo: 319.0 points; ustekinumab: 327.6 points). The same pattern was observed for baseline median C-reactive protein (CRP) levels, which ranged from 8.5 mg/L to 9.9 mg/L in UNITI-1 and 7.8 mg/L to 8.5 mg/L in UNITI-2. A majority of patients in both studies had disease activity in both the ileum and the colon, although the proportions were lower in UNITI-2 (56% to 61%) than in UNITI-1 68% to 69%). More patients in UNITI-1 than in UNITI-2 had a history of Crohn’s disease complications, such as intra-abdominal abscess in 14% to 15% (versus 11% to 12% in UNITI-2), current or prior sinus tracts or perforation in 7% to 9% (versus 4% to 6% in UNITI-2), current or prior fistulizing disease in 45% to 51% (versus 35% to 37% in UNITI-2), and current or past stricturing in 44% to 46% (versus 28% to 35% in UNITI-2). Half of the patients in both studies (UNITI-1: 49% to 51%; UNITI-2: 56% to 57%) had at least one extra-intestinal manifestation of Crohn’s disease, with the most prevalent being arthritis or arthralgia (data not shown).
At least 70% of patients were receiving one or more concomitant medications for Crohn’s disease at baseline of UNITI-1 and UNITI-2 (Table 11). The proportion of patients receiving corticosteroids (including budesonide) was similar in both studies (approximately 44%), except in the placebo group in UNITI-2 (36%). Approximately one-third of patients in both trials were receiving immunomodulators (AZA, 6-MP, or MTX) at baseline. ▬ In general, the proportions of patients receiving each class of Crohn’s disease medication at baseline were balanced across the treatment groups in both studies.
Table 11
History of Medication Use for Crohn’s Disease in the Induction Studies.
Prior exposure to Crohn’s disease treatments for the induction studies is also summarized in Table 11. In accordance with the study designs, prior TNF antagonist exposure was reported for essentially all patients in UNITI-1 and approximately one-third of the patients in UNITI-2. Of the patients in UNITI-1 with prior exposure to a TNF antagonist, inadequate initial response with, loss of response to, or intolerance to one TNF antagonist was reported for approximately one-half of patients in both treatment groups; slightly smaller proportions of patients (placebo: 44%; ustekinumab: 41%) reported inadequate initial response with, loss of response to, or intolerance to two TNF antagonists. In keeping with the inclusion criteria, none of the patients randomized to treatment groups in UNITI-2 had an inadequate initial response with, loss of response to, or intolerance to TNF antagonists. ▬
Maintenance Study
Key baseline demographic and Crohn’s disease characteristics from the maintenance phase study are summarized in Table 12. Baseline data were reported in the clinical study report for IM-UNITI as the baseline values from the induction study (UNITI-1 or UNITI-2) in which patients were enrolled.
Table 12
Summary of Baseline Characteristics From the Maintenance Study.
IM-UNITI included a higher proportion of women (approximately 57%) than men, with a mean age ranging from 38 to 40 years. ▬
The mean duration of Crohn’s disease was approximately 10 years in IM-UNITI. Patients randomized to ustekinumab every eight weeks appeared to have somewhat less disease activity based on a lower mean CDAI score (313.1) and median CRP (8.8 mg/L) at baseline versus those randomized to ustekinumab every 12 weeks (CDAI score: 320.4; CRP: 9.1) or placebo (CDAI score: 319.1; CRP: 9.6). Patients in IM-UNITI had predominantly ileocolonic disease. ▬
At week 0 of IM-UNITI, 80 (approximately 60%) of patients in each of the three treatment groups had a clinical remission.
The proportions of patients receiving concomitant Crohn’s disease medications (at induction study baseline and enrolment in IM-UNITI), including corticosteroids, immunomodulators, and aminosalicylates, were generally similar across treatment groups (Table 13). Approximately 80% of patients were receiving one or more concomitant Crohn’s disease medications at baseline. Higher proportions of patients in the ustekinumab every eight weeks treatment group were receiving corticosteroids (including budesonide) and MTX (▬%) at baseline than in the ustekinumab every 12 weeks (44% and ▬%, respectively) and placebo groups (44% and ▬%, respectively). ▬
Table 13
Summary of Concomitant Crohn’s Disease Medications at Baseline From the Maintenance Study.
▬
Of the 397 randomized patients in IM-UNITI, approximately 45% had had a failure of (inadequate response or intolerance to) TNF antagonist therapy; the proportions were similar across treatment groups.
Phase II Study
Baseline characteristics of patients randomized to treatment in CERTIFI are presented in Table 14. As in the other studies, a majority of patients enrolled in CERTIFI were women; the proportion of women was higher in the ustekinumab 6 mg/kg group (63%) than in the placebo group (52%). The mean age was similar between groups, at 39 years, and most patients were recruited from North America, with study sites in Canada. The proportion of current smokers was higher in the ustekinumab group (32%) than in the placebo group (23%).
Table 14
Summary of Baseline Characteristics From the Phase II Study.
Patients in the ustekinumab group appeared to have worse markers of Crohn’s disease severity, based on baseline mean CDAI scores and median CRP. Consistent with the other studies, most patients had ileocolonic disease (placebo: 42%; ustekinumab: 47%) and extra-intestinal manifestations (placebo: 52%; ustekinumab: 63%). The proportion of patients who reported a history of Crohn’s disease–related surgery (total or subtotal colectomy and small or large bowel resection) was similar between treatment groups.
Table 15 summarizes the baseline concomitant and prior medications used for Crohn’s disease by the patients enrolled in CERTIFI. The proportion of patients randomized to ustekinumab 6 mg/kg who were receiving concomitant oral corticosteroids (including budesonide) at baseline was 45% versus 55% for those randomized to placebo. Conversely, the proportion of ustekinumab-treated patients receiving immunomodulators was greater than that for placebo patients (27% versus 23%, respectively), mainly driven by more patients receiving 6-MP or AZA.
Table 15
Summary of Concomitant Crohn’s Disease Medications at Baseline From Phase II Study.
Although more patients in the ustekinumab group reported having been previously treated with oral corticosteroids and immunomodulators than in the placebo group, the proportion of patients who reported having a failure with these treatments (i.e., inadequate response, intolerance, or contraindication) was greater in the placebo group than in the ustekinumab group. One-half or more patients had an inadequate initial response with, loss of response to, or intolerance to treatment with one TNF antagonist, while more than one-third reported treatment failure with two TNF antagonists; there were numerical differences between the groups regarding prior TNF antagonist response.
3.2.3. Interventions
a. Study Treatments
Induction Studies
All patients received one IV administration of placebo or ustekinumab at week 0. Two dose regimens of IV ustekinumab were used: 130 mg and a tiered dose approach approximating 6 mg/kg that allowed administration of complete vials (130 mg per vial) to patients to simplify dose calculation:
- Ustekinumab 260 mg (weight ≤ 55 kg)
- Ustekinumab 390 mg (weight > 55 kg and ≤ 85 kg)
- Ustekinumab 520 mg (weight > 85 kg).
Only the results for the 6 mg/kg tiered regimen group are approved by Health Canada and reported in the CDR review.
The manufacturer stated that the placebo preparation was identical in appearance to ustekinumab.
IV administration was chosen for induction over SC administration based on findings from a manufacturer-sponsored crossover, proof-of-concept study (study C0379T07) in which a single IV and a single SC administration of ustekinumab were evaluated.26 This study suggested that serum concentrations of inflammation-related markers decreased more rapidly after IV administration than after SC administration. Therefore, IV administration was used in the phase III induction trials of ustekinumab in Crohn’s disease. The IV induction doses were based on the results of CERTIFI.
Maintenance Study
Patients who had a clinical response to ustekinumab induction treatment were randomized in a 1:1:1 ratio at week 0 of IM-UNITI/week 8 of the UNITI studies to receive one of the following SC regimens:
- Placebo
- Ustekinumab 90 mg SC every 12 weeks (with last dose at week 36)
- Ustekinumab 90 mg SC every eight weeks (with last dose at week 40).
Randomized patients who lost response at any scheduled visit between week 8 and week 32 (visits occurred every four weeks) were eligible to receive ustekinumab as follows:
- Randomized to placebo: Patients’ dosage adjusted to receive ustekinumab 90 mg SC every eight weeks
- Randomized to ustekinumab 90 mg every 12 weeks: Patients’ dosage adjusted to receive ustekinumab 90 mg SC every eight weeks
- Randomized to ustekinumab 90 mg every eight weeks: Patients continued on ustekinumab 90 mg SC every eight weeks.
Patients who had their dosage adjusted were assessed 16 weeks after the visit at which the criteria for loss of response were met. Patients who did not show improvement in Crohn’s disease activity at that time (as assessed by the investigator) discontinued from study drug administration. Patients assessed by the investigator to have clinical improvement continued to receive the same adjusted dose in a blinded manner; however, patients who had their dosage adjusted between weeks 8 and 32 were coded as nonresponders for the primary analyses.
A total of 27.5% (109 patients) of the randomized population had a dosage adjustment as follows:
- Among patients randomized to placebo, 38.3% (51 patients) had a dosage adjustment to an ustekinumab 90 mg SC every eight weeks regimen
- Among patients randomized to ustekinumab 90 mg SC every 12 weeks, 22.0% (29 patients) had a dosage adjustment to an ustekinumab 90 mg SC every eight weeks regimen
- Among patients randomized to ustekinumab 90 mg SC every eight weeks, 22.0% (29 patients) continued on the same regimen.
▬
Phase II Study
Patients in CERTIFI were randomized (1:1:1:1) to receive one of the following IV induction regimens at week 0:
- Placebo
- Ustekinumab 1 mg/kg
- Ustekinumab 3 mg/kg
- Ustekinumab 6 mg/kg.
In the maintenance phase, patients who were in clinical response to IV ustekinumab induction (irrespective of dose) at week 6 were re-randomized (1:1 ratio) at week 8 to receive an SC maintenance regimen of placebo (N = 73) or ustekinumab 90 mg (N = 72) administered at week 8 and week 16. Hence, the maintenance phase consisted of patients who received induction treatment with dosages that are not Health Canada–approved; therefore, only data from the induction phase of CERTIFI are reported in this review.
Placebo administrations were reported as having the same appearance as the respective ustekinumab administrations.
Treatment adherence was controlled by the study staff administering the drug as an SC injection and/or an IV infusion.
b. Concomitant Medications
Induction Studies
Patients were permitted to receive the following concomitant medications to treat Crohn’s disease:
- Oral aminosalicylates (i.e., 5-ASA)
- Oral corticosteroids at a prednisone-equivalent dose of ≥ 40 mg/day or ≤ 9 mg/day of budesonide
- Immunomodulators (i.e., AZA, 6-MP, or MTX)
- Antibiotics, as a primary treatment for Crohn’s disease.
Patients had to be receiving stable doses for at least three weeks (four weeks in the case of immunomodulators) and to be taking them for a total of 12 weeks or more before baseline. Patients were required to maintain a stable dose of these concomitant drugs throughout the induction studies.
Patients were not allowed to start any of the aforementioned medications or total parenteral nutrition as a treatment for Crohn’s disease during the studies.
Maintenance Study
Patients were permitted to receive the same concomitant medications as in the induction studies, with the same conditions regarding maintaining stable dosages (except for oral corticosteroids) and the same prohibition against starting therapy at any point during IM-UNITI. Patients were permitted to transiently use (i.e., for four weeks or less) increased doses of oral corticosteroids for reasons other than loss of response to treatment for Crohn’s disease (e.g., for surgery, asthma, adrenocortical insufficiency, etc.). Patients receiving corticosteroids at the start of IM-UNITI who had a clinical response to ustekinumab had their corticosteroid tapered. Tapering was mandatory and followed this schedule:
- Oral corticosteroids (other than budesonide)
- Dose > 15 mg/day prednisone or equivalent: taper daily dose by 5 mg/week until receiving 10 mg/day, then continue tapering by 2.5 mg/week until discontinued.
- Dose 11 to 15 mg/day prednisone or equivalent: taper daily dose to 10 mg/day for 1 week, then continue tapering by 2.5 mg/week until discontinued.
- Dose ≤ 10 mg/day prednisone or equivalent: taper daily dose by 2.5 mg/week until discontinued.
- Oral budesonide
- Daily dose tapered by 3 mg every 3 weeks until discontinued.
Tapering could be suspended if a patient experienced a worsening of disease activity; tapering could then be resumed within four weeks.
3.2.4. Outcomes
The outcomes analyzed in the studies and relevant to this review according to the protocol (Section 2) are summarized in Table 6, Table 7, and Table 8 for the induction, maintenance, and phase II studies, respectively. The following are brief descriptions of the outcomes from the studies. Detailed descriptions may be found in Appendix 5: Validity of Outcomes.
a. Crohn’s Disease Activity Index
The CDAI is an instrument used to evaluate and quantify the severity of symptoms for patients with Crohn’s disease. The CDAI consists of the following eight factors, each of which is summed after adjustment with a weighting factor:
- Number of liquid or soft stools each day for seven days
- Abdominal pain each day for seven days (0 [none] to 3 [severe])
- General well-being each day for seven days (0 [well] to 4 [terrible])
- Presence of complications
- Requirement to take diphenoxylate/atropine or opiates for diarrhea
- Presence of an abdominal mass (0 [none], 2 [questionable], 5 [definite])
- Hematocrit of < 0.47 in men and < 0.42 in women
- Percentage deviation from standard weight.
The total CDAI score ranges from 0 to 600, with higher scores indicating greater Crohn’s disease activity.
Clinical Remission
Clinical remission was defined as a CDAI score < 150 points. However, patients who had any of the following events before the clinical remission assessment end point were not considered in clinical remission, regardless of the CDAI score:
- specified changes in concomitant Crohn’s disease medications
- a Crohn’s disease–related surgery (with the exception of drainage of a cutaneous or perianal abscess or seton placement)
- discontinuation of treatment due to lack of efficacy or due to an adverse event of worsening Crohn’s disease
- in IM-UNITI, loss of clinical response, defined as a CDAI score ≥ 220 points and a ≥ 100-point increase from CDAI score from the week 0 to week 8 in the induction study.
In addition, patients who did not return for evaluation or who had insufficient data to assess their clinical response status (i.e., four or fewer components of the CDAI are available) were also not considered to have achieved clinical response.
Clinical remission at week 44 was the primary outcome of IM-UNITI, and a key secondary outcome at week 8 of the UNITI and CERTIFI studies.
Those who could discontinue corticosteroids and were in clinical remission (i.e., had a CDAI score ≤ 150) were considered to have corticosteroid-free clinical remission. This was a key secondary outcome at week 44 in IM-UNITI.
Clinical Response
Clinical response was defined as a reduction from baseline in the CDAI score of ≥ 100 points. In the patients with a baseline CDAI score of 220 to 248, clinical response was considered to be achieved if the CDAI score was reduced to less than 150. In addition, treatment failure rules were applied to determine each patient’s final response status. Patients who had any of the following events before the clinical response assessment end point were not considered to have a clinical response, regardless of the CDAI score:
- a Crohn’s disease–related surgery (with the exception of drainage of a cutaneous or perianal abscess or seton placement) that was thought to be a result of lack of efficacy of study treatment
- specified changes in concomitant Crohn’s disease medications.
In addition, patients who did not return for evaluation or who had insufficient data to assess their clinical response status (i.e., four or fewer components of the CDAI are available) were not considered to have a clinical response.
Clinical response at week 6 was the primary outcome in the UNITI and CERTIFI studies and a key secondary outcome at week 44 in IM-UNITI.
b. Inflammatory Bowel Disease Questionnaire
The Inflammatory Bowel Disease Questionnaire (IBDQ) is a 32-item questionnaire that aims to capture how the patient felt during the two weeks before the measurement time point. Questions are related to the symptoms of Crohn’s disease, how the patient felt in general and his/her mood over the previous two weeks, and social or employment problems that may have resulted from Crohn’s disease.32,33 Patients are asked to recall symptoms and quality of life from the last two weeks, with response graded on a seven-point Likert scale (1 being the worst situation, 7 being the best) with the total IBDQ score ranging between 32 and 224. An increase in IBDQ score indicates an improvement in health-related quality of life, while a decrease indicates deterioration. Scores of patients in remission typically range from 170 to 190. The minimal clinically important difference (MCID) for the IBDQ is considered 16 points.34 IBDQ was a secondary outcome assessment tool in all of the included studies.
c. Short Form (36) Health Survey
The Short Form (36) Health Survey (SF-36) is a generic instrument that was used to assess health-related quality of life in the UNITI and IM-UNITI studies. It assesses eight domains of health-related quality of life: physical function, role physical, general health, mental health, role emotional, social functioning, and vitality. The eight domains are aggregated to create two component summaries: the physical component summary (PCS) and the mental component summary (MCS), with scores ranging from 0 to 100, with higher scores indicating better health-related quality of life. The MCID for the PCS and the MCS has been estimated at 4.1 and 3.9, respectively, in the Crohn’s disease patient population.35 However, there is uncertainty as to the validity of these estimates. In general use, the MCIDs are two points for the PCS and three points for the MCS.36
d. Work Limitations Questionnaire
The Work Limitations Questionnaire (WLQ) is a 25-item self-reported questionnaire that asks respondents to rate their level of difficulty or ability to perform specific job demands to assess health-related loss of work productivity. 37–39 The 25 items are aggregated into four scales: time management, physical demands, mental-interpersonal, and output. Scale scores range from 0 (limited none of the time) to 100 (limited all of the time) and represent the reported amount of time in the prior two weeks that respondents were limited in functioning on the job. The MCID has been estimated in non-Crohn’s disease conditions to range from 3.2 to 4.40,41 The WLQ was a secondary outcome measure in the UNITI and IM-UNITI studies.
e. Productivity Visual Analogue Scale
The impact of disease on patients’ daily productivity was measured using a visual analogue scale (VAS, 0 = no impact at all to 10 = impacts productivity very much). No other information about this measure was identified. This was a secondary outcome in all of the included studies.
f. Time Lost From Work
Time lost from work was collected by asking patients, “How many days did you miss from work due to your Crohn’s disease in the last four weeks?” No other information about this measure was identified. This was a secondary outcome in all of the included studies.
g. Mucosal Healing
Mucosal healing was defined as the complete absence of mucosal ulcerations. Mucosal healing was assessed using endoscopy (ileocolonoscopy) in a subset of patients from specific study sites enrolled in the UNITI and IM-UNITI studies (endoscopic substudy). Patients who participated in the endoscopic substudy underwent endoscopic assessments at baseline and at week 8 of the UNITI studies, and at the end of the IM-UNITI (week 44 of maintenance). In addition, biopsies were collected to support exploratory histologic evaluation. Video endoscopies were assessed by a central facility that was blinded to treatment group.26 Changes in the Simplified Endoscopic Disease Severity Score for Crohn’s Disease (SES-CD), in addition to endoscopic detection of presence or absence of mucosal ulceration, were used to evaluate mucosal healing. The SES-CD consists of four endoscopic variables: presence and size of ulcers, proportion of surface covered by ulcers, proportion of surface affected by disease, and presence and severity of stenosis.42 Each endoscopic variable is scored by colon segment (scores range 0 to 3), and the total SES-CD score ranges from 0 to 56, with higher SES-CD scores indicating more severe disease activity.42 The MCID was not identified.
h. Need for Surgery
Crohn’s disease–related surgery included, but was not limited to, total or subtotal colectomy or other partial bowel resection, and perianal surgery. Minor procedures such as placement of a seton or cutaneous drainage of an abscess were excluded. Crohn’s disease–related surgery was evaluated in each included study.
i. Safety
Treatment safety was assessed in all included studies by collecting adverse events (AEs), serious AEs (SAEs), withdrawals due to AEs (WDAEs), vital signs, AEs related to drug-administration reactions, hematology and chemistry parameters, physical examinations, and 12-lead electrocardiograms. None of the studies was designed to specifically evaluate safety outcomes.
The frequency of AEs throughout was summarized by treatment group, the Medical Dictionary for Regulatory Activities (MedDRA) system-organ class, and preferred term. All reported AEs with onset during the treatment phase (i.e., treatment-emergent AEs, and AEs that had worsened since baseline) were included in the analysis.
3.2.5. Statistical Analysis
a. Primary Outcome
Sample Size and Power Calculations
Sample size considerations for all four studies are summarized in Table 16.
Table 16
Summary of Sample Size Consideration From the Included Studies.
The sample size and power calculations for all four studies were based on the chi-square test (two-sided) for detecting a significant difference between patients receiving the higher dosage of ustekinumab (tiered weight-based dosage approximating ustekinumab 6 mg/kg IV in the UNITI studies, 90 mg SC every eight weeks in IM-UNITI, and ustekinumab 6 mg/kg IV in CERTIFI) and those receiving placebo. The assumptions for sample size and power calculations for UNITI-1 were based on data from CERTIFI, and those for UNITI-2 and CERTIFI were based the phase IIa manufacturer-sponsored crossover, proof-of-concept study, study C0379T07;26 additional assumptions were made based on “recent [inflammatory bowel disease] literature” for CERTIFI.28 Assumptions for IM-UNITI sample size were based on clinical remission data from randomized controlled trials of maintenance treatment for Crohn’s disease with infliximab and adalimumab.43,44
Primary Outcome Analysis
The proportion of patients in clinical response at week 6 (UNITI studies and CERTIFI) or clinical remission at week 44 (IM-UNITI) was compared between the ustekinumab treatment group and the placebo group using a two-sided Cochran–Mantel–Haenszel chi-square test, stratified by study region (Asia, Eastern Europe, or rest of world) and CDAI score (≤ 300 or > 300) in UNITI-1 and UNITI-2, and by initial response to TNF antagonist therapy (yes or no) in UNITI-1; the latter was the only stratification factor for the CERTIFI analysis. Stratification factors in IM-UNITI were clinical remission status at week 0 (yes or no), ustekinumab induction dose (130 mg or tiered dosage approximating ustekinumab 6 mg/kg), and induction study (UNITI-1 or UNITI-2). Comparisons were made with a significance level of 0.05.
Patients randomized to treatment before UNITI studies were restarted were not included in the primary analysis population of the UNITI studies or IM-UNITI. In addition, data for one patient at a single study site in UNITI-2 (▬) were excluded because the patient was randomized despite major protocol deviations (e.g., not meeting the inclusion criteria for active Crohn’s disease).
In the UNITI studies, the CDAI score was calculated for a visit only if four or more of the eight components were available at that visit. Any missing components were imputed by carrying forward the last non-missing component, with the exception of a missing hematocrit value, when at least four of the eight components were available. If the CDAI score could not be calculated (i.e., four or fewer components available) at a visit, the CDAI score was considered missing. Patients with a missing CDAI score at week 6 were not considered to have achieved clinical response at week 6. (See Section 3.5 for more discussion on missing data.)
Sensitivity Analyses
The manufacturer conducted several sensitivity analyses to examine the robustness of the primary outcome analysis in each study. Sensitivity analyses of the primary outcome were conducted using the following data methods: observed case, last observation carried forward (LOCF), multiple imputation, and worst case missing data methods. Treatment failure rules superseded missing data rules, meaning that if a patient had both a treatment failure (i.e., Crohn’s disease–related surgery, specified changes in concomitant Crohn’s disease medications, discontinued treatment due to lack of efficacy or due to an adverse event, or, in IM-UNITI, a loss of response) before end point assessment and had a missing CDAI score at the end point assessment (i.e., four or fewer components of the CDAI available), the patient was considered a nonresponder in the sensitivity analysis regardless of whether CDAI data were present.
Subgroup Analyses
Subgroup analyses of the efficacy of ustekinumab versus placebo for the primary outcome were carried out for demographic baseline disease characteristics (baseline of the UNITI studies for IM-UNITI), Crohn’s disease medication history, concomitant Crohn’s disease medication use at baseline (baseline of the UNITI studies for IM-UNITI), study site location, and initial response to TNF antagonist therapy (except UNITI-2). It was reported that subgroup analyses were planned when the number of patients in the subgroups permitted; however, the threshold number of patients was not specified. The odds ratios of each ustekinumab dosage group versus placebo and corresponding 95% confidence intervals were provided for each of the subgroups.
b. Secondary Outcomes
Categorical secondary outcomes (e.g., the proportion of patients with a Crohn’s disease–related surgery) were also compared between ustekinumab and placebo using a two-sided Cochran–Mantel–Haenszel chi-square test (or chi-square test where appropriate; circumstances in which this occurred were not specified), with the same data-handling protocol specified for the primary outcome analysis.
Continuous secondary outcomes were compared using analysis of variance or covariance models. An analysis of variance or covariance on the van der Waerden normal scores or a nonparametric test such as the Kruskal–Wallis test was used if the normality assumption was uncertain. Covariates for the analyses of change from baseline in productivity VAS and WLQ were: study region (Asia, Eastern Europe, or rest of world), baseline CDAI score (≤ 300 or > 300), and initial response to TNF antagonist therapy (yes or no) (except UNITI-2). Covariates for the change from baseline in IBDQ score and SF-36 were not stated.
The IBDQ score and the PCS and MCS scores of the SF-36 were analyzed as change from baseline and as the proportion of patients achieving at least a 16-point improvement (IBDQ) or at least a five-point improvement from baseline (SF-36 components). If any one of the dimensions within the IBDQ could not be calculated (e.g., due to missing items), then the total IBDQ score was not calculated. For the SF-36, if < 50% of the items that make up a subscale were available, the subscale was not calculated. And, if any of the individual subscales that constitute the PCS or the MCS were missing, then the PCS or MCS summary scores were not calculated.
▬
For IBDQ, SF-36, productivity VAS, WLQ, and surgery, treatment failure rules used for the primary analysis applied only to the daily productivity outcome, and no imputation was performed for missing values; missing values were coded as missing.
c. Multiple Comparisons
The primary efficacy comparisons in all the included studies used a fixed-sequence testing procedure to control the overall type I error rate at a significance level of 0.05 (two-sided).
For the induction analyses (UNITI and CERTIFI studies), the comparison between the ustekinumab high-dose group (dose approximating 6 mg/kg ustekinumab) and placebo was made first; if the ustekinumab high-dose group was statistically significantly different from the placebo group, then the ustekinumab low-dose group (130 mg ustekinumab in UNITI; 3 mg/kg followed by 1 mg/kg ustekinumab in CERTIFI) was compared with the placebo group at the two-sided 0.05 level of significance. However, given that this review considers only the Health Canada–approved ustekinumab 6 mg/kg IV induction dose, this procedure has no impact on the interpretation of the results from the induction phase studies because the lower doses are out of scope.
For the IM-UNITI primary analysis, however, this procedure is relevant, as both ustekinumab dosages are approved. In IM-UNITI, the 90 mg every eight weeks ustekinumab group was compared with the placebo group first. The 90 mg every 12 weeks ustekinumab group was compared with the placebo group only if the comparison of the 90 mg every eight weeks group with placebo was statistically significant at the two-sided 0.05 level of significance.
In all included studies, major secondary outcomes (Table 17) were tested in a hierarchical fashion. The first major secondary outcome was tested only if the primary outcome was positive, and the subsequent outcomes were tested only if the preceding outcome in the hierarchy was positive. Within each major secondary outcome, the fixed-sequence testing procedure was applied: the low dose for a major secondary outcome could not be tested unless the low dose tested positive for the preceding major secondary outcome (or the primary outcome if the outcome being tested was the first major secondary outcome).
Table 17
Major Secondary Outcomes.
It was not reported whether other secondary outcomes (e.g., change in IBDQ score) were included in the hierarchical analysis procedure, and it is assumed that they were not.
d. Endoscopy Substudy
The primary objectives of the endoscopy substudy were to evaluate:
- The efficacy of ustekinumab compared with placebo to induce endoscopic mucosal healing
- The efficacy of ustekinumab maintenance treatment compared with placebo on the achievement of endoscopic healing of the mucosa among patients who had a clinical response to ustekinumab induction.
The pre-specified primary analysis population for endoscopy outcomes in the induction phase was the combined populations from UNITI-1 and UNITI-2, and the ustekinumab induction-treatment groups (130 mg and ~6 mg/kg) were pooled. This pooled ustekinumab induction-treatment group was compared with the placebo induction-treatment group. For analyses of maintenance endoscopy outcomes, the ustekinumab maintenance-treatment groups (90 mg every 12 weeks and 90 mg every eight weeks) were combined. This pooled ustekinumab maintenance-treatment group was compared with the maintenance placebo group.
Patients had to have the following in order to be eligible for the endoscopic analyses:
- A SES-CD score of three or more at baseline (for evaluation of SES-CD–based end points), or
- Evidence of ulceration in any segment of the colon at baseline (for evaluation of mucosal healing).
The primary outcome in the endoscopy substudy was the change from baseline in the SES-CD score at week 8 of induction. The major secondary outcomes were (in hierarchical order according to the hierarchical testing procedure for the substudy):
- The change from induction baseline in the SES-CD score at week 44 of maintenance
- The proportion of patients with mucosal healing at week 44 of maintenance
- The proportion of patients with mucosal healing at week 8 of induction.
The change from baseline in the SES-CD score at week 8 of induction was compared between the pooled ustekinumab group and the placebo group using an analysis of covariance on the van der Waerden normal scores, with baseline SES-CD score and study as covariates, at a significance level of 0.05. Missing data rules and treatment failure rules were applied. (Note that the manufacturer-provided documents did not specify whether these rules were the same as those applied in the UNITI and IM-UNITI studies.)
The manufacturer reported that the substudy also included pre-specified subgroup analyses of key endoscopic end points by individual induction study, by induction ustekinumab dose, and by maintenance ustekinumab dosage, as well as sensitivity analyses to evaluate the impact of different missing data rules. However, data for the subgroup and sensitivity analyses were not provided in the materials submitted for this review.
e. Analysis Populations
The manufacturer stated that efficacy analyses included patients randomized at week 0 of each study, and were based on an intention-to-treat principle. Therefore, the efficacy data for each patient were analyzed according to the assigned treatment, regardless of the actual treatment received. See Section 3.5 Critical Appraisal for comments on this.
Safety analyses were based on patients who received at least one dose of study treatment. Patients were analyzed according to the actual treatment received.
3.3. Patient Disposition
3.3.1. Induction Studies
The number of patients screened for eligibility to enter the induction studies was not reported in the clinical study reports.
Greater than 90% of patients randomly assigned to ustekinumab or placebo completed both induction studies (Table 18). A higher proportion of patients treated with ustekinumab than given placebo entered the maintenance study (IM-UNITI). More patients in the ustekinumab group in UNITI-1 (6%; placebo: 4%) and more patients in the placebo group in UNITI-2 (6%; ustekinumab: 1%) discontinued the study.
Table 18
Patient Disposition From the Induction Studies.
3.3.2. Maintenance Study
A total of 1,281 patients who completed the ustekinumab induction studies UNITI-1 and UNITI-2 were enrolled in the IM-UNITI maintenance-treatment study (Table 19). A total of 397 of these patients (31.0%) had a clinical response to ustekinumab induction and were randomized to the primary population. ▬
Table 19
Patient Disposition From the Maintenance Study.
Over 44 weeks of treatment, greater than 20% of patients in each treatment group discontinued treatment, and from 7% (ustekinumab every 12 weeks) to 11% (ustekinumab every eight weeks) discontinued the study. The most common reason for discontinuing treatment was lack of efficacy, followed by AEs.
3.3.3. Phase II Study
The number of patients screened for eligibility to enter CERTIFI was not reported in the clinical study report.
A total of 526 patients were randomized to treatment in CERTIFI, with 132 and 131 patients randomized to placebo and ustekinumab 6 mg/kg, respectively (Table 20). A larger proportion of patients receiving placebo (14%) than receiving ustekinumab (6%) discontinued treatment in the eight-week induction phase. Lack of efficacy was the primary reason, as well as the related worsening of Crohn’s disease, captured as an AE.
Table 20
Patient Disposition From the Phase II Study Induction Phase.
3.4. Exposure to Study Treatments
Patients enrolled in the UNITI studies and in the induction phase of CERTIFI received a single IV infusion of the treatment they were randomized to, administered by study staff.
Patients enrolled in IM-UNITI in the primary analysis population who were randomized to ustekinumab received treatment as follows (up to the point of meeting criteria for dosage adjustment due to loss of response):
- 90 mg every 12 weeks: 132 patients received a median cumulative dose of 360.0 mg
- 90 mg every eight weeks: 131 patients received a median cumulative dose of 540.0 mg.
Patients in IM-UNITI had a mean duration of follow-up of 32, 37, and 35 weeks in the placebo, ustekinumab every 12 weeks, and ustekinumab every eight weeks treatment groups. (Note: standard deviations for these means were not reported in the clinical study reports.)
The median average daily prednisone-equivalent oral corticosteroid dosage (excluding budesonide) at IM-UNITI baseline was the same for the ustekinumab groups (20.0 mg/day [IQR: 5.0 to 30.0 mg/day for every 12 weeks group; 10.0 to 25.0 mg/day for every eight weeks group]) and lower in the placebo group (15.0 mg/day [IQR: 10.0 to 25.0 mg/day]). The extent of exposure to oral corticosteroids during the 44 week study was not reported. ▬
3.5. Critical Appraisal
3.5.1. Internal Validity
There were a number of potential differences between studies and between the placebo and ustekinumab groups within studies with respect to certain baseline characteristics (Section 3.2.2.2). A notable potential imbalance was in the concomitant use of oral corticosteroids at baseline in UNITI-2, in which 44% of patients treated with ustekinumab and 36% of patients on placebo were receiving these drugs (Table 11). ▬ The extent of corticosteroid exposure during UNITI-2 was not reported; however, the study protocol required that patients maintain their dose at a stable level throughout. This imbalance in the proportion of patients receiving oral corticosteroids ▬ and given the documented efficacy of corticosteroids in inducing response and remission in Crohn’s disease,45 could have led to an overestimation of the response and remission outcomes for ustekinumab versus placebo, although there is no direct evidence that this occurred.
The IM-UNITI study protocol included a pre-specified regimen for tapering patients’ oral corticosteroid if they had a clinical response and were receiving corticosteroids at week 0. Corticosteroid tapering is recommended by guidance from the European Medicines Agency (EMA) on the design of trials evaluating treatments for Crohn’s disease.46 The clinical expert consulted by CDR noted that the tapering regimen used in the IM-UNITI trial was a reasonable reflection of clinical practice in Canada. The median average daily prednisone-equivalent oral corticosteroid dose (excluding budesonide) at IM-UNITI baseline was higher in the ustekinumab groups than the placebo group. However, the extent of exposure to oral corticosteroids during the course of the study was not reported, ▬ There is no direct evidence that oral corticosteroid exposure in IM-UNITI affected outcomes.
The manufacturer indicated that an adaptive randomization approach (Pocock and Simon minimization method) was used in CERTIFI because many sites were expected to enroll very few participants, making it difficult to achieve balance in treatment assignment within each site if a traditional permuted-block randomization were used.27,28 While the Pocock and Simon minimization method is a recognized approach to adaptive randomization, using adaptive randomization in general creates challenges in interpretation of the study outcome relative to a fixed randomization procedure, and trials of this design are considered to be “less well understood” by the FDA for this reason.47 Specifically, concern has been expressed that changes to the randomization probabilities could create imbalances in known and unknown patient characteristics at the end of the study, thereby increasing the risk of bias. The FDA recommends caution with the use of adaptive randomization in confirmatory trials.47
The IM-UNITI study used re-randomization at week 8 for patients receiving ustekinumab who responded to induction therapy in the UNITI studies. The strength of this design is that it allows evaluation of whether the response is maintained in the absence or presence of continued ustekinumab therapy. The use of separate induction and maintenance studies is consistent with EMA guidance for the development of drugs for the treatment of Crohn’s disease.46 However, a limitation of this approach is that all patients enrolled in IM-UNITI are a selected population: they were responders to induction therapy in the UNITI studies and could tolerate treatment with ustekinumab. However, this design is reasonable because these are also the patients who would be continued on treatment in clinical practice; nonetheless, from a research perspective, it may obscure the true effectiveness and occurrence of AEs.
Study treatments were administered in a double-blind manner in all of the included studies. Given that the maintenance study IM-UNITI included two dosage regimens of ustekinumab (i.e., 90 mg every 12 weeks and 90 mg every eight weeks), patients received an SC administration of study treatment (either placebo or ustekinumab) every four weeks from week 0 to week 40 with the exception of week 4. It is difficult to ascertain from the provided data whether the adverse event profile of ustekinumab could have compromised blinding in IM-UNITI (or the induction studies), given that there were few evident differences between groups regarding frequency of AEs, including the proportion of patients who experienced administration-related reactions.
Greater than 20% of patients prematurely discontinued treatment in IM-UNITI. For all analyses related to clinical remission and clinical response, patients who discontinued for any reason were considered to have failed treatment. This is a common approach to handling missing data in these types of trials, but may bias results in the case of differential withdrawal rates. In IM-UNITI, the overall proportion of withdrawals and the reasons for discontinuation were generally similar between the placebo and ustekinumab groups. Lack of efficacy was the most commonly cited reason for discontinuation in both the placebo and ustekinumab groups (11% in each group), suggesting that classifying patients who discontinued as having failed treatment may be an accurate reflection for half of those who failed to complete the study. High rates of withdrawal are not unusual in longer-term studies for Crohn’s disease and are consistent with the high rates of withdrawal (or early escape) reported in previous pivotal studies for TNF antagonists in the maintenance treatment of Crohn’s disease. The primary analysis was supported by a number of sensitivity analyses to investigate alternative approaches for imputing missing data (e.g., observed case, LOCF, multiple imputation, and the worst case missing data method). In general, these analyses yielded results similar to the primary analysis.
The protocols for each study indicated that the primary and major secondary outcome analyses were based on the intention-to-treat principle. However, one patient randomized to treatment in UNITI-2 was excluded from the analysis because of major protocol deviations. This is reasonable, given that it was discovered that the patient did not meet the inclusion criteria for active Crohn’s disease, and loss of one patient is unlikely to have affected the validity of the results in that study. However, for certain outcome analyses relevant to this review (i.e., IBDQ, SF-36, WLQ, and surgery) the intention-to-treat principle was not used.
As mentioned previously, the protocols for the UNITI studies and IM-UNITI stated that the CDAI score was calculated for a visit only if four or more of the eight components were available at that visit. When at least four of the eight components were available, any missing components were imputed by carrying forward the last non-missing component (with the exception of a missing hematocrit value). If the CDAI score could not be calculated (i.e., four or fewer components available) at a visit, the CDAI score was considered missing. Patients with a missing CDAI score at study end point were not considered to have achieved clinical response or remission, depending on the study. In UNITI-1, 13 (5%) patients each in the placebo and ustekinumab groups had missing CDAI scores at week 6. In UNITI-2, eight (4%) placebo-treated patients and three (1.4%) ustekinumab-treated patients had missing CDAI scores at week 6. In IM-UNITI, eight (6%), one (2%), and 10 (8%) patients in each of the placebo, every 12 weeks, and every eight weeks groups had missing CDAI scores at week 44; however, almost all of these patients were already coded as treatment failures (i.e., because of Crohn’s disease–related surgery or medication adjustment before week 44). Differences between treatment groups may introduce bias; however, only in UNITI-2 and the ustekinumab every 12 weeks group in IM-UNITI were differences in missing data notably different. It is uncertain whether these differences had a measurable effect on the validity of the results in those two studies.
In the UNITI studies and IM-UNITI, analyses of health-related quality of life and functional status were limited for several reasons. ▬ However, the direction of potential bias is unclear.
There was limited adjustment for multiple comparisons in the UNITI studies and IM-UNITI. The comparisons between treatment groups for health-related quality of life and functional outcomes, as well as surgery and subgroup analyses, were included in the pre-specified hierarchical analysis plan (Table 17). Given the number of outcomes and statistical comparisons made in each study, this is a potential limitation when interpreting study results.
The endoscopic substudy was limited by several factors. First, patients were enrolled only from select study sites within the UNITI study programs. Only 334 patients (142 from UNITI-1 and 192 from UNITI-2) of the total 1,409 enrolled in the UNITI studies were included in the endoscopy substudy. Only 252 of 334 patients met the inclusion criterion of a baseline SES-CD score of three or more points. Second, it was not described how patients were selected, and what impact this had on maintaining randomization. The manufacturer reported potential differences between the pooled ustekinumab group and placebo group with respect to certain baseline characteristics (median CDAI score, median CRP level, and extra-intestinal manifestations of Crohn’s disease). It is possible that certain patient or disease factors were no longer adequately balanced between groups, which may have acted as important confounders or effect modifiers of the endoscopy analysis. Third, the primary outcome of the endoscopy substudy was the change from baseline in the SES-CD score at week 8 of induction. Studies have been conducted to determine the instrument’s reliability and validity.42,48 However, a recent Cochrane systematic review reported that, although the SES-CD is increasingly used in randomized controlled trials of interventions for Crohn’s disease, its clinical relevance has not been fully elucidated.49 In particular, the overall validity of the SES-CD has not been fully established, and SES-CD cut-off points used for endoscopic remission and response require additional study. Furthermore, the MCID for the change in SES-CD needs to be determined. Last, the SES-CD was centrally assessed by a single evaluator for all video endoscopies. Although intra- and inter-rater reliability have been reported as high for the SES-CD (interclass correlation coefficients of > 0.9), it has been noted that there were potentially important biases associated with these analyses.49 Therefore, there is some uncertainty as to how reliable the assessments were.
3.5.2. External Validity
Inclusion and exclusion criteria for both the induction and maintenance studies were generally reflective of patients who would be considered candidates for treatment with ustekinumab in Canada. In its clinical practice guidelines on the use of TNF antagonists in the treatment of Crohn’s disease, the Canadian Association of Gastroenterology (CAG) states that moderate-to-severe Crohn’s disease should be defined as a CDAI score between 220 and 400.50 This is consistent with the inclusion criteria of the UNITI studies and CERTIFI.
The study protocols for the included studies specifically defined inclusion criteria for inadequate response, loss of response, or intolerance to a previous TNF antagonist, immunomodulator, or corticosteroid (Table 9). The protocols stated that patients could be considered primary nonresponders to treatment with adalimumab after receiving one 80 mg dose followed by one 40 mg dose. This is half the dose of adalimumab recommended in the Canadian product monograph for inducing remission (i.e., 160 mg at week 0 and 80 mg at week 2).20 Similarly, patients could be considered primary nonresponders following treatment with infliximab if they had received two or three doses of 5 mg/kg; the Canadian product monograph recommends three doses of 5 mg/kg for induction with infliximab (i.e., at weeks 0, 2, and 6).18,19 Dosage recommendations in the CAG guidelines on the use of TNF antagonists in the treatment of Crohn’s disease are consistent with those noted in the product monographs.50 The clinical expert consulted by CDR noted that more recent understanding of drug levels has changed clinical practice. For patients with no or poor clinical response after two induction doses (week 0 and 2) of infliximab, most clinicians would provide a rescue dose of 10 mg/kg at weeks 4 and 6 before deciding whether the patient is a primary nonresponder. A similar approach would be taken with adalimumab in practice. In addition, patients could be eligible for enrolment in the UNITI and CERTIFI studies if they failed treatment with certolizumab pegol. Certolizumab has been approved for use in the treatment of Crohn’s disease by the FDA, but not by Health Canada or the EMA. The CAG guidelines on the use of TNF antagonists state that certolizumab has been shown to be clinically effective in the treatment of Crohn’s disease.50 However, the FDA and the CAG guidelines recommend that certolizumab (400 mg SC) be administered at weeks 0, 2, and 4 for induction;50,51 the criteria set by the manufacturer allow primary nonresponse to certolizumab to be declared after only two doses.
Differences between the UNITI studies with respect to baseline disease characteristics (e.g., higher baseline mean CDAI score, CRP, and proportion of patients with Crohn’s disease complications in UNITI-1) likely reflect the main difference in the patient populations: namely, that patients with more advanced disease, based on their having failed TNF antagonist therapy, were enrolled in UNITI-1.
As well, a larger proportion of patients in UNITI-2 were receiving concomitant aminosalicylates at baseline (42% to 45%) than in UNITI-1 (20% to 22%). ▬ However, aminosalicylates are at present not recommended for the treatment of Crohn’s disease. 45,52 The clinical expert consulted by CDR did not consider this imbalance likely to be an important source of bias.
The lack of an active control group is a limitation of the studies. There are three other biologics indicated for the treatment of Crohn’s disease in Canada that could be considered relevant comparators: infliximab, adalimumab, and vedolizumab. Infliximab and adalimumab are long-established TNF antagonists in the treatment of Crohn’s disease, and studies could have been conducted to compare ustekinumab with these. The Crohn’s disease development programs for ustekinumab and vedolizumab overlapped, so designing a study comparing these was likely not feasible. In the absence of a study directly comparing ustekinumab with other biologics, the manufacturer submitted an indirect comparison, and CDR identified two other indirect comparisons in addition (0).
Another key limitation is that the maintenance-treatment phases (IM-UNITI and the maintenance phase of CERTIFI) randomized or re-randomized patients who had a response or remission with various strengths of ustekinumab. The UNITI induction studies included two dose regimens for IV ustekinumab: 130 mg and a weight-based tiered dose approximating 6 mg/kg. CERTIFI included three IV ustekinumab doses: 1 mg/kg, 3 mg/kg, and 6 mg/kg. The product monograph for ustekinumab indicates that the approved induction regimen is the weight-based tiered dose approximating 6 mg/kg. However, IM-UNITI and the maintenance phase of CERTIFI randomized patients from the induction studies or phase regardless of the induction dose of ustekinumab used. Table 34 shows the distribution of patients in the IM-UNITI treatment groups by ustekinumab induction dose. ▬ The proportions of patients who achieved clinical response at week six with ustekinumab 130 mg were 34% and 52%, and with 6 mg/kg were 34% and 56% in UNITI-1 and UNITI-2, respectively. Similarly, the proportions of patients who achieved clinical remission at week eight with ustekinumab 130 mg were 16% and 31%, and with 6 mg/kg were 21% and 40% in UNITI-1 and UNITI-2, respectively. Hence, randomizing patients from the ustekinumab 130 mg IV induction-treatment groups in the UNITI studies to IM-UNITI limits the generalizability of the IM-UNITI results to clinical practice. In practice, the 130 mg dose is not expected to be used because it is not the approved regimen. Of note, the maintenance phase of CERTIFI included patients who had a response and remission with doses not approved by Health Canada. For this reason, as well as others already mentioned, only data from the induction phase of CERTIFI were included in this review.
The CDAI has been validated within the Crohn’s disease population (Appendix 5). The clinical expert consulted by CDR noted that CDAI scores are not calculated in clinical practice, although all of the various individual components of the scale are evaluated when assessing the status of a patient with Crohn’s disease. The definition of clinical remission (i.e., CDAI score < 150) used in the studies is consistent with guidance from regulatory authorities46,53 and with guidance from CAG.50
The combined duration of the UNITI induction and IM-UNITI maintenance studies was 52 weeks, which is consistent with guidance from regulatory authorities.46,53 The duration of the pivotal studies may not have provided sufficient exposure to ustekinumab to allow adequate assessment of some of the more rare AEs (e.g., malignancy, progressive multifocal leukoencephalopathy, and serious infections).54
The generalizability of the results of the endoscopic substudy is unclear, given the small and select sample, pooling of ustekinumab induction and maintenance dosages, pooling of patients from two populations (i.e., inadequate response to conventional therapy plus inadequate response to TNF antagonists), and single centralized evaluator.
3.6. Efficacy
Only those efficacy outcomes identified in the review protocol (Section 2.2) are reported in this section. See 0 for additional efficacy data.
3.6.1. Clinical Remission
a. Induction Studies
A statistically significantly higher proportion of patients treated with ustekinumab (20.9% and 40.2%) than with placebo (7.3% to 19.6%; P < 0.001) had a remission at week 8 in UNITI-1 and UNITI-2, respectively (Table 21).
Table 21
Efficacy Outcomes for Induction Studies.
Subgroup analyses (based on those specified in the review protocol) are presented in Appendix 4 (Table 27). In general, differences between treatment groups with respect to the proportion of patients achieving remission at week 8 were consistent with those for remission in the overall study population.
The odds ratios for achieving remission were statistically significantly in favour of ustekinumab versus placebo at week 8, irrespective of baseline Crohn’s disease severity (subgroups of CDAI score ≤ 300 or > 300) in both UNITI studies. Likewise, among patients in UNITI-1, a statistically significantly higher proportion of patients treated with ustekinumab, compared with placebo, were in remission at week 8, irrespective of response to previous treatment with conventional therapies. With respect to subgroups according to TNF antagonist use history in UNITI-1, remission rates were in favour of ustekinumab versus placebo, but statistically significant among those who had an initial response with, secondary nonresponse to, or intolerance to these drugs. Ustekinumab was also statistically significantly superior to placebo in achieving remission at eight weeks, regardless of whether patients enrolled in UNITI-2 had or had not received previous treatment with TNF antagonist(s).
b. Maintenance Study
Statistically significantly higher proportions of patients treated with ustekinumab every 12 weeks (48.8% and 42.6%; P = 0.04 and P = 0.035, respectively) and ustekinumab every eight weeks (53.1% and 46.9%; P = 0.005 and P = 0.004) were in clinical remission and corticosteroid-free remission, respectively, at week 44 of IM-UNITI than with placebo (35.9% and 29.8%) (Table 22). Among patients who were in remission at the start of IM-UNITI, 56.4% (44/78), 66.7% (52/78), and 45.6% (36/79) treated with the ustekinumab every 12 weeks regimen, ustekinumab every eight weeks, and placebo, respectively, were still in remission at week 44; however, the difference versus placebo was statistically significant only for the ustekinumab every eight weeks group.
Several pre-specified sensitivity analyses were performed on the remission end point. ▬
Subgroup analyses (based on those specified in the review protocol) are presented in Appendix 4 (Table 28). In general, differences between treatment groups with respect to the proportion of patients achieving remission at week 44 were consistent with those for remission in the overall study population.
▬ With respect to subgroups according to TNF antagonist use history, remission rates were in favour of ustekinumab versus placebo, but statistically significant only among those treated with ustekinumab every eight weeks and enrolled from UNITI-2, and those treated with ustekinumab every eight weeks who had not previously received TNF antagonist(s).
c. Phase II Study
The proportion of patients who had a remission in CERTIFI at week 6 was not statistically significantly different between the placebo (10.6%) and ustekinumab 6 mg/kg groups (12.2%) (Table 23).
3.6.2. Clinical Response
a. Induction Studies
The primary outcome for the UNITI induction studies was achieved: the proportion of patients in clinical response at week 6 was statistically significantly higher with the ustekinumab group (33.7% and 55.5%; P = 0.003 and P < 0.001, respectively) than with the placebo group (21.5% and 28.7%) in UNITI-1 and UNITI-2, respectively (Table 21).
In the sensitivity analyses performed for the primary outcome by observed case, LOCF, multiple imputation, and worst case, as well as excluding patients who were randomized but never treated, the results were consistent with those of the primary analysis.
Subgroup analyses (based on those specified in the review protocol) are presented in Appendix 4 (Table 29). In general, differences between treatment groups with respect to the proportion of patients achieving clinical response at week 6 were consistent with those for response in the overall study population.
The odds ratios for achieving clinical response were statistically significantly in favour of ustekinumab versus placebo at week 6, irrespective of baseline Crohn’s disease severity (subgroups of CDAI score ≤ 300 or > 300) in both UNITI studies. Among patients in UNITI-1, a statistically significantly higher proportion of patients treated with ustekinumab compared with placebo were responders at week 6 among those who had failed to respond to previous treatment with conventional therapies; the between-group difference for response was not statistically significant among the subgroup of patients who had not failed conventional therapies. With respect to subgroups according to TNF antagonist use history in UNITI-1, remission rates were in favour of ustekinumab versus placebo, but statistically significant among those who had an initial response with, secondary nonresponse to, or intolerance to these drugs. Ustekinumab was also statistically significantly superior to placebo in achieving remission at eight weeks regardless of whether patients enrolled in UNITI-2 had or had not received previous treatment with TNF antagonist(s).
b. Maintenance Study
Almost 60% of patients randomized to ustekinumab maintenance treatments were responders at week 44, whereas 44% of those assigned to placebo achieved clinical response. The comparison versus placebo was statistically significant for both ustekinumab regimens (P = 0.033 [every 12 weeks] and P = 0.018 [every eight weeks]) (Table 22).
c. Phase II Study
The proportion of patients who had a clinical response in CERTIFI at week 6 was statistically significantly different between the placebo (23.5%) and ustekinumab 6 mg/kg group (39.7%) (Table 23).
3.6.3. Health-Related Quality of Life, Functional and Disability Outcomes
None of the following analyses were adjusted for multiple comparisons and should be interpreted with this in mind.
a. Induction Studies
Ustekinumab-treated patients demonstrated statistically significantly greater improvements in IBDQ total score from baseline to week 8 in both UNITI-1 and UNITI-2 compared with patients receiving placebo (Table 21).
The mean scores for all four IBDQ dimension scores (bowel, emotional, systemic, and social) were statistically significantly improved from baseline with ustekinumab versus placebo through to week 8 of both induction studies (Table 30). The mean changes from baseline were higher in the ustekinumab group in UNITI-2 than in UNITI-1. Treatment differences appeared to be clinically significant only in UNITI-2 (MCID = 16 points).
A statistically significantly greater proportion of patients in the ustekinumab group (54.8%) had a ≥ 16-point improvement from baseline in the IBDQ score at week 8 compared with the placebo group (36.5%) in UNITI-1. Similarly, a statistically significantly greater proportion of patients in the ustekinumab group (68.1%) had a ≥ 16-point improvement from baseline in the IBDQ score at week 8 compared with the placebo group (41.1%) in UNITI-2 (Table 31).
As shown in Table 21, the mean change from baseline in the SF-36 PCS and MCS scores to week 8 were statistically significantly in favour of ustekinumab versus placebo, except for the PCS score change in UNITI-1. ▬
Statistically significantly greater proportions of ustekinumab-treated patients compared with placebo patients had at least a five-point improvement from baseline in the PCS and MCS scores of the SF-36 at week 8 in both studies, except on the PCS score in UNITI-1 (Table 31).
▬ (Table 21).
b. Maintenance Study
The IBDQ total scores were statistically significantly higher at week 44 of IM-UNITI for both ustekinumab treatment regimens versus placebo (Table 22). IBDQ total scores decreased in all three treatment groups from baseline to week 44. Differences between groups were not considered clinically significant based on a MCID of 16 points.
As shown in Table 22, ▬
▬ (Table 33).
▬ (Table 22).
3.6.4. Mucosal Healing
The mean change from baseline in SES-CD score at week 8 of induction (endoscopy substudy primary outcome) was statistically significantly improved (decreased) in the pooled ustekinumab group (−2.8 points, standard deviation 5.7; P = 0.012) than in the placebo group (−0.7 points, standard deviation 5.0) (Table 35). The manufacturer reported that results of sensitivity analyses regarding approaches to handling missing data, and results across subgroup analyses by induction study and by induction dose, were consistent with the primary analysis; however, the data for these analyses were not included in the submitted materials.
The results of the first two key secondary outcomes in the endoscopy substudy — the change from induction baseline in the SES-CD score at week 44 of maintenance and the proportion of patients with mucosal healing at week 44 of maintenance — were not reported in the submitted materials. The manufacturer noted that the efficacy of ustekinumab maintenance for endoscopic outcomes could not be determined, primarily because of the very small sample size (N = 70).
The proportion of patients with mucosal healing at week 8 of induction was 9.0% and 4.1% for the pooled ustekinumab and placebo groups, respectively (P = 0.141).
3.6.5. Need for Surgery for Crohn’s Disease
a. Induction Studies
▬ (Table 21).
b. Maintenance Study
▬ (Table 22).
c. Maintenance Study
Table 22Efficacy Outcomes for the Maintenance Study
| Parameter, n (%) | IM-UNITI | ||
|---|---|---|---|
| PLA (N = 131) | UST q.12.w (N = 129) | UST q.8.w. W (N = 128) | |
| Clinical remission at week 44, n (%) | 47 (35.9) | 63 (48.8) | 68 (53.1) |
| P value | 0.04 | 0.005 | |
| CS-free clinical remission at week 44, n (%) | 39 (29.8) | 55 (42.6) | 60 (46.9) |
| P value | 0.035 | 0.004 | |
| Clinical remission at week 44 among patients in clinical remission at start of maintenance study | N = 79 | N = 78 | N = 78 |
| n (%) | 36 (45.6) | 44 (56.4) | 52 (66.7) |
| P value | 0.189 | 0.007 | |
| Clinical response at week 44, n (%) | 58 (44.3) | 75 (58.1) | 76 (59.4) |
| P value | 0.033 | 0.018 | |
| Change in IBDQ total score at week 44 | N = 130 | N = 129 | N = 128 |
| Baseline score, median (IQR) | 167.0 (▬) | 172.0 (▬ ) | 176.5 (▬) |
| Change from baseline, median (IQR)a | −14.5 ▬) | −2.5 (▬) | −2.0 (▬) |
| P value | ▬ | ▬ | |
| Change in SF-36 PCS score at week 44 | ▬ | ▬ | ▬ |
| Baseline score, mean (SD) | ▬ | ▬ | ▬ |
| Change from baseline, mean (SD) a | ▬ | ▬ | ▬ |
| P value | ▬ | ▬ | |
| Change in SF-36 MCS score at week 44 | ▬ | ▬ | ▬ |
| Baseline score, mean (SD) | ▬ | ▬ | ▬ |
| Change from baseline, mean (SD) a | ▬ | ▬ | ▬ |
| P value | ▬ | ▬ | |
| Time lost from work (during the previous 4 weeks) at week 44 (days) | ▬ | ▬ | ▬ |
| Mean (SD) | ▬ | ▬ | ▬ |
| P value | ▬ | ▬ | |
| Change in daily productivity (VAS) at week 44 | ▬ | ▬ | ▬ |
| Baseline score, median (IQR) | ▬ | ▬ | ▬ |
| Change from baseline, median (IQR) | ▬ | ▬ | ▬ |
| P value | ▬ | ▬ | |
| Change in WLQ index at week 44 | ▬ | ▬ | ▬ |
| Baseline score, median (IRQ) | ▬ | ▬ | ▬ |
| Change from baseline, median (IQR) | ▬ | ▬ | ▬ |
| P value | ▬ | ▬ | ▬ |
| Number of patients with CD-related surgery through week 44 | |||
| n (%) | ▬ | ▬ | ▬ |
| P value | ▬ | ▬ | |
CS = corticosteroid; IBDQ = Inflammatory Bowel Disease Questionnaire; IQR = interquartile range; MCS = mental component score; PCS = physical component score; PLA = placebo; q.8.w. = every 8 weeks; q.12.w. = every 12 weeks; SD = standard deviation; SF-36 = Short Form (36) Health Survey; UST = ustekinumab; VAS = visual analogue scale; WLQ = Work Limitations Questionnaire.
- a
Negative values indicate worsening.
Source: Clinical Study Report for IM-UNITI.9
Table 23Clinical Remission and Response in the Phase II Study Induction Phase
| Parameter | CERTIFI | |
|---|---|---|
| PLA (N = 132) | UST 6 mg/kg (N = 131) | |
| Clinical remission at week 6, n (%) | 14 (10.6) | 16 (12.2) |
| P value | 0.682 | |
| Clinical response at week 6, n (%) | 31 (23.5) | 52 (39.7) |
| P value | 0.005 | |
PLA = placebo; UST = ustekinumab.
Source: Clinical Study Report for CERTIFI.10
3.7. Harms
Only those harms identified in the review protocol (Section 2.2) are reported in this section.
3.7.1. Adverse Events
a. Induction Studies
Almost two-thirds and greater than one-half of patients randomized to placebo or ustekinumab in UNITI-1 and UNITI-2, respectively, experienced an AE. In UNITI-1, the most commonly experienced AEs (in more than 5% of ustekinumab patients) were headache, arthralgia, pyrexia, nausea, and abdominal pain. In UNITI-2, these were nasopharyngitis, nausea, and pyrexia (Table 24).
Table 24
Harms From the Induction Studies.
b. Maintenance Study
Greater than 80% of patients in IM-UNITI reported an AE across the treatment groups. The most commonly experienced AEs (in more than 5% of patients receiving ustekinumab every eight weeks) were arthralgia, Crohn’s disease symptoms, headache, nasopharyngitis, upper respiratory infections, abdominal pain, pyrexia, cough, rash, and injection-site erythema (Table 25).
Table 25
Harms From the Maintenance Study.
c. Phase II Study
A higher proportion of patients in the placebo group (71%) had an AE than in the ustekinumab group (61%). Gastrointestinal disorders and infections were the most common AEs, both more common in the placebo group (Table 26).
Table 26
Harms From the Phase II Study Induction Phase (Baseline to Week 8).
3.7.2. Serious Adverse Events
a. Induction Studies
The proportion of patients reporting SAEs in the treatment groups of both UNITI studies ranged from 3% to 7% over eight weeks (Table 24). Gastrointestinal disorders were the system-organ class in which the highest proportion of patients experienced an SAE: 3% to 4% of patients in the placebo groups and 2% to 3% of patients in the ustekinumab groups of UNITI-1 and UNITI-2. These SAEs were predominantly events of Crohn’s disease or related symptoms and complications.
b. Maintenance Study
The proportion of patients reporting SAEs at week 44 in the treatment groups of IM-UNITI ranged from 10% (ustekinumab every eight weeks group) to 15% (placebo group). As in the induction studies, the most commonly experienced SAEs were gastrointestinal and occurred more frequently in the placebo group (Table 25).
c. Phase II Study
The proportion of patients reporting SAEs in CERTIFI were 8% for placebo and 7% for ustekinumab. Gastrointestinal events were the most commonly reported SAEs (Table 26).
3.7.3. Withdrawal Due to Adverse Events
a. Induction Studies
▬ The main AE was related to Crohn’s disease (Table 24).
b. Maintenance Study
The proportion patients with a WDAE was lowest in the ustekinumab every eight weeks group (3.1%), compared with 6% for the placebo group and 7.6% for the ustekinumab every 12 weeks group. The primary AEs experienced were gastrointestinal disorders (Table 25).
c. Phase II Study
The proportion of patients with a WDAE in the induction phase of CERTIFI was similar to that in the UNITI studies (Table 26).
3.7.4. Mortality
There were no deaths in the included studies.
3.7.5. Notable Harms
a. Induction Studies
Less than 5% of patients in the UNITI studies experienced an infusion reaction, and no instances of anaphylaxis were reported. There was no clear difference between groups within the studies regarding infusion reactions (Table 24).
Infections were common, with approximately one-quarter of patients in each group in both UNITI studies reporting an infection. There were few patients with a serious infection over eight weeks of treatment; no cases of TB were reported in both studies.
None of the patients in UNITI-1 or UNITI-2 were reported as having developed cancer or experienced a major cardiovascular event.
Neurological AEs (primarily headaches) were relatively common in both studies.
b. Maintenance Study
Two per cent to 7% of patients treated with ustekinumab experienced an injection-site reaction versus less than 1% of those in the placebo group. No instances of anaphylaxis were reported (Table 25).
Infections were common, with approximately one-half of patients in each group reporting an infection. There were few patients with a serious infection, although a higher proportion was observed in the ustekinumab every 12 weeks regimen group (5.3%) versus the placebo and ustekinumab every eight weeks groups (both 2.3%). There was one suspected case of TB in a patient who received IV induction with 130 mg ustekinumab and was randomized to placebo in IM-UNITI.
None of the patients in IM-UNITI experienced a major cardiovascular event, but one patient randomized to each of placebo and ustekinumab every eight weeks group developed cancer over 44 weeks of maintenance therapy; in both cases, the diagnosis was basal cell carcinoma.
▬
c. Phase II Study
Approximately 5% of patients in CERTIFI experienced an induction infusion reaction, and no instances of anaphylaxis were reported. There was no clear difference between groups regarding infusion reactions (Table 26).
Approximately one-quarter of patients in each group reported an infection, primarily nasopharyngitis. There were few patients with a serious infection during the induction phase, although there appeared to be a higher proportion of patients in the ustekinumab group (3.8%) than in the placebo group (0.8%). No one cause of infection was more frequently reported, and there were no reports of TB.
None of the patients was reported to develop cancer or experience a major cardiovascular event.
▬
- RESULTS - Ustekinumab (Stelara)RESULTS - Ustekinumab (Stelara)
- ISSUES FOR CONSIDERATION - Glycerol Phenylbutyrate (Ravicti)ISSUES FOR CONSIDERATION - Glycerol Phenylbutyrate (Ravicti)
- Clinical Review Report: Icosapent Ethyl (Vascepa)Clinical Review Report: Icosapent Ethyl (Vascepa)
Your browsing activity is empty.
Activity recording is turned off.
See more...