Patient Group Input
This section was prepared by CADTH staff based on the input provided by patient groups.
About the Patient Groups and Information Gathered
One response to CADTH’s call for patient input was received for the review of HP/TAZ. The response was a joint submission by CPN, CSPA, and CAPP.
All three patient groups are national, not-for-profit organizations that are dedicated to improving care and quality of life of patients living with psoriatic diseases. These organizations aim to do this by advocating and educating for psoriatic patients and their caregivers. The CSPA extends this mandate into patients living with all dermatological conditions, including psoriasis.
A disclosure of any conflicts of interest for all three organizations is available on the CADTH website. Information for this submission was collected through three surveys. First, a survey distributed by CPN through their membership list and social media channels, which resulted in 61 responses distributed across eight provinces. Second, a survey about gaps in topical treatments for psoriasis promoted by CAPP on their social media channels, which resulted in 212 responses distributed across all the provinces. Half (52.1%) of respondents were 55 years or older, while 46% were working age adults, 35% were from Ontario, 15.6% from Nova Scotia, and 14.2% from British Columbia. Third, a survey targeting people who had experience with HP/TAZ distributed by CPN and CAPP on both their social media channels, featured on CPN’s website, and included in an e-newsletter by the National Psoriasis Foundation in the US. Last, this submission was informed through information gathered for a report titled “Journey to Stability,” created by CPN and CAPP.
Disease Experience
The patient groups describe psoriasis as a chronic inflammatory skin condition which causes skin cells to regenerate approximately seven times faster than average. As a result, skin cells are not shed normally and instead pile up on the skin’s surface creating sores or lesions, commonly called plaques. The patient groups describe inflamed, thick, silvery scales that can form on top of the plaques, which can be itchy and painful. Psoriasis can range from a few dandruff-like scales to widespread patches that cover large areas of skin. Patients with psoriasis experience periods of flares where the disease is aggravated, areas affected may become itchy and painful, and even crack and bleed. Afterwards, patients may go into a period of remission where symptoms are decreased. Many oscillate between these experiences throughout the course of their lives with the condition. Psoriasis usually affects the elbows, knees, scalp, palms of hands, soles of feet, nails, genitals, and torso.
Nearly half (44.8%) of the respondents to CAPP’s survey indicated that they had lived with psoriasis for more than 20 years, while 25.2% lived with the disease for between 10 to 20 years, 16% for between five to 10 years, and nearly 14% for fewer than five years. Within CPN’s survey, half of survey respondents (51%) identified their condition as “well-controlled,” 30% said “poorly controlled,” and 5% said “not controlled at all.” The rest indicated that they fall somewhere in between.
Many patients reported feelings of low self-esteem, loss of sleep, anxiety, depression, fear of intimacy, and avoidance of social activities as part of their experience living with this condition. It was also reported that patients living with this condition feel like they have to “miss social events and refrain from wearing certain types of clothing.” In addition, CPN’s survey reported that people with psoriasis are at higher risk of certain health conditions compared to the general population, 46.7% of respondents experience joint pain, 23.2% experience depression, 13.3% live with heart disease, and 11.7% reported having diabetes.
CPN’s survey also described the impact that psoriasis has on family members and caregivers. Three-fifths (61.2%) of respondents identified that family members experience worry related to their condition, 34.7% experience intimacy challenges, and 24.5% have avoided activities. Patients also reported feelings of stress, time issues, and frustration due to their condition and the effects on their family and interpersonal relationships, as their family had to “clean up plaques around the house after they fell off” and that they “didn’t want people to see or even know about the more private areas where [they] have psoriasis.”
Experience With Treatments
CAPP’s survey informed that 85% of respondents were currently using topical treatments such as Dovobet (17.4%), BD (14.8%), clobetasol (11.4%), Enstilar (7.4%), Dovonex (6%), cortisone (5.4%), Diprosalic (percentage not available for latter treatments), Elidel, Lamisil, Lyderm, Ultravate, Fucidin, and tar. Responses also included non-prescription moisturizers, essential and coconut oils, Epsom salt baths, and other products. Several respondents indicated they have tried multiple products, and one-tenth (9.2%) of respondents came to their current topical treatment through their own trial and error. Of the 55 individuals who responded to a question about alternate psoriasis treatments they have used, 42.3% said they have used over-the-counter topicals, phototherapy (49.1%), oral medications (41.8%), and biologics (41.8%).
When questioned about what challenges patients experienced with their current topical treatments within CPN’s survey, answers ranged from: inconvenient (e.g., greasy, time-consuming, 78.6%), experienced side effects (e.g., redness, soreness, thinning skin, pain/burning, 46.4%), and ineffective after prolonged use (46.4%). CAPP’s survey informed that the most common reason for discontinuation of a topical treatment was: lack of efficacy (56.4%), side effects (13.7%), difficulty of use (8.5%), lack of efficacy after prolonged use (7.7%), cost (6%), and unavailability of the product (3.4%).
Patients also reported issues with the cost of treatment (50.3% of respondents felt that their topical was expensive) and ease of administration, for example, 10.9% felt that their topical was difficult to use and 48% felt that their topical was messy.
The closest phototherapy is more than an hour away from where I live (in rural Manitoba) and I cannot afford the gas to go there the two or three times a week that I would need to — phototherapy worked best for me. I am trying to get some benefit from [the] tanning bed in town and there is some improvement…
Moreover, some patients’ treatment options are limited due to contraindications: “As a cancer survivor, I can’t take any biologics so my options are limited…” Notably, one-tenth of respondents indicated that they did not have any needs that have not been met by currently available treatments.
As there were no clinical trials for HP/TAZ conducted in Canada, responses from three patients who had experience with HP/TAZ currently residing in the US informed this submission. Two of the respondents were aged 45 to 54 years and had been living with psoriasis for more than 20 years. One respondent was aged 19 to 24 and had been living with psoriasis for less than one year. All three respondents identified itching and appearance of plaques (in no particular order) as the most important aspects of the disease to control. The respondent aged 19 to 24 also identified anxiety and/or social stigma, whereas one older respondent identified pain as additional important aspects of psoriasis to control. The respondents living with psoriasis for more than 20 years indicated that HP/TAZ did a better job of managing the appearance of plaques (number, size, thickness, and scaling) than previous treatments they have used, and there were no symptoms that HP/TAZ did not manage as well as other treatments. The respondent living with psoriasis for under one year indicated that it managed their itchiness better, and that it was easier to apply although it “takes longer to become efficacious [sic] when compared to other steroid treatments.” This is in contrast to one of the respondents with psoriasis for more than 20 years who indicated that it “works fast.” When asked “did you experience any side effects when using HP/TAZ?” the two respondents aged 45 to 54 experienced skin thinning, and the respondent aged 19 to 24 experienced stretch marks, skin irritation, and dryness.
Improved Outcomes
In a survey run by CPN, patients were asked “What aspects of psoriasis are the most important to control in your opinion?” and the majority (88.3%) of respondents selected the appearance of plaques, 81.5% selected redness, itching (76.7%), burning sensation (75%), joint pain (41.7%), general pain (26.7%), depression/anxiety (30%), stigma (25%), and bleeding (25%), and one-fourth selected related conditions such as diabetes and heart disease. Moreover, 61.2% of respondents to CAPP’s survey indicated they wanted a topical treatment to address all their symptoms, or a cure.
Generally, the patient groups described the single most important outcome was the resolution of plaques. In terms of other specific outcomes and factors affecting their use of topical treatment, respondents to CAPP’s survey indicated that the treatment should resolve symptoms faster, reduce side effects (e.g., thinning of the skin), “works better on scalps,” work for both the rash and the pain, and be “a more effective natural approach.” As previously noted, patients also stressed the importance of a treatment which had a better applicator, is not messy, greasy, or smelly, and is more affordable especially for patients which have to treat large areas of their body.
The effectiveness of treatments within individuals, and the way in which individuals view their condition varies. Thus, the needs of patients vary across the disease population and across the course of disease progression. The organizations stress that having access to a variety of treatments which are safe, effective, and affordable is of fundamental importance to patients. Currently, the symptoms of psoriasis are not being properly treated and there needs to be better new medicines to treat itchiness, redness, flakes, and other symptoms. However, patients ultimately want a cure for psoriasis.
Clinician Input
All CADTH review teams include at least one clinical specialist with expertise regarding the diagnosis and management of the condition for which the drug is indicated. Clinical experts are a critical part of the review team and are involved in all phases of the review process (e.g., providing guidance on the development of the review protocol, assisting in the critical appraisal of clinical evidence, interpreting the clinical relevance of the results, and providing guidance on the potential place in therapy). The following input was provided by one clinical specialist with expertise in the diagnosis and management of plaque psoriasis.
Unmet Needs
The clinical expert identified a number of needs in topical therapy for plaque psoriasis that are not being met by the presently available medications in Canada. Adherence is a major issue in topical therapy for plaque psoriasis. Patients are unlikely to use medications that leave a visible residue on exposed sites, stain clothing or skin, produce irritation, have an objectionable odour, or are difficult to spread onto plaques. Efficacy is improved when multiple topical agents are used in plaque psoriasis. When using multiple agents, it is preferred that they be used in a combination preparation rather than as separate prescriptions. This greatly enhances the ease of use and improves adherence.
The clinical expert consulted by CADTH considered that an ideal treatment in plaque psoriasis would produce a sustained PASI 100 response in all patients with little or no risk of adverse effects. This treatment would be easily accessed by the patient and convenient to administer. The PASI 100 response would translate to a DLQI score of near zero. An ideal treatment would also benefit one or more of the comorbidities, particularly psoriatic arthritis. An ideal medication would produce remission without the need for continuous long-term administration or could be administered intermittently as required when the patient reaches a predetermined PASI score after interruption of therapy. Clearly, the ideal treatment is not currently available.
Place in Therapy
The clinical expert does not expect HP/TAZ to cause a shift in the current Canadian treatment paradigm for moderate and severe plaque psoriasis; however, they feel it will be a welcome addition to the lineup of currently available topical therapies. It may offer protection against the local and systemic adverse effects of superpotent topical corticosteroids. The clinical expert noted that it is efficacious and cosmetically elegant and will likely be well accepted by patients resulting in high levels of adherence. They also mentioned that the base allows convenient application to hairy areas, however, HP/TAZ is not recommended for use on the scalp.9
According to the clinical expert, HP/TAZ is an appropriate first choice of topical therapy for patients with relatively limited areas of psoriatic involvement, noting that the efficacy and safety of HP/TAZ in patients with more than 12% of BSA affected by plaque psoriasis has not been established.30 They also felt it would be an appropriate adjunctive therapy in those patients who require systemic drugs.
The clinical expert did not think it would be appropriate to recommend that patients try other treatments before initiating treatment with HP/TAZ. They believe that HP/TAZ would be an appropriate choice of first topical agent in all patients with plaque psoriasis, excluding pregnant women and children and those who have facial and/or intertriginous involvement only. Of note, HP/TAZ is contraindicated in pregnant women and there is no evidence to support its use in children. Further, HP/TAZ is not recommended for use on the face or intertriginous areas.
Patient Population
In the opinion of the clinical expert consulted for this review, HP/TAZ would be most suitable as monotherapy for patients with relatively limited areas of psoriasis, noting that the 50 g per week limitation would exclude use in patients with large areas of involvement. HP/TAZ would also be appropriate for patients on systemic therapy, both biologics and non-biologics, who have residual plaques, or those who have previously been completely controlled with systemics and are now having flares.
Patients best suited for treatment with HP/TAZ would be recognized by clinical examination by a dermatologist. Psoriasis is generally not a challenging diagnosis for a dermatologist, so misdiagnosis is unlikely at the level of the dermatologist. No lab testing is required to make the diagnosis and routine lab testing will not be required to follow a patient on HP/TAZ.
The clinical expert anticipated that HP/TAZ will be prescribed in Canada for the treatment of mild psoriasis by family physicians and nurse practitioners. In that setting, they would anticipate some instances of misdiagnosis or inappropriate prescribing of HP/TAZ.
The clinical expert suggested that patients who would be least suitable for treatment with HP/TAZ are those who have a history of suspected hypersensitivity to HP or TAZ, as well as children and pregnant women. Treatment with HP/TAZ would not be appropriate for use as monotherapy in patients with large areas of plaque psoriasis where the 50 g per week limit would be insufficient. It would also not be appropriate in psoriasis with eczematous features, pustular psoriasis, or erythrodermic psoriasis. It would not be appropriate for use on the face or intertriginous areas.
The clinical expert does not think it would be possible to identify patients who are more likely to exhibit a response to treatment with HP/TAZ accurately. They would expect those who have a clearly documented lack of response to superpotent corticosteroids to do less well.
Assessing Response to Treatment
The clinical expert stated that the outcomes used in clinical practice are not aligned with the outcomes typically used in clinical trials. It is unlikely that PASI, IGA, and DLQI scores will be calculated and recorded in clinical practice. What is more likely to be recorded is an informal combination of the prescriber’s and patient’s view of improvement in erythema, scaling, thickness, area of involvement, and pruritus at four to eight weeks. It is expected that many patients with mild psoriasis for whom a dermatologist prescribes HP/TAZ will actually be sent back to the referring physician for continued follow-up.
A clinically meaningful response to treatment judged at four to eight weeks would require joint input from the patient and the clinician. Response would not generally be graded on a formal scale, but rather be an assessment as to whether there has been a significant improvement in the appearance of the plaques (area, scaling, thickness, and redness) and a reduction in pruritus and/or pain. There would also be observations of atrophy, irritation, and folliculitis.
It is very likely that there would be considerable variability in the assessment of response from clinician to clinician. As well, patients vary significantly in their expectations of response to topical therapy.
Ideally, when any superpotent topical corticosteroid is prescribed, the first follow-up assessment should take place at or before one month. Given the severe shortage of dermatologists in Canada, many patients will be returned to the care of their family physician and will not be followed by the dermatologist. For those patients who will remain under the care of a dermatologist, it is unlikely that a follow-up appointment can be booked prior to eight weeks after starting HP/TAZ.
Discontinuing Treatment
The clinical expert stated three factors that would influence the decision to stop the treatment, including lack of adequate disease response; presence of significant skin atrophy, folliculitis, irritation, suspected irritant, or allergic contact dermatitis; and the treatment being cost-prohibitive (i.e., the patient cannot afford continued use of HP/TAZ).
Prescribing Conditions
As per input from the clinical expert, the drug under review would be dispensed by the patient’s community pharmacist who would likely offer some written information with the medication. The drug would be applied by the patient, generally in the patient’s home.
The clinical expert did not feel that a dermatologist would be required to diagnose, treat, or monitor patients who might receive HP/TAZ. The clinical expert anticipates significant prescribing by family doctors, nurse practitioners, and perhaps by other specialists (e.g., rheumatology and internal medicine).
Additional Considerations
The clinical expert noted that HP/TAZ is unlikely to be used as monotherapy in patients with moderate-to-severe plaque psoriasis and is more likely to be an adjunct to systemic therapy. It is appropriate as monotherapy in mild psoriasis and thus, significant prescribing by non-dermatologists is anticipated.
Systematic Review (Pivotal and Protocol Selected Studies)
Objectives
To perform a systematic review of the beneficial and harmful effects of 0.01% w/w HP/TAZ topical lotion for improving the signs and symptoms of plaque psoriasis in adult patients with moderate-to-severe plaque psoriasis.
Methods
Studies selected for inclusion in the systematic review included pivotal studies provided in the sponsor’s submission to CADTH and Health Canada, as well as those meeting the selection criteria presented in .
Inclusion Criteria for the Systematic Review.
Of note, the systematic review protocol presented below was established prior to the granting of a Notice of Compliance from Health Canada.
The literature search for clinical studies was performed by an information specialist using a peer-reviewed search strategy according to the PRESS Peer Review of Electronic Search Strategies checklist (https://www.cadth.ca/resources/finding-evidence/press 2016).31
Published literature was identified by searching the following bibliographic databases: MEDLINE All (1946– ) via Ovid, Embase (1974– ) via Ovid, and PubMed. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were Monoferric (iron isomaltoside). Clinical trial registries were searched: the US National Institutes of Health’s clinicaltrials.gov and the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) search portal. No filters were applied to limit the retrieval by study type. Retrieval was not limited by publication date or by language. Conference abstracts were excluded from the search results. See Appendix 1 for the detailed search strategies.
The initial search was completed on February 12, 2020. Regular alerts updated the search until the meeting of the CADTH Canadian Drug Expert Committee (CDEC) on June 17, 2020.
Grey literature (literature that is not commercially published) was identified by searching relevant websites from the following sections of the Grey Matters: A Practical Tool For Searching Health-Related Grey Literature checklist (https://www.cadth.ca/grey-matters):32 Health Technology Assessment (HTA) Agencies, Health Economics, Clinical Practice Guidelines, Drug and Device Regulatory Approvals, Advisories and Warnings, Drug Class Reviews, Clinical Trials Registries, and Databases (free). Google was used to search for additional internet-based materials. In addition, the sponsor of the drug was contacted for information regarding unpublished studies. See Appendix 1 for more information on the grey literature search strategy.
Two CADTH clinical reviewers independently selected studies for inclusion in the review based on titles and abstracts, according to the predetermined protocol. Full text articles of all citations considered potentially relevant by at least one reviewer were acquired. Reviewers independently made the final selection of studies to be included in the review, and differences were resolved through discussion.
Findings From the Literature
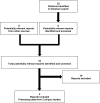
A total of two studies were identified for inclusion in the systematic review (). The included studies are summarized in . A list of excluded studies is presented in Appendix 2.
Flow Diagram for Inclusion and Exclusion of Studies.
Details of Included Studies.
Description of Studies
The two identically designed pivotal trials submitted by the sponsor, Study 301 (N = 203) and Study 302 (N = 215), met the inclusion criteria for the systematic review. Details of the included studies and study designs are provided in .
The objective of Study 301 and Study 302 was to evaluate the safety and efficacy of topical HP/TAZ in comparison to vehicle when applied once daily to adult patients with moderate-to-severe plaque psoriasis. Overall, 418 patients were included in the two studies. Each of the pivotal trials included 16 study sites, all of which were located in the US and carried out between August 2015 and November 2016.
The two trials were multi-centre, double-blind, randomized, parallel-group studies comparing HP/TAZ to vehicle. Following a screening visit that occurred between one and 35 days prior to randomization, patients were randomized in a 2:1 ratio to receive HP/TAZ or vehicle. The method of randomization used involved a unique subject number assigned to patients by the investigational centre that was used to assign patients to a study drug kit that was dispensed by an interactive response technology system. Randomization was not stratified.
The area affected by plaque psoriasis that would be treated was determined by the investigator at baseline, and patients were to treat this area with the study drug once daily for eight weeks. There were four on-treatment post-baseline study visits that occurred at week 2, 4, 6, and 8. Patients were also asked to return for a follow-up visit four weeks after treatment cessation at week 12. Patients who discontinued early were asked to complete all week 8 assessments as appropriate before starting any alternative therapy for psoriasis.
Populations
Inclusion and Exclusion Criteria
A list of key inclusion and exclusion criteria is available in .
To be eligible for participation in Study 301 and Study 302, patients were required to have an area of plaque psoriasis that was appropriate for topical treatment and covered a BSA of between 3% and 12% (inclusive). Patients were also required to have a clinical diagnosis of psoriasis at baseline with an IGA score of 3 or 4. The calculation of BSA and IGA did not include the face, scalp, palms, soles, axillae, and intertriginous areas. The patient needed to have a target lesion that measured between 16 cm2 and 100 cm2 (inclusive), was not located on areas covering bony prominences, and met the psoriasis signs scoring criteria noted in .
Patients were excluded from Study 301 and Study 302 if they presented with psoriasis that was treated with and failed to respond to prescription medication, even partially or temporarily. Patients were also excluded if they had received treatment with: any topical antipsoriatic drug within 14 days prior to the study baseline visit; had used phototherapy, photochemotherapy, or non-biologic systemic therapies for psoriasis within four weeks of the study baseline visit; and biologics known to affect psoriasis within three months of baseline visit.
Baseline Characteristics
The baseline characteristics for randomized patients in Study 301 and Study 302 are summarized in . Patients were a mean age of 48.8 (SD = 13.3) and 51.8 (SD = 14.3) years in Study 301 and Study 302, respectively. The majority of patients were male (67.0% and 63.3%) and White (89.7% and 81.9%) in Study 301 and Study 302, respectively, and 32.0% in Study 301 and 23.3% in Study 302 were Hispanic or Latino. In Study 301, a greater proportion of patients randomized to vehicle were male (69.1% vs. 65.9%) and Black or African American (6.7% vs. 2.9%), and a smaller proportion of patients randomized to vehicle were Hispanic or Latino (29.4% vs. 33.3%). In Study 302, a greater proportion of patients randomized to vehicle were male (67.6% vs. 61.0%), White (85.1% vs. 80.1%), and Black or African American (9.5% vs. 6.4%), and a smaller proportion were Asian (4.1% vs. 9.2%).
Summary of Baseline Characteristics (ITT Set).
At baseline, most of the patients randomized to Study 301 and Study 302 had moderate disease (82.8% and 87.4%, respectively), indicated by an IGA score of 3. The rest of the patients in both studies were classified as having severe disease by an IGA score of 4. Percent BSA affected by psoriasis was a mean of 6.2% (SD = 2.9%) and 5.6% (SD = 2.6%) for patients in Study 301 and Study 302, respectively, which was also similar between treatment groups. Overall, the disease characteristics of patients in both pivotal trials were similar between the HP/TAZ and vehicle treatment groups, with a few differences to note. In Study 301, patients randomized to vehicle had ▬▬▬▬▬ than patients receiving HP/TAZ ▬▬▬▬▬. In Study 302, patients randomized to HP/TAZ, the mean (SD) size of the target lesion for treatment was ▬▬▬▬▬, and the erythema signs of psoriasis for the target lesion were ▬▬▬▬▬.
A summary of prior medication used by the patients included in Study 301 and Study 302 is available in . The most common medications previously used in Study 301 and Study 302 were ▬▬▬▬▬
Prior Medication Use (ITT Set).
Interventions
Patients enrolled in Study 301 and Study 302 were randomized in a 2:1 ratio to receive HP/TAZ (HP 0.01%, TAZ 0.045%) lotion or vehicle lotion, respectively. All patients were to apply the study drug once daily to the affected area for eight weeks, with a maximum weekly usage of 50 g. The affected area was determined by the investigator at baseline. Study drug was to be applied as a thin layer to the entire selected treatment area as indicated on a body diagram, and patients were instructed to avoid or minimize exposure to direct sunlight and artificial ultraviolet light sources during the study. The investigator instructed patients on how to apply the study drug to affected treatment areas at the baseline visit. Patients were also provided with written instructions. Further, patients were asked to refrain from applying study drug on the day of a clinic visit in order to observe their study drug application technique and retrain if necessary. Both HP/TAZ and vehicle lotion were packaged and labelled identically and dispensed in tube containers of 45 g, two at a time.
Certain concomitant treatments were permitted during the two pivotal studies. A summary of concomitant medication use is available in . Non-medicated cleansers, moisturizers, and sunscreens that were approved by the sponsor were permitted for use on the treatment areas during Study 301 and Study 302. Patients could treat areas that were excluded from the trials, such as the face, scalp, axillae, and intertriginous areas, with over-the-counter 1% hydrocortisone cream, tar shampoos, or moisturizers that were permitted. Palms of the hands and soles of the feet were not included in assessments of BSA or IGA for the studies, but patients were permitted to use study drug to treat these areas and could be evaluated by the investigator for improvement.
Concomitant Medication Use (ITT Set).
If a patient was using a concomitant therapy that may interfere with the interpretation of study results, they were discontinued from the concomitant product rather than withdrawn from the study. Topical treatments (other than those previously described) were not permitted.
The investigators were to try to minimize study drug interruptions; however, if signs or symptoms of the treatment areas developed while being treated with study drug that impacted daily activities or caused discomfort during application, a “drug holiday,” or temporary interruption of study drug, could be implemented. An effort was made to limit the drug holidays to four days, but if they exceeded this limit, further use of study drug was reconsidered. The use of study drug could also be delayed or halted at any time due to safety evaluations of concern.
Outcomes
A list of efficacy end points identified in the CADTH review protocol that were assessed in the clinical trials included in this review is provided in . These end points are further summarized in this section. A detailed discussion and critical appraisal of the outcome measures is provided in Appendix 4.
Dermatology Life Quality Index
The DLQI is a widely used dermatology-specific HRQoL instrument designed to assess the impact of skin disease.33 It is a ten-item questionnaire that covers six domains: symptoms and feeling, daily activities, leisure, work and school, personal relationships, and bother with psoriasis treatment. A four-point Likert scale is used to measure how much the skin disease affects a patient’s life over the past week, in which not at all or not relevant are scored as 0, a little is scored as 1, a lot is scored as 2, and very much is scored as 3. The overall DLQI score is a numeric score derived from a sum of the 10 items, for a total score that ranges from zero to 30. A lower score indicates greater HRQoL. At least 80% of the questions must be answered for a score to be reported.33,34 The final numeric score translates to the effect of the patient’s disease on their HRQoL where 0 to 1 indicates no effect, 2 to 5 indicates a small effect, 6 to 10 indicates a moderate effect, 11 to 20 indicates a very large effect, and 21 to 30 indicates an extremely large effect.
There is evidence of validity, reliability, and responsiveness of the DLQI in patients with psoriasis, as described in Appendix 4. In addition, multiple sources in the literature report a within-group minimal important difference (MID) for patients with psoriasis, which ranges from 2.2 to 6.9.
The DLQI was used to assess patient’s HRQoL at baseline, week 4, 8, and 12, and the mean (SD) change from baseline was reported as an exploratory outcome.
Investigator’s Global Assessment
The IGA is a subjective measurement of the clinical signs of psoriasis. In the two studies under review, a five-point, static version of the IGA was used.10,11 To generate the IGA score, psoriatic lesions are graded for erythema, thickness, and scaling based on a scale of 0 to 4, that are then averaged across all lesions to obtain a single estimate of the patient’s overall severity of disease at a given point in time. The three items are given equal weighting. The sum of the three scales are added and then divided by three [(E + I + S)/3] for a final IGA score of 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), or 4 (severe) corresponding to increasing severity of disease. Although the five-point static IGA scale was used in the pivotal trials to evaluate treatment efficacy, there are no studies evaluating validity, reliability, and responsiveness of this five-point scale. However, the six-point IGA scale has been validated in terms of its validity and reliability. No MID for the IGA in patients with psoriasis has been identified in the literature.
The IGA score was used to describe the severity of psoriasis of the treatable areas in Study 301 and Study 302. The face, scalp, palms, soles, axillae, and intertriginous areas were excluded from the IGA assessment. The primary outcome was the percentage of patients with treatment success at week 8, where treatment success was defined as at least a two-grade improvement from baseline in the IGA score and an IGA score of clear or almost clear (0 or 1). This definition of treatment success was used at weeks 2, 4, 6, and 12 as a secondary outcome. In addition, the percentage of patients with at least a two-grade improvement form baseline in the IGA score was reported as an exploratory outcome.
Body Surface Area
BSA is used to determine the extent of psoriasis coverage within a patient. The 1% rule was used to calculate BSA in the HP/TAZ pivotal trials. This estimation uses a flat palm in which the subject’s palm represents approximately 1% of the total BSA. The subject or investigator then uses their flat palm to estimate the percentage BSA affected by psoriasis.35 The BSA calculation in the pivotal trials did not include areas of the face, scalp, palms, soles, axillae, and other intertriginous areas.10,11 It is generally accepted that if a patient presents with an affected BSA of 0% to 3% or less this is considered low BSA affected, 3% to 10% or less is considered medium BSA affected, and an affected BSA of greater than 10% is considered a high amount of BSA involvement.36
Evidence of a MID for reduction in BSA was not identified in the literature for patients with psoriasis. The clinical expert consulted by CADTH for this review indicated that a one-third reduction in BSA is a clinically important difference for patients.
The BSA was used to assess patients at baseline, week 2, 4, 6, 8, and 12 and the mean (SD) change from baseline was reported as an exploratory outcome.
Psoriasis Signs
The signs of psoriasis, which include erythema, plaque elevation, and scaling, were assessed for the selected target lesion in the two pivotal trials. Each of the signs are evaluated using a subjective scale that ranges from 0 or none (no signs of psoriasis) to 4 or severe. According to the sponsor, the results from the psoriasis signs scale are used to detect specific changes of a patient’s lesions. In addition, they have suggested there are many similar scales that are widely used for psoriasis as erythema, plaque elevation, and scaling are basic characteristics of psoriasis lesions. Currently, there is no further information on the construct of the grading for this assessment, including evidence on its validity, reliability, responsiveness to change, and clinical relevance. The clinical expert consulted by CADTH for this review indicated that a decrease of two points in any of the signs of psoriasis would be considered a clinically important outcome for patients.
The signs of psoriasis were used as an exploratory outcome in Study 301 and Study 302. Similar to the IGA score, the psoriasis signs were used to describe treatment success, defined as at least a two-grade improvement from baseline for each of the three signs for the target lesion. To help distinguish this outcome from treatment success by IGA, treatment success based on the signs of psoriasis will be referred to as “improvement in the signs of psoriasis” throughout the report. The percentage of patients with an improvement in the signs of psoriasis was reported at weeks 2, 4, 6, 8, and 12. Additionally, a summary of the score for each of the signs of psoriasis was reported.
Harms
Treatment-emergent AEs, or those that occurred between, on, or after the date of first study drug application, were summarized at week 8 (end-of-treatment period) and at week 12 (follow-up period). Serious AEs, WDAEs, and deaths were also reported.
In addition, the frequency of local skin reactions summarized by treatment group and visit were reported as a measure of tolerability. Itching and burning/stinging were patient reported based on a 24-hour recall period and dryness was assessed by the investigator. Local skin reactions that required use of concomitant therapy or study drug interruption or discontinuation were reported as an AE.
Statistical Analysis
In both trials, the primary outcome (percentage of patients with treatment success) was analyzed using Cochran-Mantel-Haenszel tests stratified by analysis centre to compare HP/TAZ to vehicle. Missing efficacy data were primarily handled using the MCMC multiple imputation method. Sensitivity analyses of the primary outcome were conducted using the last observation carried forward (LOCF) method and repeated measures analysis on observed data.
The secondary outcomes (IGA treatment success) and exploratory outcomes related to improvement in the signs of psoriasis were analyzed using the same Cochran-Mantel-Haenszel model as the primary outcome. Missing data for these outcomes was also handled using the MCMC multiple imputation method.
A gated sequential testing procedure, where the testing process was terminated if a value that was not statistically significant was observed, was used for the secondary outcomes regarding treatment success by the IGA score. The order was as follows: the percentage of patients who show two or greater grade improvement in the IGA score, and reach clear or almost clear at week 12 for HP/TAZ versus vehicle, followed by the same outcome and comparison at week 6, week 4, and finally week 2. Aside from these secondary outcomes and the primary outcome, none of the other outcomes were adjusted for multiplicity.
Only descriptive statistics were reported for the remaining exploratory efficacy outcomes (DLQI, two-grade improvement from baseline in IGA score, and BSA outcomes) and missing data were not imputed. For per-protocol analyses, a LOCF approach was used to impute missing values. Data were not imputed for missing safety data.
Each trial was estimated to have greater than 95% power to detect a statistically significant outcome for a two-sided test with an alpha level of 0.05 based on a total of 140 patients in the HP/TAZ group and 70 patients in with vehicle group. The power calculations were based on observed IGA efficacy data at week 8 using the ITT analysis set from the phase II study for HP/TAZ (Study 201).
Subgroup analyses for the primary outcome (ITT population) were conducted and descriptive statistics were reported. The following subgroups were included: baseline IGA, sex, age (less than vs. greater than the median age of patients), ethnicity, and race. The subgroup analyses appear to be pre-planned, although treatment randomization was not stratified by any of these subgroups at baseline.
Statistical Analysis of Efficacy Endpoints.
Analysis Populations
All patients who were randomized and dispensed study drug were included in the ITT analysis set.
The per-protocol analysis set included all subjects in the ITT set who completed the week 8 study visit without any major protocol violations, which included: violating inclusion/exclusion criteria, use of an interfering concomitant medication, not attending the week 8 visit, missing more than one visit between baseline and week 8, not being compliant with the dosing regimen, and attending the week 8 study visit more or less than five days from the intended visit date.
The safety analysis set was defined by subjects who were randomized, received at least one confirmed dose of study drug, and completed at least one post-baseline safety assessment.
The ITT analysis set was used for the primary evaluation of efficacy and the per-protocol set was used for supportive analyses. The safety set was used for all safety analyses.
Results
Patient Disposition
A summary of patient disposition including the number of patients included in each analysis set is presented in .
Patient Disposition (All Randomized Patients).
The number of patients screened for Study 301 and Study 302 was not reported. A total of 83.3% of randomized patients completed Study 301 and 84.2% of patients completed Study 302. Overall, study discontinuation rates were 16.7% and 15.8% in Study 301 and Study 302, respectively. Reasons for discontinuation were similar between the two treatment groups in both studies, with the exception of discontinuation due to AEs in Study 301, which was reported as 4.4% of patients treated with HP/TAZ and 0% for patients who received vehicle. The most common reasons for discontinuation from study were patient request (6.9% in Study 301 and 7.0% in Study 302), lost to follow-up (4.9% and 2.5%, respectively), and AEs (3.0% and 4.2%, respectively). Discontinuation rates were also similar across the two pivotal trials.
Exposure to Study Treatments
A summary of exposure to study drug during Study 301 and Study 302 is provided in .
Exposure to Study Drug (Safety Set).
The amount of study drug used, number of days exposed to study drug, and total number of applications of study drug were ▬▬▬▬▬ in Study 301. Patients used about ▬▬▬▬▬. The number of days exposed to study drug and number of applications were ▬▬▬▬▬. Patients receiving HP/TAZ used ▬▬▬▬▬ and patients receiving vehicle used ▬▬▬▬▬.
Compliance was defined by using between 80% and 120% of the expected applications of study drug while enrolled in the study. Briefly, based on the ITT population, ▬ of patients receiving HP/TAZ ▬ of patients receiving vehicle were compliant in Study 301. In Study 302, ▬ of patients treated with HP/TAZ and vehicle, respectively, were compliant. Data related to extent of exposure was summarized earlier in .
Efficacy
Only those efficacy outcomes and analyses of subgroups identified in the review protocol are reported below. See Appendix 3 for detailed efficacy data.
Health-Related Quality of Life
In both Study 301 and Study 302, HRQoL was evaluated as an exploratory outcome and assessed at week 4, 8, and 12 using the DLQI. This was the only measure of HRQoL included in the two studies. The DLQI scores at baseline and week 8, and change from baseline scores, are presented in . Data at week 8 was missing for 12% and 16% of patients in Study 301 and Study 302, respectively. The corresponding results for week 4 and week 12 are available in Appendix 3 (Table 43).
Change From Baseline in DLQI Score (ITT Set).
At the week 8 (end-of-treatment) study visit, the DLQI score for patients in Study 301 had ▬ by a mean (SD) ▬▬▬▬▬ in the HP/TAZ and vehicle treatment groups, respectively. In Study 302, the mean (SD) change from baseline in the DLQI score was ▬ in the HP/TAZ and vehicle treatment groups, respectively. In the absence of formal statistical testing, no conclusions can be made as to whether DLQI was actually reduced in either group or whether there were any differences in DLQI between the two treatment groups.
Skin Clearance
Skin clearance was assessed by the proportion of patients achieving treatment success by the IGA and BSA affected by plaque psoriasis in both of the pivotal trials. The IGA score by study visit was also reported and is available following the presentation of treatment success by IGA.
Treatment Success by IGA
The primary end point was treatment success at week 8, defined by at least a two-grade improvement from baseline in the IGA score in addition to an IGA score equal to clear or almost clear. This definition of treatment success was also assessed at week 12, 6, 4, and 2 as secondary end points. A summary of this data is available in . At week 8, 35.8% and 7.0% of patients treated with HP/TAZ and vehicle, respectively, had treatment success in Study 301. In Study 302, 45.3% and12.5% of patients treated with HP/TAZ and vehicle, respectively, had treatment success. In both trials, the difference between HP/TAZ and vehicle in the proportion of patients achieving treatment success (P < 0.001) was in favour of HP/TAZ. Two sensitivity analyses were carried out for the primary outcome: imputing missing values using LOCF and a repeated measures analysis on observed data. In both trials, the sensitivity analyses were consistent with the primary analysis.
Treatment Success by IGA (ITT Set).
The secondary end points were analyzed following a gated sequential testing procedure, beginning with treatment success at week 12, followed by week 6, 4, and finally week 2. The results are available in . Of note, week 8 was the last visit where the patient received study drug and week 12 was a post-treatment follow-up visit. The difference between HP/TAZ and vehicle in the proportion of patients with treatment success was in favour of HP/TAZ (P < 0.05) at week 12, 6, and 4 in both studies. The difference in favour of HP/TAZ was also observed at week 2 in Study 302 (P = 0.004), but not in Study 301 (P = 0.098).
A subgroup analysis by disease severity at baseline was performed for the primary end point in both trials (). Since no formal testing was carried out for this subgroup, the results are descriptive in nature. The proportion of the subgroup patients with moderate disease (IGA = 3) who achieved treatment success at week 8 was 34.9% and 8.5% for the HP/TAZ and vehicle treatment groups in Study 301, respectively, and 47.6% and 14.7% for the HP/TAZ and vehicle treatment groups in Study 302, respectively. The proportion of patients with treatment success in either treatment group was numerically similar to the results for the overall treatment population for both trials (Study 301: HP/TAZ 35.8% and vehicle 7.0%; Study 302: HP/TAZ 45.3% and vehicle 12.5%). For patients with severe disease (IGA = 4), the proportion of patients treated with HP/TAZ that achieved treatment success at week 8 was numerically similar to that of the overall population in Study 301 (40.1% vs. 35.76%). In Study 302, the treatment success among patients with severe disease who were treated with HP/TAZ was numerically less than the overall population (27.7% vs. 45.33%). In both trials, none of the patients in the vehicle group with severe disease achieved treatment success at week 8.
Subgroup Analysis by Disease Severity (ITT Set).
IGA Score by Study Visit
The IGA score by study visit was descriptively reported in Study 301 and Study 302 and the results have been presented in . Since this outcome was exploratory and not formally tested, no formal conclusions can be made based on these results.
IGA Score by Study Visit (ITT Set).
As per the inclusion criteria of the two trials, the disease severity of all patients was moderate or severe (IGA score of 3 or 4) at baseline. In Study 301, the proportion of patients with an IGA score of 0 or 1 at week 4 was 24.9% for patients randomized to HP/TAZ and 9.3% of patients randomized to vehicle. At week 8, 35.8% and 7.0% of patients treated with HP/TAZ and vehicle, respectively, had an IGA score of 0 or 1. At week 12 (four weeks following end of treatment), the proportion of patients with an IGA score of 0 or 1 in the HP/TAZ and vehicle treatment groups was 33.3% and 8.5%, respectively.
In Study 302, the proportion of patients with an IGA score of 0 or 1 at week 4 was 27.0% for patients randomized to HP/TAZ and 1.4% of patients randomized to vehicle. At week 8, 45.3% and 12.5% of patients treated with HP/TAZ and vehicle, respectively, had an IGA score of 0 or 1. At week 12, the proportion of patients with an IGA score of 0 or 1 in the HP/TAZ and vehicle treatment groups was 33.4% and 8.8%, respectively.
Percentage BSA and percentage change from baseline in BSA at week 8 are presented in . The corresponding results from week 2 through week 12 are available in Appendix 3 (Table 44). The BSA outcomes were included as exploratory end points in both trials and only descriptive statistics were presented for this outcome. At week 8, data were missing for 12% and 15% of patients in Study 301 and Study 302, respectively, and missing data were not imputed.
Percentage BSA Affected by Psoriasis (ITT Set).
Patients in the HP/TAZ and vehicle treatment groups in Study 301 had a mean 6.5% (SD = 3.0%) and 5.5% (SD = 2.6%) of their BSA affected by psoriasis at baseline. At week 8, the percent BSA affected by psoriasis had decreased to a mean of 4.4% (SD = 3.3%) and 5.3% (SD = 3.7%), respectively, which corresponded to a 32.8% (SD = 40.8%) change for the HP/TAZ group and a 2.3% (SD = 83.0%) change for the vehicle group. In Study 302, the mean percent of BSA affected by psoriasis at baseline was 5.4% (SD = 2.6%) and 5.9% (SD = 2.5%) for the HP/TAZ and vehicle treatment groups. This corresponded to a mean change of 42.5% (SD = 37.7%) and 8.3% (SD = 27.2%) for the two treatment groups, respectively.
Psoriasis-Related Signs and Symptoms
Improvement in the signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) was reported as an exploratory outcome and not adjusted for multiplicity in Study 301 or Study 302. Improvement in the signs of psoriasis was a measure of treatment success, which was defined as at least a two-grade improvement from baseline for each of the three signs of psoriasis, and it was assessed at week 2, 4, 6, 8, and 12. The results are available in . A summary of the severity of the signs of psoriasis individually and by visit are also available in Appendix 3 (Table 45, Table 46, and Table 47).
Improvement in the Signs of Psoriasis by Study Visit (ITT Set).
In Study 301, ▬ in the signs of psoriasis at week 8 was reported for ▬ of patients in the HP/TAZ and vehicle treatment groups, respectively. At week 12 in Study 301, ▬ in the signs of psoriasis was reported for ▬ of patients in the HP/TAZ treatment group and ▬ of patients in the vehicle treatment group. In Study 302, ▬ in the signs of psoriasis at week 8 was reported for ▬ of patients in the HP/TAZ and vehicle treatment groups, respectively, and at week 12, ▬ in the signs of psoriasis was reported for ▬ of patients in the HP/TAZ and vehicle treatment groups, respectively.
Productivity
Data related to productivity was not assessed in Study 301 or Study 302.
Treatment Adherence
Treatment adherence was not explicitly measured as an efficacy outcome, but dosing compliance was reported as a safety assessment and has been presented in-text following in the Exposure to Study Treatments section of this report.
Harms
Only those harms identified in the review protocol are reported below. See for detailed harms data that were reported up until the week 8 study visit.
Summary of Harms, up to Week 8 Study Visit (Safety Set).
Adverse Events
In Study 301, the percentage of patients that reported at least one AE was 36.8% and 19.4% for the HP/TAZ treatment group and vehicle treatment group, respectively. In Study 302, 35.0% and 23.3% of patients in the HP/TAZ and vehicle treatment groups, respectively, reported at least one AE. The most commonly reported AE in both studies was contact dermatitis, which only occurred in patients in the HP/TAZ treatment groups (5.3% in Study 301 and 9.5% in Study 302).
Serious Adverse Events
At week 8, SAEs were reported by three (2.3%) patients in the HP/TAZ group in Study 301. No SAE was observed in more than one patient. No SAEs were reported in Study 302.
Withdrawals Due to Adverse Events
Overall, 7.5% of patients in the HP/TAZ treatment group and zero patients in the vehicle treatment group reported a WDAE in Study 301. In Study 302, 5.1% of patients in the HP/TAZ group and 6.8% of patients in the vehicle group reported a WDAE. The most common reason for WDAE was contact dermatitis, which only occurred in the HP/TAZ treatment groups (2.3% of patients in Study 301 and 1.5% of patients in Study 302).
Mortality
No deaths were reported in either of the two pivotal trials.
Notable Harms
Of the notable harms included in the CADTH review protocol, pruritus was the most frequently occurring. In Study 301, it was reported among 3.0% of patients in the HP/TAZ treatment group and zero patients in the vehicle treatment group. In Study 302, pruritus was reported by 2.9% and 5.5% of patients in the HP/TAZ and vehicle treatment groups, respectively. Skin atrophy and folliculitis were also reported in both studies. In Study 301, skin atrophy and folliculitis were reported in 3.0% and 1.5%, respectively, of patients in the HP/TAZ treatment group, and zero patients in the vehicle treatment group. In Study 302, skin atrophy was reported in 0.7% of patients in the HP/TAZ treatment group and folliculitis was reported in 2.2% of the HP/TAZ group. Neither harm was reported in the vehicle treatment group in Study 302. In addition, events of burning sensation and skin irritation were reported in a small percentage of patients (≤ 1.5%) in both treatment groups of Study 301. Hypersensitivity was reported in one patient in each of the HP/TAZ and vehicle treatment groups of Study 302, as well as one skin irritation AE in the HP/TAZ group and one burning sensation AE in the vehicle treatment group. Severe dryness and HPA axis suppression were also included as notable harms in the CADTH review protocol, but neither harm was reported on in either study.
Local signs and symptoms were assessed as a measure of tolerability and summarized as the proportion of patients with a treatment-emergent grade 3 (severe) local skin reaction. This was defined as follows: itching = intense itching that may interrupt daily activities and/or sleep, dryness = marked roughness, and burning/stinging = hot burning sensation that causes definite discomfort and may interrupt daily activities and/or sleep. In Study 301, the proportion of patients in the HP/TAZ and vehicle treatment groups with a severe treatment-emergent skin reaction was: itching, 17.4% versus 19.4%; dryness, 2.3% versus 10.4%; and burning/stinging, 10.6% versus 14.9%. In Study 302, severe treatment-emergent skin reactions were reported as follows for the HP/TAZ treatment group compared to vehicle: itching, 11.7% versus 21.9%; dryness, 5.1% versus 16.4%; and burning/stinging, 5.8% versus 13.7%. In addition, skin atrophy and folliculitis were reported for 3.3% and 2.5%, respectively, of patients receiving HP/TAZ in Study 301 and 2.5% and 3.3%, respectively, in Study 302, compared to 0% of patients receiving vehicle in both studies.
Critical Appraisal
Internal Validity
Both trials used an acceptable method of randomization and concealment of treatment allocation. An interactive response technology system was used to randomize patients and randomization was not stratified. The two trials were double blind and vehicle controlled, where the HP/TAZ lotion and the vehicle lotion were identically packaged and labelled. Blinding was not identified as an issue in either of the two trials.
At baseline, there were some differences between the treatment groups in terms of demographic characteristics of patients in the two trials, such as a higher proportion of males in the vehicle group of Study 302 (67.6% vs. 61.0%) and a higher proportion of White patients in the vehicle group in both studies (301, 92.5% vs. 88.1%, and 302, 85.1% vs. 80.1%); however, demographic differences were not expected to affect treatment efficacy according to the clinical expert consulted for this review. As for the disease characteristics of patients at baseline, they appeared to be similar between treatment groups in Study 301 overall, with some slight differences in severity of the signs of psoriasis. In Study 302, the patients in the HP/TAZ group appeared to have milder disease in terms of IGA (11.3% had severe disease compared to 14.9% in the vehicle group), and a ▬ target lesion (the mean [SD] size for HP/TAZ was ▬; vehicle was ▬). These differences are minor but should be considered when interpreting the efficacy results in both trials due to potential bias in the results in favour of the HP/TAZ groups. In terms of prior medication use, the vehicle treatment groups included a ▬ of patients that reported prior use of ▬▬▬▬▬.
The pivotal trials were designed to compare HP/TAZ lotion to vehicle lotion, administered once daily to the affected area (which was determined by the investigator) for eight weeks, with a maximum weekly usage of 50 g. Concomitant treatments were permitted in the two studies and was similar between treatment groups in the two trials overall. Therefore, it was unlikely to have a differential impact on treatment efficacy; however, patients who had used concomitant therapy that could interfere with the interpretation of the study results were discontinued from the concomitant product rather than withdrawn from study. Specific details about the number of patients who violated the protocol that continued in the two studies was not available and therefore the impact of this issue on the results is uncertain.
Exclusion criteria regarding the use of prior treatment with certain therapies for psoriasis was implemented in the two trials, as noted in the Populations section of this report. The clinical expert consulted on this review noted that the washout period for phototherapy and biologics was too short to be certain that they did not have an impact on treatment effect. In the trials, patients were excluded if they had used phototherapy within four weeks prior to baseline, and if they had used biologics within three months of the baseline visit. The clinical expert suggested that for older therapies such as etanercept, adalimumab, and infliximab, three months is a sufficient washout period; however, a more appropriate washout period would have been eight weeks for phototherapy and four to six months for therapies such as ustekinumab, tildrakizumab, guselkumab, and risankizumab, as per a longer half-life with these therapies. The British Association of Dermatologists guidelines for biologic therapy for psoriasis also notes a washout period of three months or four times the terminal half-life for biologics, whichever is greater.37 Reported prior use of ▬▬▬▬▬ and likely not of concern for the two trials; ▬▬▬▬▬. Types of ▬ that patients reported having previously used included: ▬▬▬▬▬.
The primary outcome in both trials was treatment success at week 8, defined as at least a two-grade improvement from baseline in the IGA score and an IGA score of clear or almost clear (0 or 1). The secondary outcomes used the same definition of treatment success for week 2, 4, 6, 8, and 12. There is evidence regarding the validity and reliability of the six-point PGA scale (the same scale as the IGA, but the assessor is a physician rather than investigator) in the literature, but no evidence was identified regarding the five-point IGA scale used in the two studies. A MID was not identified in the literature for either version of the IGA scale. Further, the IGA has been shown to be reliable based on test-retest data and internal consistency, but is also a subjective measure that has demonstrated poor inter-rater reliability.12 The limitations of this scale introduce uncertainty to the interpretation of the primary and secondary outcomes, as well as the magnitude of observed treatment response. The two trials also assessed the improvement in the signs of psoriasis and the percentage BSA affected by psoriasis. A MID was not identified for either outcome and only evidence of reliability was available for the BSA. The DLQI was used to evaluate HRQoL in the two trials. The DLQI is a widely used instrument that captures different aspects of patients’ lives that are affected by skin disease and is considered valid and reliable, with an estimated MID in the range of 2.2 to 6.9.34,38 It has also shown good internal consistency reliability (with Cronbach alpha coefficients ranging from 0.75 to 0.92).38
The primary and secondary outcomes were controlled for multiplicity using a gatekeeping sequential procedure. Statistical testing was also conducted for the signs of psoriasis, but this should be interpreted considering the potential for risk of increased type I error. The DLQI and BSA outcomes were outside statistical testing procedures and reported descriptively without between-group comparisons, therefore conclusions that can be drawn from this data are limited. Missing data were imputed for the primary, secondary, and signs of psoriasis (treatment success) outcomes using the MCMC multiple imputation method, which is considered an appropriate approach. Missing data were not imputed for the DLQI and BSA outcomes, where between 12% and 16% of data were missing at week 8. Sensitivity analyses were conducted on the primary outcome using the LOCF method for missing data and a repeated measures analysis on observed data, and both were consistent with the primary analysis. As such, missing data were unlikely to be an issue for the analysis of treatment success by the IGA.
Subgroup analyses by various demographic characteristics that appear to be pre-planned were performed, but treatment randomization was not stratified by any of the subgroups at baseline and only descriptive results were provided. Further, the sample size of patients with severe disease was very small, including less than 20% of the overall population (17.2% and 12.6% of patients in Study 301 and Study 302, respectively, had severe disease). Thus, any conclusions that can be drawn regarding efficacy by baseline disease severity are limited. The CADTH review protocol included two additional subgroups of interest, namely, patients that used HP/TAZ as adjunct therapy compared to monotherapy, and patients with previous experience with treatments for plaque psoriasis (topical or systemic); however, no evidence was identified regarding these subgroups at the time of this review.
External Validity
The two trials include a number of limitations regarding generalizability to the target population identified by the indication and Canadian clinical practice.
All of the study centres were located in the US and the demographic characteristics of patients included in the two trials differ from what would be seen in practice in Canada. More specifically, there is a higher proportion of Hispanic and Black/African American patients, and a lower proportion of Asian patients than what is typically seen in Canadian clinical practice. There is also a higher proportion of male patients included in the clinical trials.
The disease severity of the patients enrolled is one issue for generalizing the results of the trials to the target population identified in the indication for HP/TAZ. Overall, the information available regarding disease severity of the patients who were included in the two trials appear to describe Canadian patients with mild-to-moderate disease rather than moderate to severe. Definitions of disease severity used in clinical trials available in the Canadian Guidelines for the Management of Plaque Psoriasis, which have suggested that a BSA of 5% is used as an upper limit for mild disease, and a BSA of 10% is used as a lower limit for moderate-to-severe psoriasis.4 The mean percent BSA of patients at baseline in the two trials was between 5.5% and 6.5% on average with upper and lower limits of 12% and 3%. However, the definitions of disease severity for psoriasis vary across international guidelines. For example, guidelines published by the American Academy of Dermatology and the National Psoriasis Foundation (US) defined disease severity by BSA involvement as less than 3% BSA considered mild, 3% to 10% BSA considered moderate, and greater than 10% BSA considered severe disease.13,14 The clinical expert consulted by CADTH acknowledged that there is lack of consensus regarding the definition of disease severity, but was of the view that the patient population in Study 301 and Study 302 was milder than what would be expected in a population of Canadian patients with moderate-to-severe psoriasis. CADTH acknowledges that patients were defined as having moderate or severe plaque psoriasis based on a baseline IGA score of at least 3; however, this was measured by the investigator or evaluator and the subjective nature of this scale and poor inter-rater reliability introduce uncertainty to this measure. Further, patients who had been treated with prescription medication and failed to respond to treatment, partially or temporarily, were excluded from the pivotal trials. Based on input from the clinical expert consulted by CADTH for this review, it is unlikely that a patient would present to care with moderate-to-severe psoriasis having not tried and failed to respond to prior treatment.
The intervention and method of administration in the clinical trials was consistent with the recommended dosing for HP/TAZ.9 The clinical expert consulted for this review also noted that in clinical practice, once a patient had achieved an IGA score of 0 or 1, treatment would be reduced or stopped. The use of certain concomitant medications was permitted during the two trials, which may be aligned with what patients do in clinical practice; however, concomitant use of phototherapy or systemic therapy was not permitted in the trials. Most patients with moderate-to-severe psoriasis would likely be treated with phototherapy or systemic treatment as per the clinical definition of moderate or severe plaque psoriasis which describes disease that cannot be, or would not be expected to be, satisfactorily controlled by routine skin care measures or topical therapy, respectively.4 To this point, it is unlikely that HP/TAZ would be used as monotherapy in patients with moderate-to-severe disease, which was confirmed by the clinical expert consulted for this review. Further, a more likely use of HP/TAZ in this population would be as an adjunct therapy to systemic treatment; however, evidence of this was not identified. This is a limitation to the generalizability of the clinical trial results for HP/TAZ.
According to feedback from the clinical expert, patients are assessed very informally in clinical practice with a focus on HRQoL and broadly on signs and symptoms of disease. Specific, structured questionnaires and scales are not typically used in clinical practice, but the goal of the assessment is similar, therefore, some of the outcomes used in the trials are relevant to clinical practice, but others such as the signs of psoriasis and percentage BSA affected by psoriasis are less relevant. The primary efficacy outcome was assessed after receiving treatment with study drug for eight weeks. As per feedback from the clinical expert consulted for this review, all patients issued a first prescription of a superpotent corticosteroid, including HP/TAZ, should be assessed after approximately four weeks for treatment response and safety-related issues, and a decision to continue or change treatment would be made at that time. The clinical expert acknowledged that the availability of a dermatologist for a four-week follow-up visit varies between practices; however, four weeks was still noted as an ideal follow-up time frame. The four-week end-of-treatment follow-up period was noted as being adequate to assess recurrence of disease.
Evidence comparing the use of HP/TAZ to other treatments for plaque psoriasis was limited to the sponsor-submitted NMA as no direct comparisons with active comparators were identified, despite the number of available treatments for this disease area. Further, the lack of direct comparative evidence is a major limitation of these trials and in the evaluation of HP/TAZ in the Canadian context. As such, the comparative effectiveness of HP/TAZ remains uncertain.