1. Introduction
1.1. Study Questions
“To determine the cost-effectiveness of dolutegravir (DTG) relative to Atripla/efavirenz (EFV), raltegravir (RAL), boosted darunavir (DRV/r), Complera/rilpivirine (RPV), Stribild/elvitegravir (EVG), boosted atazanavir (ATZ/r) and boosted lopinavir (LPV/r).”
(Manufacturer’s submission: Cost-Effectiveness Model for Dolutegravir (ARAMIS-DTG) in Treatment-Naive HIV Patients, page 11.)
“To determine the cost-effectiveness of DTG relative to raltegravir (RAL) in integrase inhibitor (INI) naive TE patients.”
(Manufacturer’s submission: Cost-Effectiveness Model for Dolutegravir (ARAMIS-DTG) in Treatment-Experienced HIV Patients, page 9.)
1.2. Treatment
a) Treatment-Naive
Treatment consisted of DTG (50 mg per day) plus NRTI backbone (according to RCT split: 60% Kivexa and 40% Truvada).
b) Treatment-Experienced
Treatment consisted of DTG (50 mg per day) plus OBT (DRV/r + tenofovir [TDF]).
1.3. Comparators
a) Treatment-Naive
Treatment consisted of Atripla, RAL plus 2 NRTIs, DRV/r plus 2 NRTIs, Complera, Stribild, ATZ/r plus 2 NRTIs, LPV/r + 2 NRTIs. The NRTI backbone (TDF plus emtricitabine [FTC] as part of the fixed-dose combination, or a blend of Truvada and Kivexa) differed according to the comparator but was considered to be equivalent in both treatment groups in the model in terms of both efficacy and safety.
b) Treatment-Experienced
Treatment consisted of RAL (800 mg per day) plus OBT (DRV/r + TDF).
1.4. Type of Economic Evaluation
A cost-utility analysis was undertaken and is appropriate according to the CADTH guidelines. The perspective utilized in the two models is that of the Canadian public payer.
1.5. Population
a) Treatment-Naive
Characteristics from patients in the DTG clinical trials (SPRING-2, SINGLE, and FLAMINGO) were used to inform transition probabilities for each simulated patient. Patients were assigned age, gender, CD4+ cell count, viral load, OI prophylaxis status, and Framingham score based on random probabilistic draws from the trial populations.
b) Treatment-Experienced
Characteristics from patients in the SAILING clinical trial were used to inform transition probabilities for each simulated patient. Patients were assigned age, gender, CD4+ cell count, viral load, OI prophylaxis status, Framingham score, and resistance status (NRTI resistance or NRTI + non-nucleoside reverse transcriptase inhibitors [NNRTI] resistance) based on random probabilistic draws from the baseline trial population.
2. Methos
Models to assess treatment of HIV are mature. They are based on models developed independent of industry to inform reimbursement decisions and have been refined over time. The model used in this submission is based on well-accepted pharmacoeconomic models (Cost-Effectiveness of Preventing AIDS Complications [CEPAC]).
Please see for a summary of the key limitations associated with the methodology used by the manufacturer.
Key Limitations of the Manufacturer’s Economic Submission.
2.1. Model Structure
a) Treatment-Naive
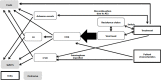
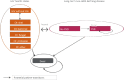
The cost-utility analysis was conducted using a micro-simulation model, where simulated patients pass through the model one at a time. This allows individual characteristics to be used, and the history of events can be incorporated and used to adjust the probability of disease progression (). Patients may transition through 12 mutually exclusive Markov health states plus death: six HIV health states (one HIV health state without OIs and five with acute OI of varying types); two cardiovascular disease health states (with and without CVD); and death (). The reference-case time horizon was lifetime with monthly cycle length and used the Canadian public-payer perspective. Individuals were assigned age, gender, CD4+ cell count, viral load, OI prophylaxis status, and Framingham score based on random probabilistic draws from the baseline characteristics of the population of the clinical trials at entry to the model. CD4+ cell count is adjusted for every model cycle using a tracker variable. The patient’s CD4+ cell count impacts the transition to the HIV-related events, including OIs and mortality. Within each cycle, individuals are at risk of developing CVD based on a probability calculated using the Framingham equation, which is based on baseline characteristics as well as changes in lipid status (which may be influenced by treatment). The model allowed for treatment switches (). Individuals could switch treatment after an acute AE, or when a treatment was failing. The model assumed a minimum time on treatment of three months, except in the case of late failures and when there was a switch due to an acute AE. Patients switching therapy were assumed to incur extra costs for an additional doctor visit.
The Interaction Between Treatment, Viral Load and CD4+ Cell Count. Source: Manufacturer’s pharmacoeconomic submission.
Markov Model. Source: Manufacturer’s pharmacoeconomic submission.
Treatment-Switching Rules. Source: Manufacturer’s pharmacoeconomic submission.
The treatment for second and subsequent therapies was based on an algorithm developed by Canadian HIV clinicians for treatment pathways that would be used in Canada. Subsequent treatment choices were influenced by the reason for discontinuation (tolerability, resistance, specific resistance mutations).
b) Treatment-Experienced
The same model was also used for treatment-experienced patients.
2.2. Clinical Inputs
a) Efficacy
Treatment-Naive
Efficacy was defined using virological suppression at 48 weeks (from trials), CD4+ cell count (from trials and modelled), and late failure (modelled). The late-failure rate is treatment-specific and was estimated by examining viral load suppression by treatment at different times (48, 96, and 240 weeks). The sources of efficacy data comparing DTG to efavirenz (EFV) were the SINGLE trial3 and the STARTMRK trials;17 SPRING-24 and STARTMRK trials for DTG versus RAL, and FLAMINGO5 and STARTMRK trials for DTG versus DRV/r. The efficacy for the other comparators was derived from the network meta-analysis (NMA). The development of resistance following virologic failure after initial treatment was derived from SINGLE, SPRING-2, and FLAMINGO clinical studies.
Treatment-Experienced
The sources of efficacy for first ART in the model for DTG and RAL were the SAILING12 and BENCHMRK studies.18 The rate of CD4+ cell count recovery was treatment-specific, while the late-failure rate was non–treatment-specific and was derived from BENCHMRK. The prevalence of resistance (NRTI resistance or NRTI plus NNRTI resistance) was based on data from the SAILING trial. The development of INI resistance following virologic failure was also derived from the SAILING trial. The development of resistance was not considered after failure of subsequent ARTs in the model.
b) Harms
Treatment-Naive
Acute AEs (grade 2 to 4) led to a switch to the next treatment based on the discontinuation rate as reported in various clinical trials. The probabilities for DTG were derived from the SINGLE, SPRING-2, and FLAMINGO trials. Only AEs leading to discontinuation were considered in the model after first therapy.
Treatment-Experienced
AEs were considered only through discontinuation of treatment due to AEs, which led to a switch to the next treatment, as no difference was observed between RAL and DTG in SAILING regarding adverse events (AEs). Discontinuation rates due to AEs were based on clinical trials. The probabilities for DTG were derived from the SAILING trial.
c) Disease Progression
Treatment-Naive
CD4+ cell count over time is dependent on treatment status. Successful treatment (based on virologic suppression) results in an increase in the CD4+ cell count over two years (based study data), although no increase is assumed to occur after five years (based on observational studies). If treatment fails, CD4+ count is assumed to be maintained as the patient changes therapy (first 12 months), and subsequent decline is dependent on the viral load with next-line therapy. The rate of decline is based on an equation derived from the Multicenter AIDS Cohort Study (MACS).19 The risk of acute OIs is dependent on the disease history of the specific OI, the CD4+ cell count, and the time on and status of treatment based on the Antiretroviral Therapy Cohort Collaboration.20 The risk of CVD was estimated from the Framingham equation for CHD supplemented with the Framingham equation for stroke. The equation was estimated every year, updating age and lipid parameters (from trials) during the first two years (Atripla and RAL) or the first year only (DVR/r). Beyond this period, the Framingham equation was updated to account for increasing age only.
Treatment-Experienced
Same methodology was used to calculate CD4+ decline rate and rate of acute OIs in the treatment-experienced patients. For the Framingham equation, age and lipid parameters (from SAILING) were updated during the first six months. Beyond this period, the equation was updated to account for increasing age only.
d) Mortality
Treatment-Naive
Mortality was modelled using four main causes of death: 1) HIV; 2) acute OIs; 3) CVD; and 4) all-cause mortality (excluding HIV and CVD-related death). General HIV mortality and acute OI mortality rates were derived from MACS (1995) and corrected for highly active antiretroviral therapy exposure stratified by baseline CD4+ cell level.21 The standard mortality ratio (SMR) was derived using the British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) study. Baseline mortality and CVD mortality were obtained from Statistics Canada.
Treatment-Experienced
Same mortality rates were used in the treatment-experienced model.
e) Quality of Life
Treatment-Naive
Although EQ-5D (EuroQol 5-dimensions questionnaire) scores were an outcome in the clinical trials, HIV utilities were derived from a Canadian study examining the relationship between HUI3-derived health preference score and CD4+ cell count.6 Utility decrements associated with CVD were derived from a US study.7 Utilities associated with OIs or AEs were not considered in the base-case analysis but were included in the sensitivity analysis.
Treatment-Experienced
The same utilities mentioned earlier were also applied in the treatment-experienced model.
More details on how the utility scores were assigned are listed in the next section.
f) Costs
Resource use was considered from the perspective of the public payer.
g) Drug Costs
Treatment-Naive
The cost of DTG ($18.50 per tablet) was obtained from the manufacturer. The costs for ART were obtained from the RAMQ List of Medications. Each first regimen used a blended backbone (60% Kivexa and 40% Truvada) based on the clinical trials for DTG. The cost for salvage therapy was defined based on data from IMS Brogan ▬.
Treatment-Experienced
The costs for ART were obtained from the RAMQ List of Medications. The cost for salvage therapy was defined based on data from IMS Brogan ▬.
h) Event Treatment Costs
Treatment-Naive
Costs associated with HIV were obtained from Canadian sources. Outpatient care costs (HIV clinic visit, HIV-related specialist visits, non-HIV physicians, lab tests) were obtained from a Canadian study using a Southern Alberta cohort.8 Costs for OI (in-patient only) were derived from a cost-effectiveness study in BC.9 The cost of death was calculated from a study in Alberta.10 The costs for OI prophylaxis treatment (trimethoprim-sulfamethoxazole and azithromycin) were sourced from the Ontario Drug Benefit Formulary. The costs of acute AEs were determined based on medication costs, physician visits, and model assumptions were based on current Canadian sources. Costs of CVD were derived from a study on Canadian outpatients with coronary heart disease, cerebrovascular disease, or peripheral artery disease.11
Treatment-Experienced
The event treatment costs used in the treatment-experienced model were the same as that used in the treatment-naive model, except for the costs of acute AE, as no difference was observed between RAL and DTG in the clinical trial.
i) Utilities
Treatment-Naive
HIV utilities were derived from the Isogai et al. study. In that study, a regression model that included CD4+ cell count (plus squared term), age (plus squared term), and sex, was used to calculate utility for each simulated patient.6 Utility decrements associated with CVD (0.0977) were derived from the Franks et al. study.7
Treatment-Experienced
The same utility allocation was used for treatment-experienced patients.
j) Time Horizon
Both models used lifetime time horizon and was appropriate according to the CADTH guideline.
k) Discounting
Costs and consequences occurring after 12 months were discounted at an annual rate of 5%, as per CADTH guidelines. The results were presented in 2013 Canadian dollars.
l) Validation
Formal information on model validation was not provided in the submission; however, the current model was based on previous models on HIV drugs (ARAMIS model, based on the original CEPAC model) which have been validated, and discussion on comparison of overall life expectancy and incremental QALYs with external estimates was provided.
3. Results
3.1. Manufacturer’s Base Case
a) Treatment-Naive
In the reference case, the manufacturer reported the total cost for DTG was $315,086, a cost saving of $7,753 compared with Atripla. Treatment with DTG resulted in 10.62 total QALYs, an additional 0.132 QALY compared with Atripla. Hence, DTG was the dominant strategy. DTG was also the dominant strategy when compared with RAL, DRV/r, Complera, Stribild, ATZ/r, and LPV/r in the manufacturer’s base case. Details on the costs and QALYs are listed in .
Summary of Results of the Manufacturer’s Base Case.
The incremental QALYs with DTG range from 0.041 to 0.132 over a lifetime horizon (or an additional 15 to 48 days of life with perfect health). The bulk of costs (87%) are from ART treatment. In all base-case scenarios, ART costs are lower for DTG, which is influenced by drug-acquisition cost (in analyses where comparators have greater drug-acquisition costs), as well as a lower likelihood of progressing to second-, third-, and fourth- to sixth-line therapy (greater drug costs occur with moving down to subsequent treatments regimens).
b) Treatment-Experienced
In the reference case, the manufacturer reported that the total cost for DTG was $353,957, a cost saving of $3,745 compared with RAL. Treatment with DTG resulted in 8.255 total QALYs, an additional 0.222 QALY (81 additional days of life with perfect health) compared with RAL. Hence, DTG was the dominant strategy.
The bulk of costs (87%) are from ART treatment driven primarily by lower drug-acquisition costs for DTG versus RAL.
3.2. Summary of the Manufacturer’s Sensitivity Analyses
As the model was a complex micro-simulation model, and running time was high, no probabilistic sensitivity analyses were carried out. Uncertainty was addressed using one-way deterministic sensitivity analyses (which varied model parameters by using alternative values) and scenario analyses.
a) One-way Sensitivity Analyses
Treatment-Naive
A series of one-way sensitivity analyses (95% confidence interval (CI) of the parameter, unless specified) were conducted by the manufacturer for DTG versus Atripla, RAL, and DRV/r where efficacy and safety data from head-to-head trials were available, including: difference in viral suppression at week 48; difference in late-failure rate between week 48 and week 96; cost of subsequent treatment (± 10%); and efficacy of subsequent treatment (± 5%).
The scenario analyses included: cost of EFV (generic versus Atripla); cost of RAL; cost of DTG plus backbone; cost of salvage (± 25%); the removal of treatment-specific effect on resistance; alternative sets of utilities (Kauf 2008); addition of disutilities due to AEs; addition of utilities related to OIs; discounting (0%, 3%); use of cohort based on an observational study in BC.
The reference-case result for DTG compared with Atripla, RAL, and DRV/r was DTG being the dominant strategy (less costly and more effective). None of the sensitivity analyses change this conclusion, except when the cost of generic EFV plus Truvada was used instead of Atripla, where the ICUR was $44,604 per QALY.
Treatment-Experienced
A series of one-way sensitivity analyses (95% CI of the parameter, unless specified) was conducted by the manufacturer including: difference in viral suppression at week 48; cost of subsequent treatment (± 10%); and efficacy of subsequent treatment (± 5%); cost of salvage (± 25%); drug-acquisition cost of RAL; removal of treatment-specific effect on resistance; alternative sets of utilities (Kauf 2008); addition of disutilities due to AEs; addition of utilities related to OIs; and discounting (0%, 3%).
The reference-case result for DTG compared with RAL found DTG to be the dominant strategy. The conclusion remained the same with all sensitivity analyses, except when a 0% discount rate was used. The ICUR was $11,787 per QALY.
b) Probabilistic Sensitivity Analysis
No probabilistic sensitivity analyses were carried out due to model complexity.
3.3. CADTH Common Drug Review Analyses
CDR has performed reanalyses based on different assumptions as listed subsequently. Due to model complexity, the CDR analysis used 10,000 simulations instead of the manufacturer’s default setting of 500,000, and this might result variability.
The QALYs gained between strategies is small (the largest incremental QALY from DTG versus RAL in the treatment-naive analyses was less than two months of perfect health [0.132]). Because of this, and given the lower number of simulations and that small incremental QALYs may lead to instability in the ICURs, it may be more meaningful to focus on incremental costs and QALYs, rather than the ICURs.
a) Treatment-Naive
Efficacy of Virologic Suppression
Since there is no head-to-head trial comparing DTG to Complera, Stribild, ATZ/r, or LPV/r, an NMA was used to inform the efficacy parameters in the model. While the NMA was appropriately conducted, uncertainty remains regarding true relative efficacy. Since the 95% CI of the OR for virological suppression at 48 weeks for Stribild and Complera cross unity, the same virological suppression is assumed (with an increase in CD4+ cell count when successful). DTG remains the dominant strategy (less costly and more effective) when the same efficacy is assumed. As the 95% CI of OR for virological suppression at 48 weeks for RAL in the NMA also crossed unity (but did not in the head-to-head trial), an analysis assuming virological suppression equivalent to as RAL was conducted. In this case, DTG remained less costly (−$28,113) but also less effective (−0.018 QALY), resulting in an ICUR of $1,586,792 for RAL per QALY due to the small incremental QALY (versus DTG).
Cardiovascular Mortality
In the current era of treatment of HIV, incremental differences in CV mortality may be less important than in the past. An exploratory analysis was performed, where CV mortality was assumed to be zero for Atripla, RAL, and DRV/r was performed. DTG remained the dominant strategy (less costly and more effective) when CV mortality is set to zero.
Death Costs
The cost of death was calculated from a Canadian study that included mostly cancer patients, which might not necessarily reflect the cost for HIV patients.10 When death costs are excluded for Atripla, RAL, and DRV/r, DTG remains the dominant strategy.
b) Treatment-Experienced
Cardiovascular Mortality
When CV mortality is assumed to be zero for RAL, DTG remains the dominant strategy (less costly and more effective) in treatment-experienced patients.
Death Costs
When death costs are excluded for RAL, DTG remains the dominant strategy.
CDR Reanalysis of ICURs for DTG in Treatment-Naive and Treatment-Experienced Patients.
Drug-Acquisition Costs
ART costs are the primary driver of total costs, although ART costs are driven by both ART acquisition costs and the probability of moving from first-line to second- through sixth-line therapy (which are more costly). While efficacy estimates support the base-case assumption that DTG is likely to lead to a lower probability of requiring second- through sixth-line therapy, this is not definitively established. Further, the predicted incremental QALY differences are small. As such, it may be informative to simply examine drug-acquisition costs of first-line therapy.
provides incremental daily and annual costs, including the range of costs depending on the NRTI backbone used (where appropriate, as some pills include triple therapy). Further information on ART costs is provided in APPENDIX 1: COST-COMPARISON TABLE FOR HIV.
ART costs of First-Line Therapy.
Price-reduction scenarios were not performed, as DTG was the dominant strategy (less costly and more effective)
4. Discussion
The economic model that this submission uses is based on previous iterations of models that were originally developed by non-industry experts to inform reimbursement of treatment for HIV by publicly funded bodies. As such, this model is relatively robust with respect to incorporating health states and outcomes, and the transitions between them. As it is a first-order Monte Carlo simulation, accounts for a variety of inputs, and considers a large number of (appropriate) comparators, this does lead to considerable model complexity; however, no major oversights in model structure or function were identified.
The key efficacy outcomes used to inform the model, and subsequent clinical outcomes, are surrogates, including viral load at 48 weeks, CD4+ cell count, etc. While existing randomized trials comparing DTG with relevant comparators are not long or large enough to evaluate hard clinical outcomes, these surrogate markers are well-accepted, and correlate well with clinical outcomes.
The relative efficacy of DTG is informed by both randomized control trials as well as a NMA. Both direct and indirect comparisons suggest that DTG is at least as effective, or more effective, than many of the comparators. While this leads to slightly improved clinical outcomes and QALYs, another impact is the subsequent reduction in the need to move to second- through sixth-line therapy (fourth line for treatment-experienced patients), due to lower failure of treatment. As subsequent therapies further down the treatment algorithm are associated with greater costs, this also contributes to lower net costs for DTG. According to the clinical expert, given the relative efficacy estimates, the reduced need for more costly therapy is a very plausible scenario. However, if this does not occur, drug costs may be higher for some of the comparators. Table 1 suggests that DTG is less costly than many comparators, but may be more costly for some.
The CDR reanalysis assessed the relative efficacy of virological suppression at 48 weeks (as there may be uncertainty regarding how a NMA is conducted, and some of the estimates crossed unity). When this efficacy parameter was set to unity, this did not change the overall conclusions of the model. Further, for the most part, neither the additional sensitivity analysis conducted by the manufacturer nor the CDR reanalysis changed the overall conclusions.
It should be noted that some of the incremental QALYs in the CDR reanalysis could be interpreted as counterintuitive, but this is likely because incremental QALYs are quite small. When a first-order Monte Carlo simulation is used, there can be a large amount of variability in the outcomes, particularly when a small number of simulations are run (which was necessary in this case given the very long time it took the model to run). The CDR reanalyses used a much smaller number of simulations than those in the manufacturer’s submission.
Issues for Consideration
According to the clinical expert, the level of adherence is a major predictor of the effectiveness of treatment. As such, the once-daily DTG might have an advantage to improve adherence over other regimens with more frequent administration, which may have an impact on real-world effectiveness.
It is possible that a triple-therapy product that includes DTG will subsequently be brought to market. The incremental cost and cost-effectiveness may be altered if priced higher. The manufacturer states that, “The Patented Medicine Prices Review Board (PMPRB) mandates that combination product cannot cost more than the price of the individual components.”
Because there is limited clinical information for patients aged 12 to 18 years, the results of the economic evaluation cannot be generalized to this population.
Patient Input
Viral suppression, quality of life, and reduced side effects are important outcomes to HIV patients that were included by the manufacturer in the economic submission.
5. Conclusions
The manufacturer-submitted pharmacoeconomic model is based on well-established methods. While the model uses surrogate outcomes and links them to hard clinical outcomes, these surrogates are well-accepted markers of future clinical events and are used by prescribers to influence treatment decisions.
The manufacturer reports that dolutegravir is dominant compared with commonly used comparators for both treatment-naive and treatment-experienced patients with HIV. Effectiveness estimates that are used to inform efficacy in the model include outcomes such as viral suppression at 48 weeks and are based on data from both randomized control trials as well as network meta-analyses. In general, dolutegravir is more effective at viral suppression than many of the comparators, which leads to a very minor increase in incremental QALYs. Also, due to a reduced probability of requiring regimens further down the treatment algorithm (e.g., second- through sixth-line drugs, which are more costly) due to treatment failure, net ART costs (the primary driver of costs in the model) are lower. The manufacturer-conducted sensitivity analysis and CDR reanalysis indicated the results were largely robust. When ART costs alone are considered, if more expensive drugs along the treatment algorithm are required, dolutegravir remains less costly than most of the comparators considered (five or seven of the eight comparators, depending on the NRTI backbone used).
The economic attractiveness of dolutegravir is driven by its pricing — it is priced lower than some (but not all) comparators, including the other INI (raltegravir) — and by its slightly greater effectiveness, which leads to reduced use of second through sixth-line treatments (which are more costly), and very small differences in QALYs.