This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.27.1
The skin epidermis, like many other epithelia, continues to self-renew throughout the life of the animals due to the presence of adult stem cells that provide new cells to replace the damaged or dead cells. Following wounding, the skin is able to regenerate itself to some degree. However, when the wound is too extensive, such as in third-degree burns or in some skin genetic diseases, the skin cannot repair itself properly without medical interventions. The purpose of this chapter is to highlight the recent medical progresses that have been developed to regenerate the skin using stem cell technologies.
Introduction
The skin is the largest organ of our body. The main function of the skin is to act as a waterproof and mechanical barrier. Beside these critical roles in the regulation of water balance and in the protection against microorganism infection, the skin plays important role in the thermoregulation and in the sensory perception of the animal's surrounding environment. In addition to these physiological functions, the skin plays also a major role in the social and reproductive behavior by providing important information concerning the gender, the age, and the social status of an individual (Blanpain and Fuchs, 2006).
The skin epidermis, like many other epithelia, continues to self-renew throughout animals’ life due to the presence of adult stem cells (SC) that provide new cells to replace the damaged or dead cells. The skin is also capable of extensive regeneration following wounding, which implicates the activation of many cell types including epidermal SC, inflammatory and endothelial cells. However, when the wound is too extensive, the skin cannot regenerate by itself without therapeutic intervention. We discuss in this chapter the recent progress that has been made in the identification of SC from the skin epidermis, the role played by epidermal SC during homeostasis and wound healing, the therapeutic use of skin stem cells to treat patients suffering from extensive burns or hereditary skin diseases. Finally, we will discuss how cellular reprogramming of skin stem cells can be envisaged as an alternative source of cells for treating non-skin diseases.
The skin stem cells
The different cell lineages of the skin epidermis
The skin epidermis is composed by the juxtaposition of pilo-sebaceous units containing one hair follicle and its sebaceous gland, which are surrounded by the interfollicular epidermis (IFE; see Figure 1).
The IFE is a stratified squamous epithelium constituted by different layers of cells. The innermost layer, called the basal layer, is strongly attached to its underlying dermis and contains mitotically active progenitor cells that divide and give rise to the differentiated suprabasal cells. The first suprabasal layer corresponds to the spinous layer, in which cells strengthen their cytoskeleton and intercellular connections to provide better resistance to mechanical stress. The outermost granular layer produces biochemical components of the skin barrier itself. Finally, the stratum corneum corresponds to dead-enucleated cells that are continuously sloughed from the skin surface (Blanpain and Fuchs, 2006; Candi et al., 2005). The IFE continuously self-renews throughout life to replace the cells that are constantly sloughed off from the skin surface. In mice and humans, it takes about 3 to 4 weeks to replace all the cells of the IFE.
The hair follicle (HF) is organized into different concentric layers of cells. In contrast to the continuous renewal of the IFE, HF alternates cycles of growth and degeneration. At the end of morphogenesis, when HF reache their final size, matrix cells located at the base of the HF cease to proliferate, and undergo apoptosis, which induces the degeneration of the lower two-thirds of the HF. After this degenerative stage (catagen phase), HF stem cells (SC) located at the base of the remaining follicle called the bulge region, enter in a quiescent stage (telogen phase). At the start of the first hair cycle, quiescent bulge SC are stimulated by signals coming from the underlying mesenchyme, they exit from the SC niche, and proliferate actively to provide the cells that will re-form the new hair (anagen phase). The mature hair follicle contains seven concentric layers of differentiated cells: the outer root sheath, which is contiguous to the basal layer of the IFE, the companion layer, 3 layers of cells forming the inner root sheath, which forms the channel of the hair and 3 layers of cells forming the hair shaft. The matrix cells located at the base of the hair follicle represent the transient-amplifying cells of the HF, which differentiate into the inner root sheath and the hair shaft cells (Blanpain and Fuchs, 2006).
The skin epidermis also contains two types of glands: the sebaceous glands (SG), which are holocrine-secreting glands attached to each HF and produce the waterproof sebum, and the sweat glands that contribute to the regulation of the body temperature.
stem cell niche in skin
For decades, stem cells of the hair follicle were though to reside in the highly proliferative matrix cellular compartments (Kligman, 1959). In the early nineties, Cotsarelis and colleagues demonstrated that prolonged administration of nucleotide analogs (pulse) followed by a chase period, results in the presence of Label Retaining Cells in the bulge region, suggesting that bulge cells are more quiescent than the rest of the epidermal cells (Cotsarelis et al., 1990). To determine the clonogenic potential of bulge cells, Barrandon and colleagues, dissected the skin epidermis into different fragments and cultured the cells originating from these different epidermal regions in vitro. Bulge cells give rise to more highly proliferative colonies, known as holoclones, than the epidermal cells coming from other regions, suggesting that bulge cells, although more quiescent in vivo, present a much greater proliferative potential during in vitro culture (Barrandon and Green, 1987; Rochat et al., 1994). When bulge cells were transplanted onto immunodeficient mice, bulge cells can differentiate into all cell lineages of the skin epidermis including HF, IFE and SG, demonstrating that bulge region contains stem cells with different potential lineages (Oshima et al., 2001). Clonal analysis of bulge cells demonstrate that the progenies of one single bulge SC can reform all the epidermal lineages of the skin epidermis, demonstrating that bulge SC are truly multipotent (Blanpain et al., 2004; Claudinot et al., 2005). Fate mapping studies also indicate that bulge cells are multipotent SC, having the capacities to give rise to all cells of the hair follicle, sebaceous gland and interfollicular epidermis (Ito et al., 2007; Morris et al., 2004; Tumbar et al., 2004). Interestingly, these studies also demonstrate that during adult homeostasis, there is a little if any contribution of bulge SC to the maintenance of IFE, suggesting that unipotent progenitors ensure the renewal of IFE (Levy et al., 2005; Morris et al., 2004). However, upon wounding, bulge SC are activated, and migrate rapidly toward the skin lesions and participate actively in the repair of epidermis (Ito et al., 2007; Levy et al., 2007; Nowak et al., 2008; Taylor et al., 2000). After the completion of epidermal repair, the flux of bulge cells stops, and the bulge cells that had migrated in the IFE will progressively disappear overtime (Ito et al., 2007).
Different techniques have been developed to isolate and characterize the molecular properties of bulge SCs. Tumbar and colleagues developed an ingenious method, which allows to perform pulse chase experiments using a fusion protein between Histone-2B and the green fluorescent protein (H2B-GFP), allowing identification and isolation of living label retaining bulge cells (Tumbar et al., 2004). Cotsarelis and colleagues developed transgenic mice expressing the GFP under a bulge specific transgene allowing the isolation of bulge SC (Morris et al., 2004). Combination of α6-integrin and CD34 monoclonal antibodies can also be used to purify specifically bulge SC during all stages of hair cycle, which greatly facilitate isolation and characterization of bulge SC (Blanpain et al., 2004; Trempus et al., 2003). Gene expression profiling of bulge SC isolated using these three different approaches yield very similar results and uncover a list of genes specifically expressed in bulge SC irrespective of the stage of hair cycle or the isolation method (Blanpain et al., 2004; Morris et al., 2004; Tumbar et al., 2004). This list of genes provides valuable information to understand the unique features of multipotent bulge SC and how bulge cells actively participate in the formation of their own niche. Also these microarray studies allow the identification of multiple and important regulators of bulge SC functions such as Wnt/βcatenin pathways, Lhx2 and NFATc1 transcription factors (Horsley et al., 2008; Lowry et al., 2005; Rhee et al., 2006).
Lineage tracing experiments demonstrated that the IFE is organized into discrete units of proliferation, known as epidermal proliferative units (EPU). EPU can be observed in lineage tracing experiments as long term IFE labeled clones forming stacks of cells extending from basal cells to the top cornified cells (Ghazizadeh and Taichman, 2001; Kolodka et al., 1998; Mackenzie, 1997; Ro and Rannala, 2005). In addition to the bulge and IFE SC, there is accumulating evidence that other types of epidermal progenitors participate in the homeostasis of other epidermal compartments such as the SG and the infundibulum, the portion of the epidermis that connects the HF to the IFE. Transplantation experiments have suggested the existence of unipotent sebaceous lineage progenitors (Ghazizadeh and Taichman, 2001). Recent lineage tracing experiments using Blimp1-Cre identified rare cells located at the juncture between HF and SG that give rise to the entire SG (Horsley et al., 2006). These findings suggest that like the IFE, SG homeostasis may be maintained by the presence of unipotent progenitors. Recently, another population of SC was identified in a region located above the bulge but below the SG, within the upper isthmus (UI; Nijhof et al., 2006). These cells were identified by their expression of MTS24 (Nijhof et al., 2006), a cell surface marker, or using a combination of different monoclonal antibodies (Jensen et al., 2008). Moreover, transplanted UI cells reformed all three epidermal lineages (Jensen et al., 2008), suggesting that UI SC may even be multipotent SC (Jensen et al., 2008; Nijhof et al., 2006). Further studies are needed to better characterize the respective contributions of these newly identified epidermal SC to the overall skin epidermis homeostasis.
Skin stem cells for the treatment of third-degree burns
Major skin injuries, resulting from extensive burns, infection or trauma, cannot repair alone and require medical interventions to heal properly. Wound healing is organized into different steps. The first step is the release of soluble mediators from the degranulated platelets and from injured blood vessels, leading to the formation of a blood clot. Few minutes after injury, the invasion of neutrophils, monocytes and leukocytes promotes migration, proliferation and survival of various cell types including keratinocyte SC, leading to the beginning of the re-epithelialization process. In parallel to the reparation of the epidermis, the injured dermis is also repaired by the recruitment and proliferation of fibroblasts producing extracellular matrix and keratinocyte growth promoting factors. While wound repair was though to require influx of inflammatory cells, mice deficient for PU.1, which lack neutrophils and macrophages, repair skin wound as well as wild-type mice (Martin et al., 2003). These results challenge the current view that inflammatory cells are required for orchestrating the different steps of skin repair. Clearly more studies are needed to better understand the respective contribution of the various types of cells involved during wound healing.
In USA, the skin loss from thermal injuries represents about 1 million person each year, whereas the skin loss from trauma and chronic ulcerations from diabetes or from venous ulcers represents 2 millions and 600.000 patients respectively (Clark et al., 2007). These numbers reveal the importance of this clinical problem and the need for further research in the treatment of skin injuries. The autologous skin grafting, consisting in the removal of a piece of skin from unaffected tissue and its transplantation to the wound area, is the most viable and aesthetic technique for the treatment of extensive skin injuries. Nevertheless, this approach has important limitations, such as only a limited fraction of the skin can be repaired by this method and it creates additional injuries at the donor sites. For these reasons, researchers look for alternative methods to treat severe skin injuries. In the eighties, Green and colleagues discovered that human keratinocyte SC could be propagated in vitro when culture on fibroblast feeder cells (Green et al., 1979; Rheinwald and Green, 1975, 1977). Cultured under these conditions, human keratinocyte SC have an enormous proliferation potential, and only few cells can regenerate sufficient keratinocytes to cover the all-human skin surface. These cultured human keratinocyte SC can differentiate and reform a functional skin barrier that can be transplanted into patients suffering from severe burnt injuries (see Figure 2).
The human keratinocyte SC are obtained from aseptic skin biopsy (Gallico et al., 1984; Pellegrini et al., 1999; Ronfard et al., 2000) dissociated with trypsin to obtain single cells. These cells are then cultivated on irradiated 3T3 fibroblasts feeders that create, through the secretion of extracellular matrix and growth factors, an artificial niche particularly suitable for the proliferation of epidermal SC. The keratinocytes are kept in culture until the formation of a stratified epithelium that can be removed to cover the wound area, while the leftover cells can be frozen for future use (Green et al., 1979). This method is extremely powerful and allows the transplantation of a large piece of autologous skin starting from the removal of only a very small piece of unaffected skin. The first limitation of this technique is the time required to grow confluent epithelial sheets in vitro, during which the patient is particularly susceptible to infections. To prevent wound contraction and to cover the burns wound during the period of cell expansion, cadaver “decellularized” skin allografts are used. The second limitation is the huge cost of this treatment, which estimates at 13.000$ per 1% total body surface, suggesting a cost of at least 500.000$ for the majority of patients that are severely injured (Clark et al., 2007).
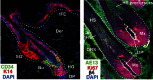
The grafted skin obtained by this method does not contain hair follicle and sweat glands (see Figure 2). The absence of hair follicle and sweat glands in third-degree burns does not result only from the destruction of multipotent hair follicle SC but also from the destruction of its underlying instructive dermis. Hair follicle morphogenesis and regeneration are critically dependent on the interaction between epidermal SC and specialized cells from the underlying mesenchyme, called the dermal papilla (DP) cells (Jahoda et al., 1984). To achieve HF regeneration in grafted epidermal sheets coming from cultured epidermal SC, DP cells will need to be transplanted together with epidermal cells in order to stimulate epidermal SC to adopt hair follicle fate. Recently progress has been accomplished to this end by the purification and genetic profiling of DP cells (Rendl et al., 2005). Using this approach, Rendl and colleagues identified BMP-6 as one of the critical factors expressed by DP cells to maintain its hair follicle inducing activity in vitro and in vivo, providing new hopes for the development of novel methods allowing the expansion and the differentiation of DP like cells in vitro (Rendl et al., 2008).
Cellular therapy for inherited genetic skin diseases
There are many theoretical advantages to use skin SC for cellular therapy in human. Skin is a highly accessible source of SC, which have an extraordinary capacity of cellular expansion in culture, and that can be stably genetically modified (Shi et al., 2006).
There are many different genetic diseases affecting the skin epidermis. Among the most devastating genetic skin diseases are the epidermolysis bullosa, which is characterized by an extreme fragility of the skin. There are thousands of patients around the world presenting these genetic diseases and suffering from repeating blistering lesions when the skin is submitted to a minor trauma. The blisters are painful and require long time to heal. In addition, possibly due to permanent state of wound healing that stimulates SC proliferation, these patients are at high risk of developing skin cancer (Eady, 2001).
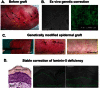
It is thus critical to develop new strategies to treat these patients. A major step has been realized recently by the group of De Luca, which demonstrated that correction of a severe form of epidermolysis bullosa could be achieved by transplantation of genetically modified keratinocyte SC (see Figure 3). Briefly, they isolated keratinocyte SC from a patient presenting epidermolysis bullosa caused by mutation in the gene encoding laminin 5, a critical component of hemidesmosomes. They expanded these skin SC in vitro and transduced these cells with a retrovirus expressing Laminin 5. They isolated cells stably expressing Laminin 5 and made genetically corrected cultured epidermal grafts that have been transplanted onto the patient skin lesions. The genetically corrected skin tissue healed perfectly well and biopsies from the graft demonstrate that the recombinant skin presents a normal histology and the expression of the transgene remains stable even a year after the treatment (see Figure 3). This beautiful study demonstrates the feasibility of gene therapy of inherited skin diseases in humans and provides new hopes that other genetic skin diseases can be treated with similar methods (Ferrari et al., 2006; Mavilio et al., 2006).
It is also conceivable to use grafted skin tissues engineered to express genes to treat non skin genetic diseases resulting for example from the absence of proteins necessary for metabolic or enzymatic activity. For example in hemophilia, it is expected that restoration of only 2–3% of the normal level of the deficient coagulation factors would result in a significant reduction of the clinical manifestations of the disease. Thus the transplantation of small patches of engineered skin tissue expressing well the non-functional proteins is expected to provide some therapeutical benefits (Herzog et al., 1999).
It has been suggested that cells residing in the bone marrow can migrate in the different tissues, “transdifferentiate” into various epithelial cell types and integrate long-term in these tissues (Krause et al., 2001). On the other hand, this possibility remains controversial today and the rarity of the “transdifferentiation” process suggests that it might not be useful clinically. Nevertheless, it has been reported that embryonic bone marrow cell transplantation can ameliorate dystrophic epidermolysis bullosa phenotype in neonatal mice (Chino et al., 2008). Clearly, more studies are needed to determine to which extent BM transplantation will be useful to treat patients with skin disorders.
Reprogramming of skin stem cells into pluripotent stem cells
Although the differentiation potential of adult SC is usually restricted to mature cells of their tissue of origin (Wagers et al., 2002; Wagers and Weissman, 2004), nuclear reprogramming of more committed cells into pluripotent SC can be now achieved using different methods (Hochedlinger and Jaenisch, 2006; Yamanaka, 2007). The first demonstration that differentiated somatic cells can be reprogrammed into pluripotent cells came from the demonstration of cloned amphibian embryo coming from the microinjection of nucleus of a differentiated cell into an enucleated oocyte (Gurdon and Byrne, 2003). To day, many domestic animals including cat, dog, cow, sheep, have been cloned using this nuclear transfer technique (Hochedlinger and Jaenisch, 2006). Nuclear transfer of isolated epidermal cells demonstrated that multipotent bulge SC could be more easily reprogrammed to pluripotent state than more committed epidermal cells, suggesting that these cells might be a better source of cells for nuclear reprogramming to pluripotent state (Li et al., 2007).
A major breakthrough came with the demonstration that exogenous addition of only four transcription factors (Oct4, Sox2, Myc and Klf4) were able to reprogram mature differentiated cells including fibroblast (Takahashi et al., 2007; Takahashi and Yamanaka, 2006), lymphocytes (Hanna et al., 2008) or liver cells (Aoi et al., 2008) into pluripotent embryonic SC, with the ability to differentiate into all cells of the body. This major finding opens new avenues for the generation of patient specific pluripotent embryonic SC and re-differentiate these cells into various defective or damaged cells types (Yamanaka, 2007). The proof of principle of the feasibility of this approach has been recently demonstrated in a mouse model of sickle cell anemia, a genetic disease that affects millions of people over the world. Jaenisch and colleagues took fibroblasts from a mouse model of sickle cell anemia, reprogrammed these cells into pluripotent SC, corrected the genetic deficiency by homologous recombination, and redirected these pluripotent cells toward the hematopoietic lineages, and transplanted these engineered cells to a lethally irradiated mice (Hanna et al., 2007). Treated animals presented many features demonstrating clinical improvement of the disease. The higher efficiency of bulge SC to be reprogrammed to pluripotent state by nuclear transfer (Li et al., 2007), together with their enormous expansion potential in vitro (Barrandon, 2007), suggest that bulge cells or other keratinocyte SC could be the ideal source of cells for nuclear reprogramming into pluripotent embryonic stem cells and the re-differentiation of these cells toward the cell types such as neurons, cardiac cells, hepatocytes, or pancreas cells that are defective or missing in human diseases.
References
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. [PubMed: 18276851] [CrossRef]
- Barrandon Y. Genetic manipulation of skin stem cells: success, hope, and challenges ahead. Mol Ther. 2007;15:443–444. [PubMed: 17311031] [CrossRef]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84:2302–2306. [PMC free article: PMC304638] [PubMed: 2436229] [CrossRef]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. [PMC free article: PMC2405915] [PubMed: 16824012] [CrossRef]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. [PMC free article: PMC2408375] [PubMed: 17289566] [CrossRef]
- Blanpain C, Lowry W.E, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. [PubMed: 15339667] [CrossRef]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. [PubMed: 15803139] [CrossRef]
- Chino T, Tamai K, Yamazaki T, Otsuru S, Kikuchi Y, Nimura K, Endo M, Nagai M, Uitto J, Kitajima Y. et al. Bone marrow cell transfer into fetal circulation can ameliorate genetic skin diseases by providing fibroblasts to the skin and inducing immune tolerance. Am J Pathol. 2008;173:803–814. [PMC free article: PMC2527073] [PubMed: 18688022] [CrossRef]
- Clark R.A, Ghosh K, Tonnesen M.G. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018–1029. [PubMed: 17435787] [CrossRef]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102:14677–14682. [PMC free article: PMC1253596] [PubMed: 16203973] [CrossRef]
- Cotsarelis G, Sun T.T, Lavker R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. [PubMed: 2364430] [CrossRef]
- Eady R.A. Epidermolysis bullosa: scientific advances and therapeutic challenges. J Dermatol. 2001;28:638–640. [PubMed: 11770723]
- Ferrari S, Pellegrini G, Matsui T, Mavilio F, De Luca M. Gene therapy in combination with tissue engineering to treat epidermolysis bullosa. Expert Opin Biol Ther. 2006;6:367–378. [PubMed: 16548763] [CrossRef]
- Gallico G.G., 3rd, O’Connor N.E, Compton C.C, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. [PubMed: 6379456]
- Ghazizadeh S, Taichman L.B. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. [PMC free article: PMC145528] [PubMed: 11250888] [CrossRef]
- Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–5668. [PMC free article: PMC411710] [PubMed: 293669] [CrossRef]
- Gurdon J.B, Byrne J.A. The first half-century of nuclear transplantation. Proc Natl Acad Sci U S A. 2003;100:8048–8052. [PMC free article: PMC166179] [PubMed: 12821779] [CrossRef]
- Hanna J, Markoulaki S, Schorderet P, Carey B.W, Beard C, Wernig M, Creyghton M.P, Steine E.J, Cassady J.P, Foreman R. et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. [PMC free article: PMC2615249] [PubMed: 18423197] [CrossRef]
- Hanna J, Wernig M, Markoulaki S, Sun C.W, Meissner A, Cassady J.P, Beard C, Brambrink T, Wu L.C, Townes T.M. et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. [PubMed: 18063756] [CrossRef]
- Herzog R.W, Yang E.Y, Couto L.B, Hagstrom J.N, Elwell D, Fields P.A, Burton M, Bellinger D.A, Read M.S, Brinkhous K.M. et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. [PubMed: 9883840] [CrossRef]
- Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. [PubMed: 16810240] [CrossRef]
- Horsley V, Aliprantis A.O, Polak L, Glimcher L.H, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. [PMC free article: PMC2546702] [PubMed: 18243104] [CrossRef]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. [PMC free article: PMC2424190] [PubMed: 16901790] [CrossRef]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar S.E, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. [PubMed: 17507982] [CrossRef]
- Jahoda C, Horne K, Oliver R. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. [PubMed: 6482967] [CrossRef]
- Jensen U.B, Yan X, Triel C, Woo S.H, Christensen R, Owens D.M. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609–617. [PMC free article: PMC2963074] [PubMed: 18252795] [CrossRef]
- Kligman A.M. The human hair cycle. J Invest Dermatol. 1959;33:307–316. [PubMed: 14409844]
- Kolodka T.M, Garlick J.A, Taichman L.B. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc Natl Acad Sci U S A. 1998;95:4356–4361. [PMC free article: PMC22493] [PubMed: 9539741] [CrossRef]
- Krause D.S, Theise N.D, Collector M.I, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis S.J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. [PubMed: 11348593] [CrossRef]
- Levy V, Lindon C, Harfe B.D, Morgan B.A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. [PubMed: 16326396] [CrossRef]
- Levy V, Lindon C, Zheng Y, Harfe B.D, Morgan B.A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. [PubMed: 17255473] [CrossRef]
- Li J, Greco V, Guasch G, Fuchs E, Mombaerts P. Mice cloned from skin cells. Proc Natl Acad Sci U S A. 2007;104:2738–2743. [PMC free article: PMC1815251] [PubMed: 17299040] [CrossRef]
- Lowry W.E, Blanpain C, Nowak J.A, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. [PMC free article: PMC1172065] [PubMed: 15961525] [CrossRef]
- Mackenzie I.C. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J Invest Dermatol. 1997;109:377–383. [PubMed: 9284108] [CrossRef]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher S.R. Wound healing in the PU.1 null mouse–tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. [PubMed: 12842011] [CrossRef]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, Maruggi G, Ferrari G, Provasi E, Bonini C. et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. [PubMed: 17115047] [CrossRef]
- Morris R.J, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin J.S, Sawicki J.A, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. [PubMed: 15024388] [CrossRef]
- Nijhof J.G, Braun K.M, Giangreco A, van Pelt C, Kawamoto H, Boyd R.L, Willemze R, Mullenders L.H, Watt F.M, de Gruijl F.R. et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. [PubMed: 16818453] [CrossRef]
- Nowak J.A, Polak L, Pasolli H.A, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. [PMC free article: PMC2877596] [PubMed: 18593557] [CrossRef]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. [PubMed: 11207364] [CrossRef]
- Pellegrini G, Ranno R, Stracuzzi G, Bondanza S, Guerra L, Zambruno G, Micali G, De Luca M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. [PubMed: 10515389] [CrossRef]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. [PMC free article: PMC1216328] [PubMed: 16162033] [CrossRef]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. [PMC free article: PMC2238674] [PubMed: 18281466] [CrossRef]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. [PMC free article: PMC2405918] [PubMed: 16809539] [CrossRef]
- Rheinwald J.G, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. [PubMed: 1052771] [CrossRef]
- Rheinwald J.G, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. [PubMed: 299924] [CrossRef]
- Ro S, Rannala B. Evidence from the stop-EGFP mouse supports a niche-sharing model of epidermal proliferative units. Exp Dermatol. 2005;14:838–843. [PubMed: 16232306] [CrossRef]
- Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. [PubMed: 8137423] [CrossRef]
- Ronfard V, Rives J.M, Neveux Y, Carsin H, Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588–1598. [PubMed: 11152220] [CrossRef]
- Shi C, Zhu Y, Su Y, Cheng T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006;24:48–52. [PubMed: 16298447] [CrossRef]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. S. Y. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. [PubMed: 18035408] [CrossRef]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [PubMed: 16904174] [CrossRef]
- Taylor G, Lehrer M.S, Jensen P.J, Sun T.T, Lavker R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. [PubMed: 10966107] [CrossRef]
- Trempus C.S, Morris R.J, Bortner C.D, Cotsarelis G, Faircloth R.S, Reece J.M, Tennant R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. [PubMed: 12648211] [CrossRef]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry W.E, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. [PMC free article: PMC2405920] [PubMed: 14671312] [CrossRef]
- Wagers A.J, Sherwood R.I, Christensen J.L, Weissman I.L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. [PubMed: 12215650] [CrossRef]
- Wagers A.J, Weissman I.L. Plasticity of adult stem cells. Cell. 2004;116:639–648. [PubMed: 15006347] [CrossRef]
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. [PubMed: 18371333] [CrossRef]
- *
Edited by Leslie Silberstein. Last revised September 18, 2008. Published November 15, 2008. This chapter should be cited as: Gaelle, L. and Cédric, B., Medical applications of epidermal stem cells (November 15, 2008), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.27.1, http://www.stembook.org.
- Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila.[PLoS One. 2013]Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila.Patterson RA, Juarez MT, Hermann A, Sasik R, Hardiman G, McGinnis W. PLoS One. 2013; 8(4):e61773. Epub 2013 Apr 24.
- Skin-derived precursors possess the ability of differentiation into the epidermal progeny and accelerate burn wound healing.[Cell Biol Int. 2017]Skin-derived precursors possess the ability of differentiation into the epidermal progeny and accelerate burn wound healing.Bayati V, Abbaspour MR, Neisi N, Hashemitabar M. Cell Biol Int. 2017 Feb; 41(2):187-196. Epub 2016 Dec 29.
- Advantages of using a bank of allogenic keratinocytes for the rapid coverage of extensive and deep second-degree burns.[Med Biol Eng Comput. 2000]Advantages of using a bank of allogenic keratinocytes for the rapid coverage of extensive and deep second-degree burns.Braye F, Pascal P, Bertin-Maghit M, Colpart JJ, Tissot E, Damour O. Med Biol Eng Comput. 2000 Mar; 38(2):248-52.
- Review Stem cells, niches and scaffolds: Applications to burns and wound care.[Adv Drug Deliv Rev. 2018]Review Stem cells, niches and scaffolds: Applications to burns and wound care.Watt SM, Pleat JM. Adv Drug Deliv Rev. 2018 Jan 1; 123:82-106. Epub 2017 Oct 26.
- Review Human skin stem cells and the ageing process.[Exp Gerontol. 2008]Review Human skin stem cells and the ageing process.Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C. Exp Gerontol. 2008 Nov; 43(11):986-97. Epub 2008 Sep 9.
- Medical applications of epidermal stem cells - StemBookMedical applications of epidermal stem cells - StemBook
- Dexamethasone effect on cultured airway smooth muscle cells: time courseDexamethasone effect on cultured airway smooth muscle cells: time courseAccession: GDS3946GEO DataSets
- Related DataSets for GEO Profiles (Select 73639670) (1)GEO DataSets
- dbVar for Gene (Select 2596) (497)dbVar
- PMC Links for Gene (Select 2596) (38)PMC
Your browsing activity is empty.
Activity recording is turned off.
See more...

 .
.