This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.49.1
Regenerative medicine with the use of stem cells has appeared as a potential therapeutic alternative for many disease states. Numerous questions remain regarding the viability and biology of transplanted stem cells in the living subject. Recent advances in molecular biology and imaging have allowed the successful non-invasive monitoring of transplanted stem cells in the living subject. In this review, different imaging strategies to study the viability and biology of transplanted stem cells are presented. Use of these strategies will be critical as the different regenerative therapies are being tested for clinical use.
Over the last decade, regenerative medicine has appeared as a powerful alternative for the treatment of many diseases (Baizabal et al., 2003; Gimble et al., 2007; Goldberg et al., 2007; Lechner, 2004). The main objective of cell-based therapies is to repopulate the damaged tissue with functional cells, with the final goal that these cells will integrate with the remaining functional native cells and contribute to the recuperation of the lost function. Regenerative medicine has been used to repair different organs/systems: endocrine (i.e., pancreas; Yamada et al., 2005), musculoskeleton (i.e., bone, joints; De Bari et al., 2001; Liu et al., 2006), and cardiovascular system (i.e. myocardium; Anversa et al., 2006; Ii et al., 2005; Rafii and Lyden, 2003), as well as adjuvant treatment for malignancies (Genre et al., 2002). While much has been learned on how stem cells function in cell culture, several questions remain regarding the biology of stem cells in living subjects. For most therapeutic applications of regenerative medicine, critical issues such as stem cell type and delivery route of stem cells remain to be elucidated. In addition, after cells are transplanted to the living subject, it becomes critical to understand the biology of transplanted cells and how they interact with the microenvironment. To answer some of these questions, it is imperative to perform these studies directly in the living subject in a longitudinal manner. Until recently those could not be performed in a reliable and accurate manner. Recent developments in molecular imaging modalities may likely permit investigators to answer some of these questions. Furthermore, some of these imaging strategies have the potential to be translated to patients, which makes them plausible to be used in the clinics.
Requisites for the ideal imaging modality for stem cell tracking
The ideal imaging agent/modality should provide the following information:
- Real-time visualization of stem cell delivery
- Determination of location(s) of cells over time
- Quantification of numbers of viable transplanted stem cells
- Long-term quantification of transplanted stem cell survival
- Study of stem cell biology:
- Interaction between stem cells
- Interaction of stem cell with its microenvironment
- Differentiation capacity of stem cells
The chosen labeling modality should not interact with the normal functions of the stem cell. Otherwise, one would not be able to accurately study the biology of these cells over time. In addition, issues such as biocompatibility, toxicity, and safety not only to the stem cell but most importantly to the individual should also be considered, and included in the decision of which modality to use.
All imaging modalities have a certain degree of background/non-specific signal (that may interfere with the signal under study). The preferred imaging modality should be one that provides a good contrast between background and the target signal under study, achieving a large signal-to-noise ratio. Furthermore, it should have good specificity (negative study in the absence of what is being studied, stem cells in this case). Only then will it be possible to use these modalities to study stem cell biology.
The main objective of regenerative medicine, and thus of stem cell imaging, is its clinical application. It is generally agreed upon that before its clinical use, therapeutic strategies should be tested in clinical models of disease. Thus, an ideal imaging modality should be flexible across different imaging modalities, both in terms of spatial resolution and system sensitivity (the lowest amount of activity or numbers of cells that can be detected by that specific modality).
Methodologies for labeling stem cells
Direct labeling
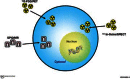
One of the most commonly used strategies for the labeling of stem cells for imaging in living subjects is that of direct labeling (Guzman et al., 2007; Thakur et al., 1976; Thakur et al., 1977). In a direct labeling strategy, labeling agents are introduced into the cells prior to transplantation (Figure 1), stem cells transplanted and then followed in the living subject (Figure 2). The strategy is then used to image the molecules previously introduced into the cell and use them as surrogate of the number of stem cells. Depending on the imaging modality to be used, cells can be labeled with quantum dots (Chakraborty et al., 2007; Lin et al., 2007; Rosen et al., 2007) or fluorophores (Askenasy et al., 2002; Keppler et al., 2006; Voura et al., 2004) for optical fluorescence imaging, superparamagnetic iron oxide particles (SPIO) for magnetic resonance imaging (MRI; Hill et al., 2003; Rogers et al., 2006), and radionuclides for single photon emission computed tomography (SPECT) or positron emission tomography (PET; Adonai et al., 2002; Bindslev et al., 2006; Kang et al., 2006; Rini et al., 2006).
Fluorescent semiconductor nanocrystals (known as quantum dots, QDs (Michalet et al., 2005) or different fluorophores (Kalchenko et al., 2006) have been used to monitor stem cells in the living subject. At each imaging time point, QDs and fluorophores are excited at predetermined wavelengths, and emit a fluorescent signal can be used for imaging of transplanted cells. This strategy has been used for tracking of stem cells in many organs (Kalchenko et al., 2006; Lin et al., 2007; Michalet et al., 2005; Seleverstov et al., 2006; Shah et al., 2007). However, fluorescence imaging has limited tissue penetration (around 2 mm) limiting the use of these techniques to superficial tissues in small animals (e.g., mice; Bengel et al., 2005; Massoud and Gambhir, 2003).
In this review, focus will be placed mainly on direct labeling modalities that have the potential to be applied clinically: MRI, SPECT, and PET.
Magnetic resonance imaging
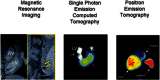
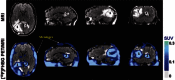
Magnetic resonance imaging has been commonly used for imaging of stem cells, mostly based on concept of the imaging of super paramagnetic iron oxide (SPIOs). SPIOs are highly magnetic particles that can elicit changes in T2 relaxivity (effect known as T2*; Arbab et al., 2003; Bos et al., 2004; Di Tucci et al., 2008; Frank et al., 2003; Himes et al., 2004; Ittrich et al., 2007), allowing their detection in vivo (see Figure 2 left). In a direct labeling strategy, SPIOs are incorporated into cells (using either electroporation or liposome-based incorporation techniques), cells then transplanted to the living subject, and subsequently MR imaging is performed (Bengel et al., 2005; Kraitchman et al., 2003) using gradient recalled echo sequences (Chen et al., 2008). The signal originated from the SPIOs is used as a surrogate for number of cells. MRI offers the advantage of high spatial resolution, resulting in detailed organ morphologic and functional information, and thus appears as a good candidate for an integrated stem cell imaging-functional assessment imaging approach in organs like the heart (Dick et al., 2003; Kraitchman et al., 2003) or the brain (Guzman et al., 2007; Watson et al., 2006). MR has also been used to monitor the delivery process. Specifically, MR fluoroscopy allows real-time assessment of the delivery of stem cells to the myocardium (Dick et al., 2003). However, the sensitivity of SPIO-based labeling is in the micromolar range (10−5 mol/L; Bengel et al., 2005; Massoud and Gambhir, 2003) and may not be sensitive enough to detect low signal levels (more than 1×105 cells are needed; Kraitchman et al., 2005). Sensitivity of the system can be increased using high-field magnets (e.g., 11 Tesla). However, for now these magnets are limited to use in small animal preclinical models. SPIO-based imaging constitutes a very good imaging strategy for initial localization of cells after transplantation, and for the co-registration of cell transplantation with areas of functional loss. After myocardial infarction, MR imaging is used to identify areas of infarction (using delayed enhancement MR techniques), and SPIO-MRI can be used to label stem cells for their localization immediately after delivery (Kraitchman et al., 2003; Figure 2 left). However, SPIO-based imaging is not well suited for long-term monitoring of stem cells (Bengel et al., 2005; Chen et al., 2008; Li et al., 2008). Several reports suggested that SPIOs may not stay in the transplanted cells over time (Li et al., 2008), but rather be incorporated by macrophages and other cell types, and some iron may remain in the interstitial space. Because the effect of iron particles on the magnetic field continues (regardless of the location and status of the transplanted cells), there is an uncoupling between the MR signal and the viability of stem cells (Li et al., 2008). This effect may not be critical for the initial localization of transplanted cells, but will preclude the use of this strategy for the monitoring of transplanted stem cells over time.
Radionuclide imaging
Radionuclide labeling of cells has also been used for cell imaging, using a strategy similar to SPIO-based techniques, which is to introduce a labeling agent to the cell prior to transplantation (see Figure 1). Radionuclides used for this purpose can have different physical half lives (e.g., 99mTc: 6 hours, 111In: 2.8 days, 18F: 109 minutes, 64Cu: 12 hours), that will determine the amount of time that cells can be monitored non-invasively after cell labeling. For example, 111In-labeled cells have been used for many years to track the homing of inflammatory cells to localize inflammatory processes (Thakur et al., 1977; Thakur et al., 1977). More recently, the methodology has been applied to the labeling of stem cells, using different isotopes (e.g., 111In for SPECT, 18F-Fluoro-Deoxyglucose (18F-FDG) for PET). Use of isotopes like 18F-FDG (physical half life = 109 minutes) may allow tracking of cells for a 6–8 hours (after correction for isotope physical decay) after transplantation (Kang et al., 2006; Figure 2 right), while use of 111In (see Figure 2 middle) may allow cell tracking longer periods of time (up to 14 days; Chin et al., 2003). In addition to its physical half-life, each radionuclide also has a biological half-life (e.g., radionuclides may go in and out of the cell), which should be taken into consideration when performing these studies. One of the major advantages of SPECT and PET imaging is their high sensitivity (nano- and femto-molar, respectively), which permits the detection of relatively low amounts of signal (Adonai et al., 2002; Wu et al., 2004). However, SPECT and PET have relatively low spatial resolution, compared to other modalities (such as MRI), which may be a relative disadvantage for signal localization. The recent development of integrated PET-Computed Tomography (CT) and SPECT-CT provides a better anatomical guide for the location of the detected signal.
Advantages and disadvantages of direct labeling. Role in the assessment of stem cell therapy
Use of SPIOs (at least at doses of 20 pg of SPIO/cell) may have an effect on the gross morphology and proliferation capacity of stem cells, which may preclude their widespread use as a labeling strategy (Bos et al., 2004; Chen et al., 2008). In the case of radionuclide labeling, cell toxicity will likely vary depending on the radionuclide and dosage used (Carr et al., 1995; Zanzonico et al., 2006).
In addition, both SPIOs and radionuclides have a biological half-life (e.g., may go in and out of the cell), which should be taken into consideration when performing these studies. Thus, when using this imaging strategy, physical as well biological properties of the labeling agent should be considered together with the properties of the cells, to accurately determine the appropriate cell imaging modality. In general, the shortest half-life (biological or physical) will determine for how long transplanted cells can be monitored.
One of the main limitations of any direct labeling strategy is that it does not account for cell viability/division (i.e., cell numbers increase after cell division, but the number of radioisotope molecules stays the same), which results in “dilution” of the signal over time. This aspect of direct labeling limits its use for long term monitoring.
In summary, direct labeling strategies (whether it is MRI, SPECT-CT or PET-CT) appear to be a good imaging strategy for detection of cells shortly after transplantation (e.g., to ensure that cells where delivered to the intended organ or region of an organ), providing a good signal-to-noise ratio, but less suited for long-term monitoring of stem cell viability.
Reporter gene imaging
There is increasing need to understand the biology of stem cells after they are transplanted to the living subject. To achieve that, one needs a strategy that can accurately monitor stem cell biology in a longitudinal manner, without interfering with the normal biology of the cells under study or of the transplant recipient. In order to accurately study the biology of stem cells, such a system should be based on the physiologic activity of transplanted cells. Advances in molecular biology and imaging modalities have resulted in the development of reporter gene strategies, a system that allows evaluation of trans-gene expression in many disease states (see Figure 3; Contag et al., 2000; Gambhir et al., 1999; Herschman, 2004; Inubushi and Tamaki, 2007; Kang and Chung, 2008; Massoud and Gambhir, 2003; Phelps, 2000; Wu et al., 2004; Zhang and Wu, 2007). Reporter genes consist of gene regulatory elements (promoters and enhancers) that drive the reporter gene DNA sequence, and a polyA sequence (which provides stabilization to the final product). Initially, reporter genes were mainly used in histology and ex-vivo studies (beta-galactosidase and luciferase) to assess the trans-gene expression in many different tissues and organs (Forss-Petter et al., 1990; Himes and Shannon, 2000; Naciff et al., 1999). Subsequently, reporter genes were used for imaging in vivo, using green fluorescent protein (GFP; Zhuo et al., 1997) or bioluminescence (Contag et al., 2000), providing one of the first evidences of the monitoring of trans-gene expression assessed in living subjects. For the application of stem cell monitoring, the reporter gene is incorporated into the cell before cell transplantation into the living subject. If the stem cells are viable (e.g., after transplantation), the reporter gene will be expressed and the protein (e.g., enzyme, cell surface receptor) will be encoded. On the other hand, if the reporter gene is not expressed due to cell death for example, no signal will be produced. At the specified imaging time point, an exogenously given substrate is administered. The interaction between the substrate and the encoded reporter protein (if present) will result in a signal, which can be detected non-invasively (using different imaging modalities, e.g. bioluminescence imaging, radionuclide imaging). The most common use of reporter genes is for the longitudinal study of stem cell viability. For this purpose, reporter genes are driven by a constitutive promoter (e.g., cytomegalovirus or CMV), which is always turned ON and, as long as the cell is viable and has the transcriptional machinery intact, will results in production of the reporter protein. However, the viral CMV promoter can undergo “gene silencing” (the gene is turned OFF) over time (Krishnan et al., 2006; a proposed mechanisms is linked to the large number of CpG repeats in the CMV promoter) that will result in reduced signal. From a practical standpoint, a decrease in the activity of the constitutive CMV promoter will result in decreased production of the reporter gene which could be mistaken for decreased stem cell survival. More recently, investigators have used promoters of constitutive mammalian cell proteins (e.g., β-actin, ubiquitin), which are proteins that are constantly produced in a cell. Most of these promoters undergo less gene silencing and stay “ON” as long as the cell is viable, and may represent better alternatives as the promoter of choice for assessment of stem cell viability. Furthermore, cells should be monitored for alterations in their phenotype and dividing capacity, although recent data suggest that the introduction of reporter genes do not seem to significantly alter the biological properties and differentiation capacity of stem cells (Cao et al., 2008; Wang et al., 2008; Wu et al., 2006).
Specific biological pathways can also be investigated using reporter genes strategies. To study a certain pathway, a specific promoter is used (e.g., a protein specific promoter), and only when the intracellular signal for the production of that protein is active, would the reporter protein be made. For example, if one wants to monitor when embryonic stem cells differentiate into myocytes, one can use a reporter gene that is driven by a specific myocyte promoter, which will only be turned “ON” when the stem cell under study has turned “ON” the signaling cascade to produce a mature cardiac protein (e.g. troponin, desmin). Then, the activation of that specific promoter will “drive” the expression of the reporter gene, and one could visualize it non-invasively using different imaging modalities.
Fluorescent reporter genes
Use of GFP constitutes one of the first examples of the application of reporter genes for in vivo imaging of trans-gene expression. Fluorescence reporter proteins are very sensitive and result in the emission of a strong signal. However, both light excitation and emission undergo significant tissue attenuation (due to absorption) and tissue refraction, which limits the tissues that can be studied (up to 2 mm in depth; Contag, 2007; Massoud and Gambhir, 2003; Shah et al., 2004). In view of this, the most used application of fluorescent reporter genes in stem cell imaging is for ex-vivo analysis, where they can be used for cell sorting. Along these lines, the Gambhir laboratory and other groups have created a triple fusion reporter gene that has a fluorescent reporter gene, a bioluminescent reporter gene, and a PET reporter gene and that results in a fusion protein (Kesarwala et al., 2006; Ray et al., 2007). Cao et al. have transduced mouse embryonic stem cells (Cao et al., 2007; Cao et al., 2006), using the fluorescent protein (red fluorescent protein, mRFP) to identify the cells that have been effectively transduced with the fusion protein (using fluorescent activated cell sorting), and then transplanted those cells to the myocardium (Cao et al., 2007; Cao et al., 2006). Subsequently, the bioluminescent (firefly luciferase, Fluc) and PET reporter genes (herpes simplex virus enzyme thymidine kinase, HSV1-tk) were used for long-term monitoring of cell viability after transplantation (see next sections for details). Another common application of fluorescent reporter genes is for use in histology (immunofluorescence) for post-mortem confirmation of imaging results (Li et al., 2007). Using a similar approach to the one previously described (bi-or tri-fusion reporter genes), one can “mark” cells with a fluorescent reporter gene, and then when the tissue is excised, the “marked” cells can be easily identified using histological methods.
Bioluminescent reporter genes
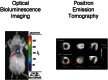
Reporter gene-bioluminescence imaging (BLI) is based on light emission and detection by specific cooled charge coupled device (CCD) cameras (Contag et al., 2000). Similar to other reporter gene strategies, the BLI signal is only emitted when cells are viable, and thus can be used for the longitudinal monitoring of stem cell survival and study of cell status (see Figure 4 left). BLI is commonly used for the tracking of stem cells delivered to organs within small living animals (Chen et al., 2009; Rodriguez-Porcel et al., 2005; Wu et al., 2003). Fluc and Renilla luciferase (Rluc) are the two most common reporter genes used for BLI. BLI-Fluc imaging (see Figure 4 left) is based on the oxidation of the substrate D-luciferin by the FLuc enzyme, a reaction that requires oxygen, magnesium, and ATP, and results in a red-shifted light emission (wavelength: 500–700nm). Imaging in the red-shifted light spectrum results in higher signal-to-background ratio, and thus makes it more “attractive” as a reporter gene for imaging in living subjects. On the other hand, Rluc does not require other cofactors and result in a lower wavelength emission (wavelength: 450– 550 nm), resulting in a lower signal-to-background ratio that makes it more challenging for imaging in the living subject. More recently, the Gambhir laboratory has developed red-shifted Rluc variants that have increased light output which can make Rluc a more attractive option for in vivo animal imaging (Loening et al., 2006; Loening et al., 2007).
Using a reporter gene strategy, BLI has been successfully used for in vivo study of cell delivery and monitoring of stem cell viability in small living animals (see Figure 5). BLI has been used for tracking and monitoring of different types of cells, such as neural cells (Okada et al., 2005), cardiomyoblasts (Chen et al., 2008; Rodriguez-Porcel et al., 2005; Wu et al., 2003) and embryonic stem cells (Cao et al., 2007; Cao et al., 2006). For the delivery of stem cells to the myocardium, investigators have used ultrasound guidance. This strategy (ultrasound guidance for the delivery of stem cells) allows the precise delivery of stem cells to the affected part of the organ (myocardium in this case; Rodriguez-Porcel et al., 2005; Springer et al., 2005). Furthermore, in these studies ultrasound guided delivery is complemented with non-invasive assessment of cardiac function (as it routinely done in the clinic). BLI has also been used to evaluate the response of stem cells transplanted to organs like the pancreas (Roth et al., 2006). Furthermore, using reporter genes, Kutscha et al have shown that modulation of the cellular and local microenvironment resulted in prolonged stem cell survival after transplantation to the myocardium (Kutschka et al., 2006), providing evidence that reporter gene imaging can be used to monitor changes in the biology of transplanted cells.
As with other imaging modalities, BLI has some drawbacks and unsolved issues. Currently, BLI is mainly a planar imaging modality, providing limited depth information and signal localization within the living subject. Also, because of the lack of tomographic information, while BLI can provide information on the trafficking of stem cells to different organs, it may not be an ideal modality to accurately assess changes in stem cell viability as cells traffic between organs. This is because changes in the depth of the originated signal may be confused with changes in cell survival. Significant efforts are being devoted to the development of tomographic BLI either by rotating the subject under study, by detecting light using 2 or more cameras, or using spectral imaging (Lv et al., 2007; Soloviev, 2007). However, these systems are still under development. Due to the limited tissue depth that can be assessed with BLI, it is a technique that is mainly restricted to small animals (rats and mice), and very superficial tissues in larger living subjects (e.g., skin, in an intraoperative setting). In the future, these strategies may also be applied in specific clinical scenarios (e.g., skin, superficial tissues). Because BLI uses imaging strategies (reporter genes) similar to clinical modalities (e.g., PET and SPECT), BLI is routinely used as a starting step in the development of novel imaging strategies, and once the efficacy of these strategies is proven they can be adapted for use in the clinic.
In summary, BLI is a very useful imaging modality for the monitoring of stem cell trafficking and survival in small animals and will play a critical role in the study of cell biology and its interaction with their microenvironment.
Positron emission tomography/single photon emission computed tomography reporter genes
Both SPECT and PET reporter gene imaging are based on the interaction between an exogenously administered probe (that contains the tracer) and the reporter gene product (e.g., enzyme, receptor), what results in the retention of the radionuclide substrate that can then be imaged non-invasively (Bengel et al., 2005; Gambhir et al., 1998; Herschman, 2004; Tjuvajev et al., 1998; Wu et al., 2004). One of the main advantages of PET and SPECT imaging is that it can provide tomographic, quantitative, and volumetric information, allowing one to better localize and quantify the detected signal within the subject under study (see Figure 4 right). In addition, the sensitivity of PET is in the femtomolar range (10−12 mol/L), higher than MR imaging (10−5 mol/L), but not as sensitive as optical imaging (at limited depths, 10−15 mol/L). There are predominantly three reporter gene systems (for PET or SPECT) that have been used for cell imaging. The system used the most is based on the production of an intracellular enzyme (e.g., HSV1-tk), that phosphorylates an exogenously administered substrate which is retained in the cell due to its negative charge. While normal cells (without the HSV1-tk) do carry the enzyme mammalian wild type thymidine kinase, it only minimally phosphorylates the radionuclide probes used in this system. On the other hand, in cells carrying the HSV1-tk, the exogenously administered probe undergoes significant phosphorylation and intracellular retention, leading to a robust signal-to-background ratio, enabling accurate monitoring of these cells. Furthermore, this strategy is very powerful because the enzyme can phosphorylate many molecules of the radionuclide substrate, increasing the signal retained in the cells of interest and improving the signal-to-background ratio. However, the probe has to cross the cell membrane, which may limit the interaction between substrate and enzyme, resulting in reduced signal. In addition, because it is a non-mammalian protein (of viral origin) it has the potential to trigger an immunological response, resulting in a reaction in the organism with decreased overall signal. Attempts to circumvent this problem have included use of a mammalian protein (dopamine receptor, see later in this paragraph) or a mammalian mitochondrial tk (Ponomarev et al., 2007), or a destabilized HSV1-tk (Hsieh et al., 2008). A second reporter gene imaging strategy is based on the imaging of dopamine receptor using PET (D2R, MacLaren et al., 1999). In this case, the reporter gene encodes for a cell membrane protein, which binds to an exogenously given probe, and the “bound probe” is then imaged non-invasively with PET. It is important to mention that the wild type D2R has the potential to elicit a downstream biological response, which can be prevented if one uses a mutant version of the dopamine receptor (Chen et al., 2004; Liang et al., 2001). Importantly, the mutant version of the D2R (Mutation of Asp80 or Ser194) maintains the affinity for the PET probe (3-(2’-[18F]-fluoroethyl)-spiperone) used. This strategy can be advantageous as the probe does not have to cross the cell membrane in order to interact with the reporter protein. However, this approach is limited by the amount of signal that can be produced, as one receptor interacts with only one molecule of the ligand. One of the most common strategies is the use of a PET-based reporter gene strategy for the visualization of transplanted cells to the myocardium and showed that it can be used to monitor the fate of stem cells after transplantation to the living subject (Li et al., 2007; Wu et al., 2003). Similarly, we have shown that transplantation of pancreatic islet cells can be followed non-invasively (Kim et al., 2006; Lu et al., 2006; Lu et al., 2006). A third approach consists on the encoding of the sodium-iodide symporter (NIS; Kang et al., 2005; Kim et al., 2005; Miyagawa et al., 2005; Terrovitis et al., 2008), which is a thyroid transmembrane protein, under physiological conditions transport iodine into the cells, in exchange for sodium. Successful sequencing and cloning of the NIS gene sequence allowed its use as a reporter gene in the thyroid and other organs. This system has been used not only for PET (with 124I as the tracer), but also for SPECT imaging (using 123I or 99Tc-perthechnetate as tracer). The somatostatin receptor reporter gene (Zinn and Chaudhuri, 2002) can also be evaluated with both SPECT and PET as imaging modalities, providing flexibility on the assessment of trans-gene expression (Schillaci, 2007; Sharma et al., 2002; Zhernosekov et al., 2005). Other less frequent reporter genes include the neurotensin receptor subtypes (Alvarez-Maya et al., 2001; Tavares et al., 1999), and cytosine deaminase (Kreuzer et al., 1996; Lee et al., 2007). Details of the use of these reporter genes are beyond the scope of this review and can be found elsewhere (Sharma et al., 2002).
The major advantage of PET and SPECT (when compared to BLI) resides in their potential for clinical use. Both PET and SPECT radionuclides are of relatively high energy (PET: 511KeV, SPECT: 80–250 KeV) and do not undergo significant tissue attenuation. While SPECT (due to its relatively low energy) does have some tissue attenuation, it does not preclude its use in patients and proof of that is its extensive clinical use.
These imaging modalities present a number of issues that need to be considered from the operational standpoint. On one side, PET has significant flexibility for the production of specific probes for the detection of different processes in the living subject (almost any compound can be labeled with a radionuclide), which is a significant advantage as it allows the researcher to first identify the molecule that needs to be studied, and then design a specific probe that will target that molecule. However, the production of PET probes is complex, needs advanced chemistry and very tight quality control. In addition, depending on the half-life of the radioisotope used, it requires an on-site (or at least near-by) cyclotron, that limits this strategy to medium to large research centers. From the imaging standpoint, all electron-positron annihilations (whether is from 18F, 64Cu or 11C) result in the production of photons of 511KeV, and as such we can not detect differences in registered signals. SPECT, on the other hand, can detect simultaneous signals of different energies (by varying the detection windows, as it is routinely done with the perfusion agents 201Tl and 99Tc). At the same time, tracer labeling is less complex (compared to PET) and, for the most part, can be done in a radionuclide pharmacy. However, the spatial resolution of SPECT is less than that of PET, and this variable may be of importance when we try to spatially localize relatively low number of cells. From the tracer perspective, SPECT labeling can be somewhat limited by its chemistry, with the result that not every compound can be easily labeled, thus providing less labeling flexibility compared to PET. In practical terms, if the compound of interest can be labeled with SPECT and answers the research questions posed by the investigator, its production and availability is more accessible to many academic and research centers.
Magnetic Resonance reporter genes
Over the last few years, significant efforts have been devoted to combined these modalities (MRI and reporter gene technology) and develop MR reporter genes (Cohen et al., 2005; Gilad et al., 2007).
MRI-based reporter genes efforts are based on the production of proteins, mostly intracellular metalloproteins (transferring, ferritin, tyrosinase; Gilad et al., 2007). As previously described, iron is a paramagnetic substance that induces changes in relaxivity (i.e., T2* effect) that can be detected using specific imaging sequences. Physiologically, iron enters cells through the transferrin receptor (TfR) that binds the transferrin protein containing two iron atoms and internalizes iron molecules. So the goal of this strategy is to express transferring, that will accumulate large quantities of iron intracellularly for non-invasive detection.
A reporter gene strategy targeted to express Ferritin has also been used for MR-based detection. Ferritin is a metalloprotein that functions as the body's iron depot and can contain up to 4000 iron atoms. Native ferritin is in essence an anti-magnetic particle, but several orders of magnitude weaker than SPIOs. Considerable efforts are being devoted to improve its relaxivity by removing its native core (oxyhydroxide) and reconstituting the protein shell with a superparamagnetic core.
Tyrosinase has also been used as a MR reporter gene (Weissleder et al., 1997). Briefly, tyrosinase participates in the production of melanin, and melanin has high affinity for iron, leading to increased relaxivity. Tyrosinase has been transfected to fibroblasts and embryomal kidney cells as well as breast cancer cells which resulted in increased signals. However, there is concern regarding potential toxicity of iron. Melanin production produces reactive oxygen species (an important and deleterious component of the oxidative stress cascade) and thus can exhibit significant toxic effects.
There are a few drawbacks in the use of metalloproteins as MR reporter genes that also deserve consideration (Gilad et al., 2007). First, the signal is dependent on the accumulation of iron inside the cells and for how long iron particles can be retained inside cells, what may result in toxicity to the cell. Second, when cells divide the signal gets diluted and the “clock” starts again, as cells need to start again to accumulate enough iron for MRI detection, making it difficult to interpret correlation between detected signal and viability of transplanted cells. From the imaging standpoint, the relaxation is dynamic and dependent on the iron loading conditions. R2 relaxivity is high at low iron doses, it decreases at intermediate iron loading conditions and when iron conditions are high, T2 relaxation remains constant, which may preclude accurate quantification of the obtained signal. As previously described for direct cell labeling, MR signal works on the basis of accumulation of iron. Similar to what happens with SPIOs, when cells die the accumulated iron continues to be present inside the cells for some time (until cells are dissolved or phagocytosed by macrophages). The remaining iron continues to induce a change in MR relaxivity (even if cells are not alive), and thus the signal is not representative nor linear of cell viability (Li et al., 2008).
How to decide which modality to use
The ideal imaging modality is one that has excellent spatial resolution and cell detection sensitivity, can guide the delivery of cells, and can serially follow stem cells and their fate. Currently, no such imaging modality exists. Each imaging modality should be chosen depending on the question that is being asked (see Figure 6, Table 1). If the objective of the study is to image the delivery and short-term homing of stem cells in different organs, a direct labeling approach may answer the posed question, taking into consideration the potential toxicity that they may have. Magnetic resonance imaging provides the highest spatial resolution and almost real-time image-guidance for cell delivery, albeit with significantly lower molecular sensitivity compared to other modalities like PET, SPECT or optical imaging. Radionuclide imaging modalities (PET, SPECT) have been successfully and extensively used, although depending on the application they may not provide sufficient spatial resolution. Another research objective may be the long-term monitoring of stem cells viability. The drawbacks of a direct labeling strategy for stem cell monitoring have been previously described. On the other hand, reporter gene imaging appears more suited for the long-term monitoring of stem cells, and can be achieved using optical imaging (bioluminescence, fluorescence) or PET/SPECT imaging. While optical imaging is more molecularly sensitive, it provides lesser anatomical localization, and is limited mainly to small animals. On the other hand, PET or SPECT provide good sensitivity with the major advantage of good anatomical localization and potential translation to human applications.
Similarly, when the goal is to study the biology of these cells and whether they do or do not express certain gene(s) or do or do not come in contact with the environment, the imaging strategy to be used has to be one that will only emit signal or even ceases to, when the biological action being studied is taking place (e.g., differentiation of a stem cell into an adult cell) and a specific pathways is being activated. At the present time, report gene imaging appears to provide the best tool to address these questions. For example, if one wants to study whether a stem cell has differentiated into an adult myocyte, use of a reporter gene that is driven by a promoter that will only be activated when the cell has the features of an adult myocyte (i.e., expresses the sarcomeric protein Troponin T) can provide that information. Again, the imaging modality to be used (PET vs SPECT vs. optical) should be decided based on the variables previously mentioned.
Translation to clinical applications
Over the last few years, the majority of our understanding on how stem cells behave in the living subject. However, before these therapies are routinely applied clinically, there are a number of questions that need to be answered, such as dose, timing of delivery, homing, etc. For most of these answers to truly address the clinical dilemma, they need to be answered either in a large animal model or directly in the patient. The ability to directly monitor and assess cell based therapies in patients will be invaluable as it will allow us for the first time to investigate these therapies directly in the living subject to which they where were intended to. In many pathophysiological states, large animal models have been shown to be similar to human in what respects to disease progression (Bloor et al., 1992). These similarities have led researchers to use large animal models for diagnosis of disease as well as evaluation of different therapies.
In addition, many large animals have comparable weight, size, and anatomy to humans. These similarities also allow a better optimization of the different imaging strategies prior to clinical applications. In diseases where the use of large animal models is not relevant or feasible, the translation from small animal models to humans may be more difficult.
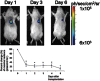
Recently, a number of research groups have used the swine model for imaging of reporter genes in the myocardium (Bengel et al., 2003; Miyagawa et al., 2004; Rodriguez-Porcel et al., 2008), showing the feasibility of applying these imaging strategies for gene monitoring in large animals. A similar strategy has been used for the imaging of stem cells (previously labeled to carry the reporter gene) in a large animal model (Willmann et al., 2009) and humans (see Figure 7; Yaghoubi et al., 2009). however, it is important to realize that when cells are exogenously delivered to a host, a myriad of mechanisms may play a role in the survival of the transplanted stem cells: a relatively hypoxic scenario, lack of constant and efficient cell-cell contact with the microenvironment, and activated immune response. In addition to the biological variables already mentioned, imaging of large animals (similar to clinical imaging) has a few technical aspects that need to be kept in mind. On one side, the sensitivity of clinical systems is lower than that of dedicated small animal imaging systems, which make imaging and signal quantitation more challenging. In addition and depending on the imaging modality used, the amount of the reporter gene/cell system to be delivered may need to be adjusted depending on the weight and other characteristic of the subject under study.
Summary
Over the last decade, we have seen a revolution in non-invasive stem cell imaging in the living subject. In this review, we have outlined some of the most important characteristics of direct- and indirect- cell labeling focusing on reporter gene technology, which will likely be the preferred methodology for long-term monitoring of stem cell biology. It is likely that not one technique will answer all the questions, but use of a multimodality approach will be the more appropriate approach to address the myriad of aspects posed in this exciting and rapidly evolving field.
Acknowledgements
NIH ICMIC P50 CA114747 and Doris Duke Foundation (SSG), and NIH HL88048 (MR-P)
References
- Adonai N, Nguyen K.N, Walsh J, Iyer M, Toyokuni T, Phelps M.E, McCarthy T, McCarthy D.W, Gambhir S.S. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci USA. 2002;99:3030–3035. [PMC free article: PMC122467] [PubMed: 11867752] [CrossRef]
- Alvarez-Maya I, Navarro-Quiroga I, Meraz-Rios M.A, Aceves J, Martinez-Fong D. In vivo gene transfer to dopamine neurons of rat substantia nigra via the high-affinity neurotensin receptor. Mol Med. 2001;7:186–192. [PMC free article: PMC1950024] [PubMed: 11471555]
- Anversa P, Leri A, Kajstura J. Cardiac regeneration. J Am Coll Cardiol. 2006;47:1769–1776. [PubMed: 16682300] [CrossRef]
- Arbab A.S, Bashaw L.A, Miller B.R, Jordan E.K, Bulte J.W, Frank J.A. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76:1123–1130. [PubMed: 14557764] [CrossRef]
- Askenasy N, Zorina T, Farkas D.L, Shalit I. Transplanted hematopoietic cells seed in clusters in recipient bone marrow in vivo. Stem Cells. 2002;20:301–310. [PubMed: 12110699] [CrossRef]
- Baizabal J.M, Furlan-Magaril M, Santa-Olalla J, Covarrubias L. Neural stem cells in development and regenerative medicine. Arch Med Res. 2003;34:572–588. [PubMed: 14734098] [CrossRef]
- Bengel F.M, Anton M, Richter T, Simoes M.V, Haubner R, Henke J, Erhardt W, Reder S, Lehner T, Brandau W, et al. Noninvasive imaging of transgene expression by use of positron emission tomography in a pig model of myocardial gene transfer. Circulation. 2003;108:2127–2133. [PubMed: 14530205] [CrossRef]
- Bengel F.M, Schachinger V, Dimmeler S. Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging. 2005;32(Suppl 2):S404–416. [PubMed: 16205898] [CrossRef]
- Bindslev L, Haack-Sorensen M, Bisgaard K, Kragh L, Mortensen S, Hesse B, Kjaer A, Kastrup J. Labelling of human mesenchymal stem cells with indium-111 for SPECT imaging: effect on cell proliferation and differentiation. Eur J Nucl Med Mol Imaging. 2006;33:1171–1177. [PubMed: 16763813] [CrossRef]
- Bloor C.M, White F.C, Roth D.M. The pig as a model of myocardial ischemia and gradual coronary artery occlusion. In Swine as models in biomedical research. In: Swindle M. M, Moody D. C, Phillips L. D, editors. Ames, Iowa: Iowa State University Press; 1992. pp. 163–175.
- Bos C, Delmas Y, Desmouliere A, Solanilla A, Hauger O, Grosset C, Dubus I, Ivanovic Z, Rosenbaum J, Charbord P, et al. In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology. 2004;233:781–789. [PubMed: 15486216] [CrossRef]
- Cao F, Drukker M, Lin S, Sheikh A.Y, Xie X, Li Z, Connolly A.J, Weissman I.L, Wu J.C. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9:107–117. [PubMed: 17386018] [CrossRef]
- Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla S.J, Connolly A.J, Chen X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. [PMC free article: PMC4701384] [PubMed: 16476845] [CrossRef]
- Cao F, Wagner R.A, Wilson K.D, Xie X, Fu J.D, Drukker M, Lee A, Li R.A, Gambhir S.S, Weissman I.L, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:e3474. [PMC free article: PMC2565131] [PubMed: 18941512] [CrossRef]
- Carr H.M, Smyth J.V, Rooney O.B, Dodd P.D, Sharma H, Walker M.G. Limitations of in-vitro labeling of endothelial cells with indium-111 oxine. Cell Transplant. 1995;4:291–296. [PubMed: 7640868] [CrossRef]
- Chakraborty S.K, Fitzpatrick J.A, Phillippi J.A, Andreko S, Waggoner A.S, Bruchez M.P, Ballou B. Cholera toxin B conjugated quantum dots for live cell labeling. Nano Lett. 2007;7:2618–2626. [PubMed: 17663586] [CrossRef]
- Chen I.Y, Greve J.M, Gheysens O, Willmann J.K, Rodriguez-Porcel M, Chu P, Sheikh A, Faranesh T, Paulmurugan R, Yang P.C, Wu J.C, Gambhir S.S. Comparison of Optical Bioluminescence Reporter Gene and Superparamagnetic Iron Oxide MR Contrast Agent as Cell Markers for Non-invasive Imaging of Cardiac Cell Transplantation. Mol Imaging Biol May-Jun; 2009;11(3):178–87. [PMC free article: PMC4155941] [PubMed: 19034584] [CrossRef]
- Chen I.Y, Wu J.C, Min J.J, Sundaresan G, Lewis X, Liang Q, Herschman H.R, Gambhir S.S. Micro-positron emission tomography imaging of cardiac gene expression in rats using bicistronic adenoviral vector-mediated gene delivery. Circulation. 2004;109:1415–1420. [PMC free article: PMC4154818] [PubMed: 15007006] [CrossRef]
- Chin B.B, Nakamoto Y, Bulte J.W, Pittenger M.F, Wahl R, Kraitchman D.L. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun. 2003;24:1149–1154. [PubMed: 14569169] [CrossRef]
- Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7:109–117. [PMC free article: PMC1501126] [PubMed: 15802016] [CrossRef]
- Contag C.H. In vivo pathology: seeing with molecular specificity and cellular resolution in the living body. Annu Rev Pathol. 2007;2:277–305. [PubMed: 18039101] [CrossRef]
- Contag C.H, Jenkins D, Contag P.R, Negrin R.S. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia. 2000;2:41–52. [PMC free article: PMC1550286] [PubMed: 10933067] [CrossRef]
- De Bari C, Dell’Accio F, Tylzanowski P, Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. [PubMed: 11508446]
- Di Tucci A.A, Matta G, Deplano S, Gabbas A, Depau C, Derudas D, Caocci G, Agus A, Angelucci E. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica. 2008;93:1385–1388. [PubMed: 18603557] [CrossRef]
- Dick A.J, Guttman M.A, Raman V.K, Peters D.C, Pessanha B.S, Hill J.M, Smith S, Scott G, McVeigh E.R, Lederman R.J. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation. 2003;108:2899–2904. [PMC free article: PMC1325104] [PubMed: 14656911] [CrossRef]
- Forss-Petter S, Danielson P.E, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe J.G. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990;5:187–197. [PubMed: 2116814] [CrossRef]
- Frank J.A, Miller B.R, Arbab A.S, Zywicke H.A, Jordan E.K, Lewis B.K, Bryant L.H Jr., Bulte J.W. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. [PubMed: 12819345] [CrossRef]
- Gambhir S.S, Barrio J.R, Phelps M.E, Iyer M, Namavari M, Satyamurthy N, Wu L, Green L.A, Bauer E, MacLaren D.C, et al. Imaging adenoviral-directed reporter gene expression in living animals with positron emission tomography. Proc Natl Acad Sci USA. 1999;96:2333–2338. [PMC free article: PMC26784] [PubMed: 10051642] [CrossRef]
- Gambhir S.S, Barrio J.R, Wu L, Iyer M, Namavari M, Satyamurthy N, Bauer E, Parrish C, MacLaren D.C, Borghei A.R, et al. Imaging of adenoviral-directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998;39:2003–2011. [PubMed: 9829598]
- Genre D, Viens P, Bertucci F, Chabannon C, Gravis G, Braud A.C, Camerlo J, Houvenaeghel G, Moutardier V, Goncalvez A, et al. Modulations of dose intensity of doxorubicin and cyclophosphamide in association with G-CSF and peripheral blood stem cells in adjuvant chemotherapy for breast cancer: comparative evaluation of completion and safety of three intensive regimens. Bone Marrow Transplant. 2002;29:881–886. [PubMed: 12080351] [CrossRef]
- Gilad A.A, Winnard P.T Jr., van Zijl P.C, Bulte J.W. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–290. [PubMed: 17451181] [CrossRef]
- Gimble J.M, Katz A.J, Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. [PMC free article: PMC5679280] [PubMed: 17495232] [CrossRef]
- Goldberg J.L, Laughlin M.J, Pompili V.J. Umbilical cord blood stem cells: implications for cardiovascular regenerative medicine. J Mol Cell Cardiol. 2007;42:912–920. [PubMed: 17368666] [CrossRef]
- Guzman R, Uchida N, Bliss T.M, He D, Christopherson K.K, Stellwagen D, Capela A, Greve J, Malenka R.C, Moseley M.E, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci USA. 2007;104:10211–10216. [PMC free article: PMC1891235] [PubMed: 17553967] [CrossRef]
- Herschman H.R. Noninvasive imaging of reporter gene expression in living subjects. Adv Cancer Res. 2004;92:29–80. [PubMed: 15530556] [CrossRef]
- Herschman H.R. PET reporter genes for noninvasive imaging of gene therapy, cell tracking and transgenic analysis. Crit Rev Oncol Hematol. 2004;51:191–204. [PubMed: 15331078] [CrossRef]
- Hill J.M, Dick A.J, Raman V.K, Thompson R.B, Yu Z.X, Hinds K.A, Pessanha B.S, Guttman M.A, Varney T.R, Martin B.J, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. [PMC free article: PMC1490325] [PubMed: 12912822] [CrossRef]
- Himes N, Min J.Y, Lee R, Brown C, Shea J, Huang X, Xiao Y.F, Morgan J.P, Burstein D, Oettgen P. In vivo MRI of embryonic stem cells in a mouse model of myocardial infarction. Magn Reson Med. 2004;52:1214–1219. [PubMed: 15508153] [CrossRef]
- Himes S.R, Shannon M.F. Assays for transcriptional activity based on the luciferase reporter gene. Methods Mol Biol. 2000;130:165–174. [PubMed: 10589430]
- Hsieh C.H, Chen F.D, Wang H.E, Hwang J.J, Chang C.W, Lee Y.J, Gelovani J.G, Liu R.S. Generation of destabilized herpes simplex virus type 1 thymidine kinase as transcription reporter for PET reporter systems in molecular genetic imaging. J Nucl Med. 2008;49:142–150. [PubMed: 18077523] [CrossRef]
- Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo D.W. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. [PubMed: 15723985] [CrossRef]
- Inubushi M, Tamaki N. Radionuclide reporter gene imaging for cardiac gene therapy. Eur J Nucl Med Mol Imaging. 2007;34(Suppl 1):S27–33. [PubMed: 17464505] [CrossRef]
- Ittrich H, Lange C, Togel F, Zander A.R, Dahnke H, Westenfelder C, Adam G, Nolte-Ernsting C. In vivo magnetic resonance imaging of iron oxide-labeled, arterially-injected mesenchymal stem cells in kidneys of rats with acute ischemic kidney injury: detection and monitoring at 3T. J Magn Reson Imaging. 2007;25:1179–1191. [PubMed: 17520738] [CrossRef]
- Kalchenko V, Shivtiel S, Malina V, Lapid K, Haramati S, Lapidot T, Brill A, Harmelin A. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J Biomed Opt. 2006;11:050507. [PubMed: 17092148] [CrossRef]
- Kang J.H, Chung J.K. Molecular-genetic imaging based on reporter gene expression. J Nucl Med. 2008;49(Suppl 2):164S–179S. [PubMed: 18523072] [CrossRef]
- Kang J.H, Lee D.S, Paeng J.C, Lee J.S, Kim Y.H, Lee Y.J, Hwang D.W, Jeong J.M, Lim S.M, Chung J.K, Lee M.C. Development of a sodium/iodide symporter (NIS)-transgenic mouse for imaging of cardiomyocyte-specific reporter gene expression. J Nucl Med. 2005;46:479–483. [PubMed: 15750162]
- Kang W.J, Kang H.J, Kim H.S, Chung J.K, Lee M.C, Lee D.S. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295–1301. [PubMed: 16883008]
- Keppler A, Arrivoli C, Sironi L, Ellenberg J. Fluorophores for live cell imaging of AGT fusion proteins across the visible spectrum. Biotechniques. 2006;41172:167–170. 174–165. [PubMed: 16925018] [CrossRef]
- Kesarwala A.H, Prior J.L, Sun J, Harpstrite S.E, Sharma V, Piwnica-Worms D. Second-generation triple reporter for bioluminescence, micro-positron emission tomography, and fluorescence imaging. Mol Imaging. 2006;5:465–474. [PubMed: 17150159]
- Kim S.J, Doudet D.J, Studenov A.R, Nian C, Ruth T.J, Gambhir S.S, McIntosh C.H. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med. 2006;12:1423–1428. [PubMed: 17143277] [CrossRef]
- Kim Y.H, Lee D.S, Kang J.H, Lee Y.J, Chung J.K, Roh J.K, Kim S.U, Lee M.C. Reversing the silencing of reporter sodium/iodide symporter transgene for stem cell tracking. J Nucl Med. 2005;46:305–311. [PubMed: 15695791]
- Kraitchman D.L, Heldman A.W, Atalar E, Amado L.C, Martin B.J, Pittenger M.F, Hare J.M, Bulte J.W. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. [PubMed: 12732608] [CrossRef]
- Kraitchman D.L, Tatsumi M, Gilson W.D, Ishimori T, Kedziorek D, Walczak P, Segars W.P, Chen H.H, Fritzges D, Izbudak I, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. [PMC free article: PMC1456731] [PubMed: 16129797] [CrossRef]
- Kreuzer J, Denger S, Reifers F, Beisel C, Haack K, Gebert J, Kubler W. Adenovirus-assisted lipofection: efficient in vitro gene transfer of luciferase and cytosine deaminase to human smooth muscle cells. Atherosclerosis. 1996;124:49–60. [PubMed: 8800493] [CrossRef]
- Krishnan M, Park J.M, Cao F, Wang D, Paulmurugan R, Tseng J.R, Gonzalgo M.L, Gambhir S.S, Wu J.C. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. Faseb J. 2006;20:106–108. [PMC free article: PMC3625424] [PubMed: 16246867]
- Kutschka I, Chen I.Y, Kofidis T, Arai T, von Degenfeld G, Sheikh A.Y, Hendry S.L, Pearl J, Hoyt G, Sista R, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–173. [PubMed: 16820568]
- Kutschka I, Kofidis T, Chen I.Y, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl D. R, Hendry S. L, Sheikh A. Y, et al. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114:I174–180. [PubMed: 16820569]
- Lechner A. Stem cells and regenerative medicine for the treatment of type 1 diabetes: the challenges lying ahead. Pediatr Diabetes. 2004;5(Suppl 2):88–93. [PubMed: 15601379] [CrossRef]
- Lee C.H, Wu C.L, Shiau A.L. Hypoxia-induced cytosine deaminase gene expression for cancer therapy. Hum Gene Ther. 2007;18:27–38. [PubMed: 17184154] [CrossRef]
- Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly A.J, Yang P.C, Wu J. C. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. [PMC free article: PMC3638035] [PubMed: 18218820] [CrossRef]
- Li Z, Wu J.C, Sheikh A.Y, Kraft D, Cao F, Xie X, Patel M, Gambhir S.S, Robbins R.C, Cooke J.P, Wu J.C. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. [PMC free article: PMC3657509] [PubMed: 17846325]
- Liang Q, Satyamurthy N, Barrio J.R, Toyokuni T, Phelps M.P, Gambhir S.S, Herschman H.R. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 2001;8:1490–1498. [PubMed: 11593362] [CrossRef]
- Lin S, Xie X, Patel M.R, Yang Y.H, Li Z, Cao F, Gheysens O, Zhang Y, Gambhir S.S, Rao J.H, Wu J.C. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007;7:67. [PMC free article: PMC2174930] [PubMed: 17925032] [CrossRef]
- Liu Y, Shu X.Z, Prestwich G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405–3416. [PubMed: 17518677] [CrossRef]
- Loening A.M, Fenn T.D, Wu A.M, Gambhir S.S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel. 2006;19:391–400. [PubMed: 16857694] [CrossRef]
- Loening A.M, Wu A.M, Gambhir S.S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods. 2007;4:641–643. [PubMed: 17618292] [CrossRef]
- Lu Y, Dang H, Middleton B, Campbell-Thompson M, Atkinson M.A, Gambhir S.S, Tian J, Kaufman D.L. Long-term monitoring of transplanted islets using positron emission tomography. Mol Ther. 2006;14:851–856. [PubMed: 16982215] [CrossRef]
- Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Stout D.B, Campbell-Thompson M, Atkinson M.A, Phelps M, Gambhir S.S, et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proc Natl Acad Sci USA. 2006;103:11294–11299. [PMC free article: PMC1544080] [PubMed: 16868090] [CrossRef]
- Lv Y, Tian J, Cong W, Wang G, Yang W, Qin C, Xu M. Spectrally resolved bioluminescence tomography with adaptive finite element analysis: methodology and simulation. Phys Med Biol. 2007;52:4497–4512. [PubMed: 17634646] [CrossRef]
- MacLaren D.C, Gambhir S.S, Satyamurthy N, Barrio J.R, Sharfstein S, Toyokuni T, Wu L, Berk A.J, Cherry S.R, Phelps M.E, Herschman H.R. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther. 1999;6:785–791. [PubMed: 10505102] [CrossRef]
- Massoud T.F, Gambhir S.S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. [PubMed: 12629038] [CrossRef]
- Michalet X, Pinaud F.F, Bentolila L.A, Tsay J.M, Doose S, Li J.J, Sundaresan G, Wu A.M, Gambhir S.S, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. [PMC free article: PMC1201471] [PubMed: 15681376] [CrossRef]
- Miyagawa M, Anton M, Haubner R, Simoes M.V, Stadele C, Erhardt W, Reder S, Lehner T, Wagner B, Noll S, et al. PET of cardiac transgene expression: comparison of 2 approaches based on herpesviral thymidine kinase reporter gene. J Nucl Med. 2004;45:1917–1923. [PubMed: 15534063]
- Miyagawa M, Anton M, Wagner B, Haubner R, Souvatzoglou M, Gansbacher B, Schwaiger M, Bengel F.M. Non-invasive imaging of cardiac transgene expression with PET: comparison of the human sodium/iodide symporter gene and HSV1-tk as the reporter gene. Eur J Nucl Med Mol Imaging. 2005;32:1108–1114. [PubMed: 15988610] [CrossRef]
- Naciff J.M, Behbehani M.M, Misawa H, Dedman J.R. Identification and transgenic analysis of a murine promoter that targets cholinergic neuron expression. J Neurochem. 1999;72:17–28. [PubMed: 9886050] [CrossRef]
- Okada S, Ishii K, Yamane J, Iwanami A, Ikegami T, Katoh H, Iwamoto Y, Nakamura M, Miyoshi H, Okano H.J, et al. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. Faseb J. 2005;19:1839–1841. [PubMed: 16141363]
- Phelps M.E. Inaugural article: positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci USA. 2000;97:9226–9233. [PMC free article: PMC16850] [PubMed: 10922074] [CrossRef]
- Ponomarev V, Doubrovin M, Shavrin A, Serganova I, Beresten T, Ageyeva L, Cai C, Balatoni J, Alauddin M, Gelovani J. A human-derived reporter gene for noninvasive imaging in humans: mitochondrial thymidine kinase type 2. J Nucl Med. 2007;48:819–826. [PubMed: 17468435] [CrossRef]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. [PubMed: 12778169] [CrossRef]
- Ray P, Tsien R, Gambhir S.S. Construction and validation of improved triple fusion reporter gene vectors for molecular imaging of living subjects. Cancer Res. 2007;67:3085–3093. [PubMed: 17409415] [CrossRef]
- Rice C.M, Halfpenny C.A, Scolding N.J. Stem cells for the treatment of neurological disease. Transfus Med. 2003;13:351–361. [PubMed: 14651741] [CrossRef]
- Rini J.N, Bhargava K.K, Tronco G.G, Singer C, Caprioli R, Marwin S.E, Richardson H.L, Nichols K.J, Pugliese P.V, Palestro C.J. PET with FDG-labeled leukocytes versus scintigraphy with 111In-oxine-labeled leukocytes for detection of infection. Radiology. 2006;238:978–987. [PubMed: 16505395] [CrossRef]
- Rodriguez-Porcel M, Brinton T.J, Chen I.Y, Gheysens O, Lyons J, Ikeno F, Willmann J.K, Wu L, Wu J.C, Yeung A.C, et al. Reporter gene imaging following percutaneous delivery in swine moving toward clinical applications. J Am Coll Cardiol. 2008;51:595–597. [PMC free article: PMC2853907] [PubMed: 18237691] [CrossRef]
- Rodriguez-Porcel M, Gheysens O, Chen I.Y, Wu J.C, Gambhir S.S. Image-guided cardiac cell delivery using high-resolution small-animal ultrasound. Mol Ther. 2005;12:1142–1147. [PubMed: 16111921] [CrossRef]
- Rogers W.J, Meyer C.H, Kramer C.M. Technology insight: in vivo cell tracking by use of MRI. Nat Clin Pract Cardiovasc Med. 2006;3:554–562. [PubMed: 16990841] [CrossRef]
- Rosen A.B, Kelly D.J, Schuldt A.J, Lu J, Potapova I.A, Doronin S.V, Robichaud K.J, Robinson R.B, Rosen M.R, Brink P.R. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128–2138. [PubMed: 17495112] [CrossRef]
- Roth D.J, Jansen E.D, Powers A.C, Wang T.G. A novel method of monitoring response to islet transplantation: bioluminescent imaging of an NF-kB transgenic mouse model. Transplantation. 2006;81:1185–1190. [PubMed: 16641606] [CrossRef]
- Schillaci O. Somatostatin receptor imaging in patients with neuroendocrine tumors: not only SPECT? J Nucl Med. 2007;48:498–500. [PubMed: 17401084] [CrossRef]
- Seleverstov O, Zabirnyk O, Zscharnack M, Bulavina L, Nowicki M, Heinrich J.M, Yezhelyev M, Emmrich F, O’Regan R, Bader A. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 2006;6:2826–2832. [PubMed: 17163713] [CrossRef]
- Shah B.S, Clark P.A, Moioli E.K, Stroscio M.A, Mao J.J. Labeling of mesenchymal stem cells by bioconjugated quantum dots. Nano Lett. 2007;7:3071–3079. [PMC free article: PMC4410692] [PubMed: 17887799] [CrossRef]
- Shah K, Jacobs A, Breakefield X.O, Weissleder R. Molecular imaging of gene therapy for cancer. Gene Ther. 2004;11:1175–1187. [PubMed: 15141158] [CrossRef]
- Sharma V, Luker G.D, Piwnica-Worms D. Molecular imaging of gene expression and protein function in vivo with PET and SPECT. J Magn Reson Imaging. 2002;16:336–351. [PubMed: 12353250] [CrossRef]
- Soloviev V.Y. Tomographic bioluminescence imaging with varying boundary conditions. Appl Opt. 2007;46:2778–2784. [PubMed: 17446927] [CrossRef]
- Springer M.L, Sievers R.E, Viswanathan M.N, Yee M.S, Foster E, Grossman W, Yeghiazarians Y. Closed-chest cell injections into mouse myocardium guided by high-resolution echocardiography. Am J Physiol Heart Circ Physiol. 2005;289:H1307–1314. [PubMed: 15908468] [CrossRef]
- Tavares D, Tully K, Dobner P.R. Sequences required for induction of neurotensin receptor gene expression during neuronal differentiation of N1E-115 neuroblastoma cells. J Biol Chem. 1999;274:30066–30079. [PubMed: 10514493] [CrossRef]
- Terrovitis J, Kwok K.F, Lautamaki R, Engles J.M, Barth A.S, Kizana E, Miake J, Leppo M.K, Fox J, Seidel J, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52:1652–1660. [PMC free article: PMC4980098] [PubMed: 18992656] [CrossRef]
- Thakur M.L, Coleman R.E, Mayhall C.G, Welch M.J Jr. Preparation and evaluation of 111In-labeled leukocytes as an abscess imaging agent in dogs. Radiology. 1976;119:731. [PubMed: 935418]
- Thakur M.L, Lavender J.P, Arnot R.N, Silvester D.J, Segal A.W. Indium-111-labeled autologous leukocytes in man. J Nucl Med. 1977;18:1014–1021. [PubMed: 409745]
- Thakur M.L, Segal A.W, Louis L, Welch M.J, Hopkins J, Peters T.J. Indium-111-labeled cellular blood components: mechanism of labeling and intracellular location in human neutrophils. J Nucl Med. 1977;18:1022–1026. [PubMed: 409746]
- Tjuvajev J.G, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, Safer M, Beattie B, DiResta G, Daghighian F, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed: 9766661]
- Troy T, Jekic-McMullen D, Sambucetti L, Rice B. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging. 2004;3:9–23. [PubMed: 15142408] [CrossRef]
- Voura E.B, Jaiswal J.K, Mattoussi H, Simon S.M. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med. 2004;10:993–998. [PubMed: 15334072] [CrossRef]
- Wang F, Dennis J. E, Awadallah A, Solchaga L. A, Molter J, Kuang Y, Salem N, Lin Y, Tian H, Kolthammer J. A, et al. Transcriptional Profiling of Human Mesenchymal Stem Cells Transduced with Reporter Genes for Imaging. Physiol Genomics. 2008 [PMC free article: PMC2661103] [PubMed: 19116247]
- Watson D.J, Walton R.M, Magnitsky S.G, Bulte J.W, Poptani H, Wolfe J.H. Structure-specific patterns of neural stem cell engraftment after transplantation in the adult mouse brain. Hum Gene Ther. 2006;17:693–704. [PubMed: 16839269] [CrossRef]
- Weissleder R, Simonova M, Bogdanova A, Bredow S, Enochs W.S, Bogdanov A Jr. MR imaging and scintigraphy of gene expression through melanin induction. Radiology. 1997;204:425–429. [PubMed: 9240530]
- Willmann J.K, Paulmurugan R, Rodriguez-Porcel M, Stein W, Brinton T.J, Connolly A.J, Nielsen C.H, Lutz A.M, Lyons J, Ikeno F, et al. Imaging Gene Expression in Human Mesenchymal Stem Cells: From Small to Large Animals. Radiology. 2009 [PMC free article: PMC2702468] [PubMed: 19366903]
- Wu J.C, Cao F, Dutta S, Xie X, Kim E, Chungfat N, Gambhir S, Mathewson S, Connolly A.J, Brown M, Wang E.W. Proteomic analysis of reporter genes for molecular imaging of transplanted embryonic stem cells. Proteomics. 2006;6:6234–6249. [PMC free article: PMC3683542] [PubMed: 17080479] [CrossRef]
- Wu J.C, Chen I.Y, Sundaresan G, Min J.J, De A, Qiao J.H, Fishbein M.C, Gambhir S.S. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. [PMC free article: PMC4154815] [PubMed: 12963637] [CrossRef]
- Wu J.C, Tseng J.R, Gambhir S.S. Molecular imaging of cardiovascular gene products. J Nucl Cardiol. 2004;11:491–505. [PubMed: 15295418] [CrossRef]
- Yaghoubi S.S, Jensen M.C, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir S.S. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. [PMC free article: PMC3526373] [PubMed: 19015650] [CrossRef]
- Yamada S, Terada K, Ueno Y, Sugiyama T, Seno M, Kojima I. Differentiation of adult hepatic stem-like cells into pancreatic endocrine cells. Cell Transplant. 2005;14:647–653. [PubMed: 16405075] [CrossRef]
- Zanzonico P, Koehne G, Gallardo H.F, Doubrovin M, Doubrovina E, Finn R, Blasberg R.G, Riviere I, O’Reilly R.J, Sadelain M, Larson S.M. [131I]FIAU labeling of genetically transduced, tumor-reactive lymphocytes: cell-level dosimetry and dose-dependent toxicity. Eur J Nucl Med Mol Imaging. 2006;33:988–997. [PubMed: 16607546] [CrossRef]
- Zhang S.J, Wu J.C. Comparison of imaging techniques for tracking cardiac stem cell therapy. J Nucl Med. 2007;48:1916–1919. [PMC free article: PMC3638042] [PubMed: 18056330] [CrossRef]
- Zhernosekov K, Aschoff P, Filosofov D, Jahn M, Jennewein M, Adrian H.J, Bihl H, Rosch F. Visualisation of a somatostatin receptor-expressing tumour with 67Ga-DOTATOC SPECT. Eur J Nucl Med Mol Imaging. 2005;32:1129. [PubMed: 16133389] [CrossRef]
- Zhuo L, Sun B, Zhang C.L, Fine A, Chiu S.Y, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. [PubMed: 9224672] [CrossRef]
- Zinn K.R, Chaudhuri T.R. The type 2 human somatostatin receptor as a platform for reporter gene imaging. Eur J Nucl Med Mol Imaging. 2002;29:388–399. [PubMed: 12002716] [CrossRef]
- *
Edited by Sangeeta Bhatia and Julia Polak. Last revised June 30, 2009. Published July 30, 2009. This chapter should be cited as: Rodriguez-Porcel, M., Wu, J.C., and Gambhir, S.S., Molecular imaging of stem cells (July 30, 2009), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.49.1, http://www.stembook.org.
- Review In vivo imaging and monitoring of transplanted stem cells: clinical applications.[Curr Cardiol Rep. 2010]Review In vivo imaging and monitoring of transplanted stem cells: clinical applications.Rodriguez-Porcel M. Curr Cardiol Rep. 2010 Jan; 12(1):51-8.
- Review Molecular imaging of cell therapy for gastroenterologic applications.[Pancreatology. 2011]Review Molecular imaging of cell therapy for gastroenterologic applications.Dimayuga VM, Rodriguez-Porcel M. Pancreatology. 2011; 11(4):414-27. Epub 2011 Sep 7.
- Review Stem Cell Tracing Through MR Molecular Imaging.[Tissue Eng Regen Med. 2018]Review Stem Cell Tracing Through MR Molecular Imaging.Yahyapour R, Farhood B, Graily G, Rezaeyan A, Rezapoor S, Abdollahi H, Cheki M, Amini P, Fallah H, Najafi M, et al. Tissue Eng Regen Med. 2018 Jun; 15(3):249-261. Epub 2018 Jan 16.
- The Myocardial Microenvironment Modulates the Biology of Transplanted Mesenchymal Stem Cells.[Mol Imaging Biol. 2020]The Myocardial Microenvironment Modulates the Biology of Transplanted Mesenchymal Stem Cells.Franchi F, Ramaswamy V, Olthoff M, Peterson KM, Paulmurugan R, Rodriguez-Porcel M. Mol Imaging Biol. 2020 Aug; 22(4):948-957.
- Tracking of Labelled Stem Cells Using Molecular MR Imaging in a Mouse Burn Model in Vivo as an Approach to Regenerative Medicine.[Adv J Mol Imaging. 2021]Tracking of Labelled Stem Cells Using Molecular MR Imaging in a Mouse Burn Model in Vivo as an Approach to Regenerative Medicine.Qadri Z, Righi V, Li S, Tzika AA. Adv J Mol Imaging. 2021 Jan; 11(1):1-15.
- Molecular imaging of stem cells - StemBookMolecular imaging of stem cells - StemBook
- ClinVar for Gene (Select 9829) (343)ClinVar
- Homo sapiens ALG13 UDP-N-acetylglucosaminyltransferase subunit (ALG13), transcri...Homo sapiens ALG13 UDP-N-acetylglucosaminyltransferase subunit (ALG13), transcript variant 3, mRNAgi|1676441551|ref|NM_001039210.5|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...