Introdution
When embryonic stem cells are differentiated in the presence of activin A in serum-free conditions, an endoderm progenitor population defined by the coexpression of either Brachyury, Foxa2 and c-Kit, or c-Kit and Cxcr4 is generated. Specification of these progenitors with bone morphogenetic protein-4 in combination with basic fibroblast growth factor and activin A results in the development of hepatic populations highly enriched (45–70%) for cells that express the alpha-fetoprotein and albumin proteins.
Protocol
- Prepare ES cells on matrigel plate to deplete MEFs.
- Prepare 1:3 matrigel plate: cool down tissue culture plate and pipettes on ice; thaw 1:3 matrigel in fridge or on ice. Carefully apply matrigel onto plate to cover the bottom and then suck off excessive matrigel. Keep everything cool when plate matrigel. Put matrigel plates on ice for 30 min. Suck off excessive matrigel again and put matrigel-coated plates in 37°C incubator for 30 min.
- Treat confluent ES cells with TrypLE for 2–3 min at 37°C, use 4 ml TrypLE for 10 cm dish or 1 ml for 1 well of 6-well plate, and then suck out TrypLE. Wash with wash media (IMDM + Glu + P/S + 0.1% BSA) for 2–3 times. Add 4 ml hES media for 10 cm dish or 1ml for 1 well of 6-well plate, and scrape ES cells off the bottom of plate with cell scraper. Pipette up and down for 3–5 times and check under microscope until you get 5–10-cell clumps.
- Plate cells onto matrigel-coated plates. Cells will get ready for differentiation in 1 day @ 1:1 split; 2 days @ 1:2 split and 3 days @ 1:3 split.
- Setup T0: Differentiation is performed at 5% O2, 5% CO2. Cells are ready to go when they are 70–80% confluent. Wash cells twice with wash media and apply appropriate volume of T0 media to cell, such as 10 ml for 10 cm dish, 2 ml for 1well in 6-well dish.
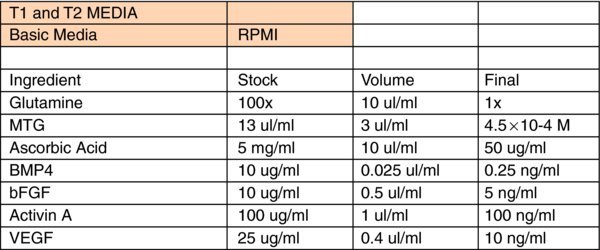
- Wash cells with wash media and change media everyday until T5. It's normal to see huge cell death in the first several days. Detailed differentiation media recipe is attached in the next page.
- To proceed to liver differentiation, dissociate T5 cells with Accutase @ 37°C for 3 min. Rinse with wash media twice. Add 1 ml wash media to each well of 6-well plate and scrape cells off the dish. Collect T5 cells, spin down @ 1200 rpm for 3 min. Resuspend cells in T5 media and seed onto 1:6 matrigel-coated 6-well plate @ 250K cells/well.
- Feed cells every two days with different differentiation media (recipe below) and harvest between 18–25 days for analysis.
Reagents and preparation
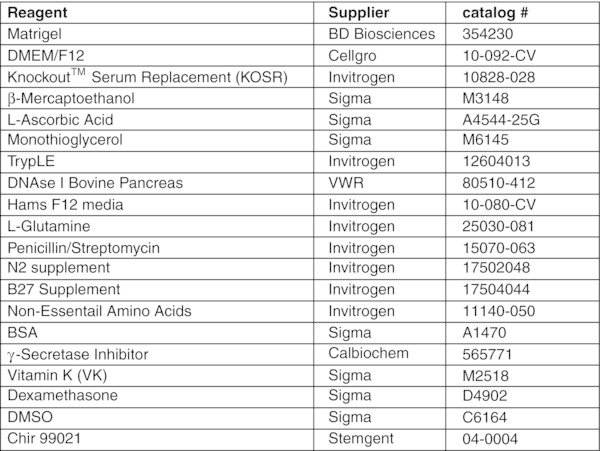
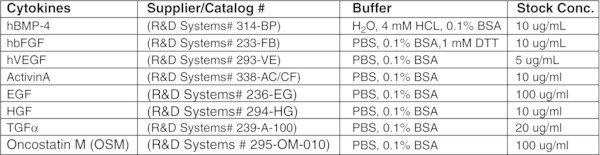
L-ASCORBIC ACID (AA) (SIGMA # A-4544)
Prepare a stock solution of 5 mg/mL in cold TC-H2O. Leave on ice and vortex periodically until completely dissolved. Filter sterilize, aliquot and store at −20ºC. Use once and discard.
MONOTHIOGLYCEROL (MTG) (SIGMA# M-6145)
The amounts of MTG indicated in our protocols are recommended concentrations. However, it is important to test each new batch of MTG as there is variability between them. MTG should be aliquoted (1 mL) and stored frozen (−20ºC). When aliquots are thawed, they can be used for several experiments and then discarded. Aliquoting of MTG is strongly recommended as it minimizes the amount of oxidation due to repeated opening.
*MATRIGEL (REDUCED FACTOR) (BD# 354230)
Each batch of matrigel has its own unique levels of endotoxin and protein concentrations. We find that the endotoxin levels should not be higher than 2 endotoxin units/mL and the protein levels should range between 7 to 10 mg/mL. If the protein levels are higher than this you may need to dilute the matrigel more than 1:1. This is determined by observing the hESC colony morphology and the ability of the hESCs to differentiate into the lineage required of them.
Caution: When working with matrigel, all tubes, plates and pipettes should be pre-chilled, as matrigel solidifies at room temperature.
MATRIGEL1:1 PREPARATION
- Thaw frozen bottles of matrigel on ice overnight in the cold room. We normally thaw 6×5-mL bottles per batch.
- The next day, make a 50% working stock by adding an equal volume of IMDM+P/S to each bottle. Resuspend gently with a pre-chilled 5 mL pipette.
- Leave the bottles on ice all day to allow the matrigel to completely equilibrate with IMDM.
- Pool 3 bottles of 1:1 matrigel (30 mL) into a pre-chilled 50 mL tube. Gently mix with a chilled 10 mL pipette and aliquot.
- Transfer 2.5 mL into pre-chilled and pre-labelled 4-mL snap cap tubes
- Store aliquots at −20ºC
hES Media (500 ml)
- DMEM/F12: 400 ml
- KOSR: 100 ml
- NEAA: 5 ml
- L-glutamine: 5 ml
- Pen/strep: 5 ml
- β-Mercaptoethanol: 3.5 ul
- bFGF: 10 ng/ml (50 ul of 100 ug/ml stock)
- L-Ascorbic Acid: 50 ug/ml –add fresh 5 mg/ml stock at each media change
Detailed differentiation media:
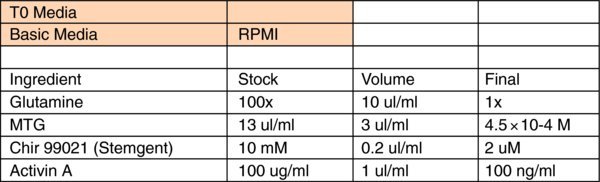
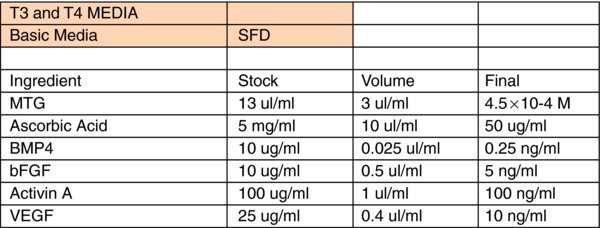
SFD: Recipe for 1 L
750 ml: homemade IMDM (Invitrogen 12200-069) (1×10 L) (+ Glutamine, + 25 mM HEPES+P/S)
250 ml: Hams F12 (Mediatech 10-080-CV)
5 ml: N2-supplement (Invitrogen 17502-048)
10 ml: B27 supplement w/o retinoic acid (Invitrogen 12587-010)
5 ml: 10% BSA in PBS (Sigma A1470, Cohn analog) (Lot tested)
Liver Differentiation Media:
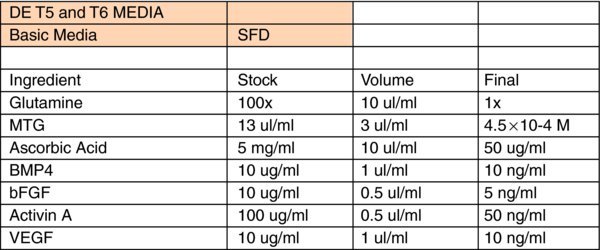
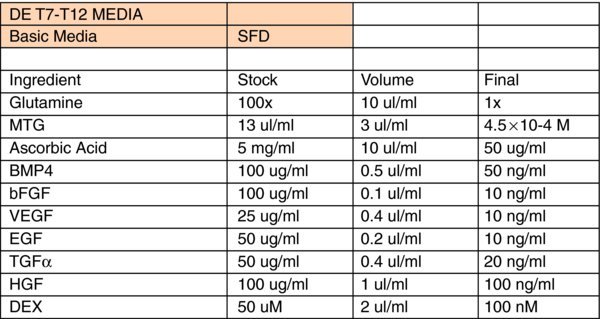
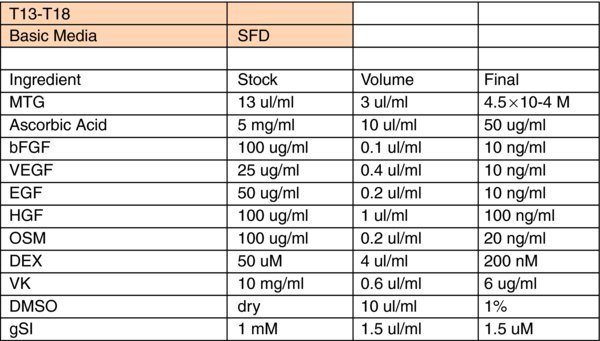
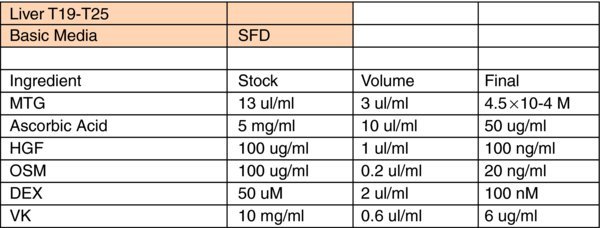
- *
Last revised March 28, 2012. Published June 10, 2012. This chapter should be cited as: Cheng, X., Ying, L., Lu, L., Galvão, A.M., Mills, J.A., Lin, H.C., Kotton, D.N., Shen, S.S., Nostro, M.C., Choi, J.K., Weiss, M.J., French, D.L., and Gadue, P. Monolayer endoderm differentiation from human ESCs (June 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.64.1, http://www
.stembook.org.
Publication Details
Author Information and Affiliations
Authors
X Cheng, L Ying, L Lu, AM Galvão, JA Mills, HC Lin, DN Kotton, SS Shen, MC Nostro, JK Choi, MJ Weiss, DL French, and P Gadue.
Affiliations
 Correspondence: E-mail: islukvin@wisc.edu
Correspondence: E-mail: islukvin@wisc.eduPublication History
Published June 10, 2012.
Copyright
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Publisher
Harvard Stem Cell Institute, Cambridge (MA)
NLM Citation
Cheng X, Ying L, Lu L, et al. Monolayer endoderm differentiation from human ESCs. 2012 Jun 10. In: StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.64.1