This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.87.1
Introduction
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), are known to be vulnerable to apoptosis upon various technical manipulation, such as single cell dissociation, freezing and thawing, etc., which hinder their use for clonal isolation in gene transfer, differentiation and FACS cell sorting.
However, Y-27632, a selective inhibitor of p160-Rho-associated coiled-coil kinase (ROCK) was found to be an effective inhibitor of apoptosis and enhanced survival of hPSCs upon single cell dissociation.
Here we describe how to propagate hPSCs in single cell dissociation using
Accutase, a ready to use cell detachment solution of proteolytic and collagenolytic enzymes and a direct replacement for trypsin solution.
Protocol
- Preparation of MEF feeder plate
- Coat culture dish with sterile 0.1% gelatin solution for about 5 min at room temperature.
- Thaw frozen MEF vial at 37°C water bath quickly.
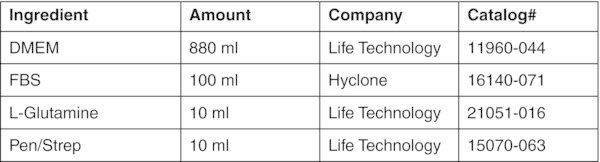
- Transfer cells into 15 ml conical tube and add fibroblast media (DMEM + 10% FBS).
- Wash cells via spinning at 1500 rpm, 5 min.
- Plate MEF at a density of 1.5–2.0 × 104/cm2.
- Let MEF settle down at least 8 hours before use.(Overnight is best and MEF can be used within 2 days)
- Splitting hPSCs with Accutase
- Replace media for MEF dish with hES media.
- Remove differentiated hPSCs colonies under microscope (optional).
- Aspirate the media of hPSCs and add appropriate amount of Accutase (ex. 2 ml in 60 mm dish).
- Incubate cells at 37°C incubator for 20 min until cells are in single cell suspension.
- Harvest cells into conical tube and add hES media for wash.Cell strainer with 40 μm-pore can be used to remove debris and cell clumps (optional).
- Centrifuge cells for 5 min at 1000 rpm in hESC media to remove any remaining Accutse solution.
- Resuspend cells with hES media and plate cells onto MEF feeder cells with density of 1.0–2.0 × 104 cells/cm2.
- Add Y-27632 with a final concentration of 10 μM.
- Incubate cells at 37°C incubator for the growth and feed with hES media everyday.
Materials
- Cell Materials

- hES media (1000 ml)
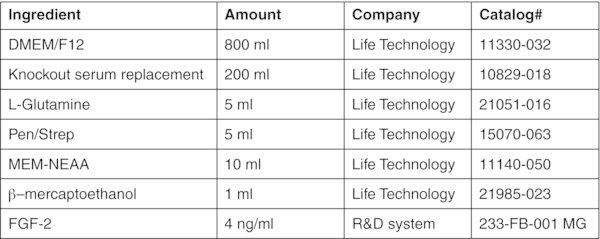
- DMEM with 10% FBS (1000 ml)
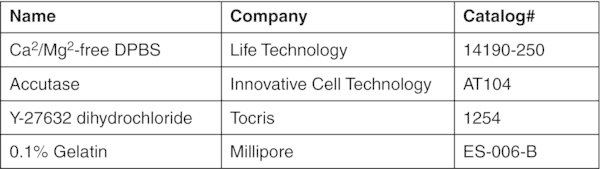
- Reagents
- STUFF
Troubleshooting
- Cells are still in clump with Accutase treatment
- Remove clumps with cell strainer
- Incubate cells enough time
- Check Accutase
- No colonies attached/survived
- Wait until single cells form visible colonies for about a couple of days
- Check Y-27632
Reference
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi J.B, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnology. 2007;25:681–686. [PubMed: 17529971] [CrossRef]
- *
Last revised August 28, 2012. Published December 10, 2012. This chapter should be cited as: Kim, H., Splitting hESC/hiPSC lines on MEF using Accutase (December 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.87.1, http://www
.stembook.org.
- PubMedLinks to PubMed
- Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells.[Stem Cells Dev. 2011]Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells.Li Z, Hu S, Ghosh Z, Han Z, Wu JC. Stem Cells Dev. 2011 Oct; 20(10):1701-10. Epub 2011 Feb 28.
- Review Splitting hESC/hiPSC lines with EDTA in feeder free conditions.[StemBook. 2008]Review Splitting hESC/hiPSC lines with EDTA in feeder free conditions.Chen G. StemBook. 2008
- Efficient propagation of single cells Accutase-dissociated human embryonic stem cells.[Mol Reprod Dev. 2008]Efficient propagation of single cells Accutase-dissociated human embryonic stem cells.Bajpai R, Lesperance J, Kim M, Terskikh AV. Mol Reprod Dev. 2008 May; 75(5):818-27.
- Gene and MicroRNA Profiling of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells.[Stem Cell Rev Rep. 2015]Gene and MicroRNA Profiling of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells.Wang L, Su W, Du W, Xu Y, Wang L, Kong D, Han Z, Zheng G, Li Z. Stem Cell Rev Rep. 2015 Apr; 11(2):219-27.
- Review Pluripotent Stem Cells to Model Degenerative Retinal Diseases: The RPE Perspective.[Adv Exp Med Biol. 2019]Review Pluripotent Stem Cells to Model Degenerative Retinal Diseases: The RPE Perspective.Dalvi S, Galloway CA, Singh R. Adv Exp Med Biol. 2019; 1186:1-31.
- Splitting hESC/hiPSC lines on MEF using Accutase - StemBookSplitting hESC/hiPSC lines on MEF using Accutase - StemBook
- PNPT1 polyribonucleotide nucleotidyltransferase 1 [Homo sapiens]PNPT1 polyribonucleotide nucleotidyltransferase 1 [Homo sapiens]Gene ID:87178Gene
- Gene Links for GEO Profiles (Select 87626629) (1)Gene
- OMIM(Genes) for MedGen (Select 1824007) (1)OMIM
- Lineage analysis of stem cells - StemBookLineage analysis of stem cells - StemBook
Your browsing activity is empty.
Activity recording is turned off.
See more...
