Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Loveman E, Cooper K, Bryant J, et al. Dasatinib, High-Dose Imatinib and Nilotinib for the Treatment of Imatinib-Resistant Chronic Myeloid Leukaemia: A Systematic Review and Economic Evaluation. Southampton (UK): NIHR Journals Library; 2012 May. (Health Technology Assessment, No. 16.23.)

Dasatinib, High-Dose Imatinib and Nilotinib for the Treatment of Imatinib-Resistant Chronic Myeloid Leukaemia: A Systematic Review and Economic Evaluation.
Show detailsQuantity and quality of research available
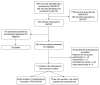
Searching by WMHTAC and SHTAC identified a total of 8760 references after deduplication. After initial screening of titles and abstracts, 242 references were retrieved for further inspection. The total number of published papers included at each stage of the systematic review is shown in the flow chart in Figure 1. In total, 11 studies met the inclusion criteria, four (three new studies, one updated publication) of which included data published since the PenTAG AR.2 The present report presents data from these four studies in order to supplement and update the PenTAG AR.2 For data from the eight studies previously reviewed, see PenTAG AR2 (pp. 55–164).
The studies included in the present report assessed dasatinib and/or high-dose imatinib in chronic-phase CML. No new studies were found by the updated search for accelerated phase or blast phase for any of the interventions. The relevant sections of the PenTAG AR2 for the clinical effectiveness of dasatinib in these subgroups can be found in Table 1. No eligible studies assessing nilotinib were identified. The results for the clinical effectiveness of nilotinib can be found in the PenTAG AR2 (see pp. 138–57).
TABLE 1
Cross-references to PenTAG AR for results of clinical effectiveness for dasatinib in accelerated- and blast-phase CML.
References for the studies retrieved for further inspection, but subsequently excluded can be seen in Appendix 3. The most common reason for exclusion was a retrospective study design. One eligible abstract was identified;5 however, this could not be included owing to insufficient reporting of methods and baseline data. The level of agreement between reviewers assessing study eligibility was generally good, although this was not formally measured.
Design and characteristics of included studies
One published update of a RCT and three new single-arm cohort studies met the inclusion criteria (Figure 1). Data extraction forms for these studies can be seen in Appendix 4. The RCT (Kantarjian and colleagues6) compared high-dose imatinib (600 or 800 mg/day) with dasatinib (140 or 180 mg/day) and was reported in detail in the context of its dasatinib intervention in the PenTAG AR2 (see p. 57, p. 59 and pp. 79–89). The update of this RCT was published in 2009,7 and longer follow-up from the dasatinib arm of this study, as well as data from the high-dose imatinib arm, are included in the present review. However, methodological flaws associated with this RCT render it of limited value as a comparative study (see PenTAG AR,2 section 3.2.4, p. 90), and the PenTAG AR2 presented the dasatinib arm as non-comparative evidence. In line with this, data from the dasatinib and high-dose imatinib arms of the RCT are presented separately and are not compared in the present systematic review (see Chapter 3, Critical appraisal of included evidence)
The single-arm cohort studies each had a single high-dose imatinib arm. In one study, by Rajappa and colleagues,8 all participants received imatinib at 800 mg/day, whereas in the remaining studies the imatinib dose varied from 600 to 800 mg/day according to whether individual participants met criteria for dose escalation or reduction. The interventions in the RCT and observational studies are summarised, respectively, in Tables 2 and 3, and can be viewed in detail in Appendix 4. None of the studies reported whether or not participants received any treatment concurrent with imatinib.
TABLE 2
Details of interventions: RCT.
TABLE 3
Details of interventions: single-arm cohort study.
The designs of the RCT and single-arm cohort studies are summarised, respectively, in Tables 4 and 5. The RCT was conducted in 58 centres in 23 countries, including the UK, Europe, the Russian Federation and Asia. The three single-arm cohort studies were conducted in single countries: Republic of Korea, Italy and India. Apart from the Korean study, which involved 19 centres, the number of centres was small or unclear (Table 5). The studies included only participants with chronic-phase CML, except for the single-arm cohort study by Koh and colleagues,10 which also included very small numbers of participants with accelerated phase and blast-crisis phase (Table 6). Inclusion and exclusion criteria were reported in detail for the RCT (Table 4), but only briefly for the single-arm cohort studies (see Table 5). The RCT required participants to be at least 18 years of age and have ‘adequate hepatic and renal function’, and excluded those with BCR–ABL (oncogene fusion protein consisting of BCR and ABL genes) mutations known to be particularly resistant to imatinib. The single-arm cohort study by Koh and colleagues10 required participants to be aged 15–75 years with ‘adequate organ function’. All other inclusion and exclusion criteria reported in the RCT and single-arm cohort studies were based on cytogenetic or molecular aspects of CML or imatinib dosing.
TABLE 4
Study design: RCT.
TABLE 5
Study design: single-arm cohort studies.
TABLE 6
Baseline characteristics of participants.
Failure on standard-dose imatinib was defined in terms of resistance and suboptimal cytogenetic, haematological and molecular response. None of the studies defined imatinib failure as intolerance (Table 6). The criteria used to define imatinib failure were slightly different in each of the four studies (Table 7).
TABLE 7
Criteria for defining imatinib failure (resistance or suboptimal response) in studies of high-dose imatinib.
Baseline characteristics of the participants in the RCT and cohort studies are summarised in Table 6. For high-dose imatinib, the proportion of male participants in the RCT (45%) was lower than in the three single-arm cohort studies (70–71%). Across the four studies,7–10 the participants ranged in age from 18 to 85 years. The cohort study by Rajappa and colleagues8 included younger participants (mean age 35.7 years) than the three other studies (median age 49–51 years). Duration of CML from diagnosis to imatinib therapy ranged from 14 to 133 months in the RCT, but was not reported in any of the single-arm cohort studies. Baseline genetic and haematological data were not consistently reported across the four studies and are therefore difficult to compare. Only the RCT provided baseline data on the proportion of participants with a major cytogenetic response or a complete haematological response (CHR). The proportion of participants with BCR–ABL mutations at baseline was slightly lower in the RCT high-dose imatinib participant group (22.4%) than in the only single-arm cohort study, by Rajappa and collegues,8 that provided comparable data (32.2%).
The previous imatinib therapy received by participants in each of the four high-dose imatinib studies is summarised in Table 8. The duration of prior imatinib therapy ranged from 0.6 to 70 months (median 18 to 36 months) in the single-arm cohort studies, and from < 1 year to > 3 years in the RCT (not reported more precisely).7 In two of the single-arm cohort studies all participants had previously received only standard-dose imatinib (400 mg/day).8,9 In the remaining single-arm cohort study the majority of participants (90%) had received 400 mg/day, although 10% (the accelerated- and blast-phase participants) received 400–600 mg/day.10 In the RCT the majority of participants (69%) had received 600 mg/day and the remainder (29%) received 400 mg/day (one participant received 500 mg/day). In addition to imatinib, the majority of participants in the RCT had received hydroxycarbamide or anagrelide (93.9%) and/ or interferon alfa (67.3%), with some (36.7%) having received chemotherapy or, in a minority of cases (4.1%), stem cell transplantation. Two single-arm cohort studies reported that, in addition to imatinib, participants had previously received interferon alfa (one study, 29.7%) or hydroxycarbamide (one study, % not stated) only.
TABLE 8
Previous therapy received by study participants.
The characteristics of the studies reviewed are shown in the PenTAG AR2 (see pp. 57–75).
Critical appraisal of included evidence
A summary of the critical appraisal of the RCT is provided in the PenTAG AR2 (see section 3.2.3.1, p. 84). As noted in the PenTAG AR,2 the RCT is flawed, which has implications for interpreting effectiveness and safety information. The updated publication by Kantarjian and colleagues7 provides new information on two aspects of the RCT methodology that were not reported in the previous publications and which therefore do not currently appear in the PenTAG AR:2
- Kantarjian and colleagues7 explained how the sample size was calculated. However, the approach, which is based on arbitrary maximum widths of confidence intervals (CIs) for the primary outcome, was not considered in relation to the statistical power of the trial. This explanation appears to be an attempt to justify the sample size retrospectively.
- Kantarjian and colleagues7 stated that dasatinib and high-dose imatinib groups were stratified by study site and cytogenetic response (CyR) on previous imatinib.
In the PenTAG AR,2 analyses conducted in the RCT were considered appropriate. Although the statistical methods used were generally appropriate, the way in which they were applied does have serious shortcomings. Specifically, the analyses were not planned a priori and were not adjusted for multiple comparisons. However, data from the individual arms are not compared in this report.
Overall, the new information available from the Kantarjian and colleagues publication7 does not alter the judgement that the RCT was substantially flawed. Of particular relevance to the high-dose imatinib arm of the RCT was that 80% of high-dose imatinib participants with inadequate responses crossed over to the dasatinib arm at a median time of 13 weeks (range 1–68 weeks). Conversely, 20% of participants with inadequate responses to dasatinib crossed over to the high-dose imatinib arm at a median time of 28 weeks (range 1–56 weeks). As a result, outcomes for the high-dose imatinib arm reported at a median of 26 months would include an unknown (not reported) proportion of participants who had predominantly received dasatinib. Interpretation of the outcome data for high-dose imatinib in the RCT is also difficult because follow-up times varied considerably and were reported only as the median and range.
Critical appraisal of the three single-arm cohort studies8–10 of high-dose imatinib is summarised in Table 9. The assessment criteria in Table 9 reflect aspects of study design relevant to interpretation of generalisability and some types of bias, which may help to assess the relative strengths and weaknesses of the individual studies. All three studies have risk of selection bias owing to a lack of any randomised procedures for allocation to study groups, and risk of performance bias owing to a lack of allocation concealment and blinding.
TABLE 9
Indicators of quality of included evidence: single-arm cohort studies.
The reporting of these single-arm cohort studies was generally superficial.8–10 Only the study by Koh and colleagues10 could be clearly identified as a prospective study, although the other two studies were judged to be prospective by reviewers.8,9 Only the study by Rajappa and colleagues8 reported whether or not participants were recruited consecutively. Breccia and colleagues9 and Rajappa and colleagues8 failed to adequately report the inclusion criteria for their studies, which is a major impediment to interpreting generalisability and selection bias. Although generalisability of the study by Koh and colleagues10 appears to be stronger than for the other two studies, none of the single-arm cohort studies was conducted in the UK. It is, therefore, unclear how relevant the findings from these studies would be to UK patients with chronic-phase CML.
In these single-arm cohort studies the participants within a study did not all receive exactly the same intervention, as dose escalations occurred at different times for individual participants, or subgroups of participants received different dose changes. It is unclear whether or not any participants received concurrent therapies alongside high-dose imatinib, as this was not reported in any of the three studies. None of the studies reported explicitly whether or not all participants allocated to treatment were analysed and whether or not the analyses included attrition [intention-to-treat (ITT) approach]. Only one of the studies, by Koh and colleagues10 adequately reported participant attrition.
Overall, owing to the inherent limitations of a single-arm study design, compounded by generally poor reporting of the methodology, the three single-arm cohort studies of high-dose imatinib appear to be at high risk of bias and limited or unclear relevance to CML patients in a UK setting.
Relationship of identified evidence to research question
The research questions could not be directly addressed by the PenTAG AR2 (see section 3.2.4, p. 90), as there was no comparative evidence available. Similarly, in our update of the PenTAG AR2 for those with imatinib-resistant CML we have not identified any comparative evidence for any of the three interventions of interest. Therefore, caution continues to be recommended in the interpretation of the evidence now presented.
Evidence reported in this review is relevant to patients with chronic-phase CML only. Owing to the paucity of data the review has been unable to consider whether or not the level of previous response to imatinib has any bearing on outcome, and the evidence does not allow the adoption of an early stopping rule to be considered.
Effectiveness of dasatinib: update of Peninsula Technology Assessment Group assessment report
As described earlier, the updated searches identified one study that provided additional data on the effectiveness of dasatinib (Kantarjian and colleagues 2009;7 Appendix 4). A 2007 publication of this RCT6 was described in the PenTAG AR2 (see pp. 91–8), and the following outcomes have been superseded by the 2009 updated publication:7
- CyR and duration of CyR
- adverse events (AEs).
In addition, the current report presents data on the following outcomes, which were not reported by the PenTAG AR:2
- molecular response
- proportion without treatment failure at 24 months.
There is no difference in the data for the following outcome reported both in 20076 and in 2009:7
- CHR (see PenTAG AR,2 pp. 106–12).
The PenTAG AR2 presented updated results from the manufacturer's submission or from conference abstracts for the following outcomes, and the update publication7 does not change the PenTAG AR2 for:
- CHR rate in participants who had no CHR at baseline (see PenTAG AR,2 p. 109)
- estimated progression-free survival at 24 months (see PenTAG AR,2 p. 118).
Cytogenetic response
Complete cytogenetic response improved from 39.6% at median 15 months' follow-up to 43.6% at median 26 months' follow-up (Table 10). Major cytogenetic response was similar between the two follow-up periods (52.5% at 15 months6 and 53.5% at 26 months7). Major cytogenetic response was similar between patients with (34/62, 55%) and without (20/39, 51%) a previous CyR on standard-dose imatinib.
TABLE 10
Cytogenetic response to dasatinib in chronic-phase CML.
Duration of major cytogenetic response
The 2007 publication reported the probability of a maintained response at 1 year as being 0.98.6 With longer follow-up, the proportion with a maintained major cytogenetic response at 18 months was 90% (95% CI 82% to 98%).7
Major molecular response
Kantarjian and colleagues7 reported a major molecular response (MMR) in 28.7% of participants (29/101) receiving dasatinib, and in 63.4% (28/44) of those who had a complete cytogenetic response and a molecular response assessment (MMR: generally defined as ≥ 3-log reduction in the level of BCR–ABL transcripts or a BCR–ABL ratio of ≤ 0.05%).
Time to treatment failure and proportion without treatment failure
Median time to treatment failure was not reached with dasatinib in the 2007 publication by Kantarjian and colleagues,6 but was not reported in the 2009 study with longer follow-up.7 However, the authors reported that the estimated proportion of participants without treatment failure at 24 months was 59%.7
Adverse events
The PenTAG AR2 (see p. 121) stated that in most of the included evidence, neutropenia and thrombocytopenia each affected in the order of 50% ± 10% of individuals taking dasatinib. The proportion of individuals affected by neutropenia in the RCT is slightly higher with longer follow-up7 (Table 11).
TABLE 11
Haematological AEs (grades 3 and 4) with dasatinib (%).
The PenTAG AR2 (see p. 122) described the AEs (of any grade) most commonly reported by its included studies as diarrhoea, dyspnoea, fatigue, headache, nausea, pleural effusion, and rash, at frequencies in the range 10%–40%. The 2009 update7 also reports fluid retention (39%), bleeding (18%), infection (14%) and upper respiratory tract infection or inflammation (11%), and an increase in superficial oedema from 15% to 20% (Table 12).
TABLE 12
Non-haematological AEs (all grades) with dasatinib (%).
The PenTAG AR2 (see p. 122) reported that grades 3–4 AEs appeared to be fairly rare in the included studies, with only dyspnoea and pleural effusion occurring in more than 5% of participants in any of the included studies. However, grades 3–4 fluid retention occurred in 7% of individuals in the 2009 update publication7 (Table 13).
TABLE 13
Non-haematological AEs (grades 3 and 4) with dasatinib (%).
Rates of treatment discontinuation due to AEs in the four studies included in the PenTAG AR2 (see p. 124) that reported this outcome were described as ranging from approximately 5% to 15%. Discontinuations due to AEs increased from 15.8% in the 2007 Kantarjian and colleagues publication6 to 22.8% in the 2009 publication7 (Table 14).
TABLE 14
Discontinuations due to AEs with dasatinib.
Summary of effectiveness of dasatinib
- No new studies of dasatinib were identified by the updated searches.
- The RCT was methodologically flawed, with a high level of crossovers between treatment arms. As such, the individual treatment arms were considered separately as non-comparative evidence. This is in line with the approach taken by the PenTAG AR.2
- A MMR was reported in 28.7% of participants.7
- Additional AEs were reported with longer follow-up (fluid retention, bleeding, infection, upper respiratory tract infection or inflammation), and grades 3–4 fluid retention occurred in 7% of individuals in the update paper.7
Effectiveness of high-dose imatinib
Cytogenetic response
Table 15 provides a summary of the available data detailing CyR to high-dose imatinib in CML (see Appendix 4 for further details).6–10 All four studies were in participants with chronic-phase CML, with the exception of one study which also included a small number of participants in accelerated-phase CML (n = 3) and blast-crisis CML (n = 4).10 Three studies reported complete, partial and major cytogenetic response rates or provided enough information to enable the deduction of each, whereas one study reported only complete cytogenetic response and major cytogenetic response. Minor and minimal responses were not reported by these studies. The definition of response for each category was consistent across studies.
TABLE 15
Cytogenetic response to high-dose imatinib.
It should be noted that some participants already had some degree of CyR at baseline. Rajappa and colleagues8 reported that 44.5% of participants had achieved major cytogenetic response (although it is unclear whether this is the proportion at study entry)8 and 43.7% of participants were in partial cytogenetic response in the study by Koh and colleagues.7 None of the participants in the high-dose imatinib arm of the RCT by Kantarjian and colleagues7 was in major cytogenetic response at baseline. However, 44% (15/34) of participants with a previous CyR on standard-dose imatinib achieved a major cytogenetic response on high-dose imatinib, whereas 7% (1/15) of those without a previous CyR on standard-dose imatinib achieved a major cytogenetic response. CyR was not reported at baseline by Breccia and colleagues.9
Complete cytogenetic response rates ranged from 18.4%7 to 36.4%.9 Major cytogenetic response rates ranged from 32.7%7 to 63.5%.9
Duration of major cytogenetic response
Kantarjian and colleagues7 reported that 74% (95% CI 49% to 100%) of individuals maintained their major cytogenetic response at 18 months. This outcome was not reported by the other three studies.8–10
Haematological response
Only two of the studies reporting CHR provided a definition,7,9 and there were minor differences between these definitions (Table 16).
TABLE 16
Definitions of CHR in high-dose imatinib studies.
Table 17 provides a summary of the available data detailing HR to high-dose imatinib in CML. Three of the included studies reported CHR, with response rates ranging from 55.5% (18-month follow-up)8 to 91.8% (36-month follow-up).9 It should be noted that 55.1% of participants in the high-dose imatinib arm of the RCT by Kantarjian and colleagues were in CHR at study entry,7 but this was not reported by the other two studies. The CHR in participants without CHR at baseline was 16/22 = 72%.7
TABLE 17
Complete haematological response to high-dose imatinib.
Duration of major or complete haematological response
These were not reported by the included studies.
Molecular response
Three studies reported molecular response and, as can be seen in Table 18, the definitions of molecular response varied between the studies. A summary of molecular response to high-dose imatinib can be seen in Table 19. Kantarjian and colleagues7 reported a MMR in 12.2% of participants receiving high-dose imatinib, and in 55.6% (5/9) of those who had a complete cytogenetic response and a molecular response assessment. A complete molecular response was found in 13.5% of participants by Breccia and colleagues,9 whereas Koh and colleagues reported that 56.3% of participants achieved a molecular reduction > 50% within 6 months.10
TABLE 18
Definitions of molecular response in high-dose imatinib studies.
TABLE 19
Molecular response to high-dose imatinib.
Time to treatment failure
Median time to treatment failure was 18.0 months (range not reported) for all participants (n = 71) in the study by Koh and colleagues.10 For chronic phase participants (n = 64) it was 27 months, for accelerated-phase participants (n = 3) it was 2.5 months and for blast-crisis-phase participants (n = 4) it was 4.0 months.10 Median time to treatment failure was 3.5 months (95% CI 3.3 to 3.8 months) with high-dose imatinib in the 2007 publication by Kantarjian and colleagues,6 but was not reported in the 2009 study with longer follow-up.7 However, the authors reported that the estimated proportion of participants without treatment failure at 24 months was 18%.7
Progression-free or event-free survival
Three of the included studies reported progression-free survival (progression-free survival) or event-free survival.6–9 These are reported together, as the definitions, although differing slightly (Table 20), appear to be measuring similar outcomes. Table 21 provides a summary of the available data detailing progression-free survival with high-dose imatinib. Rajappa and colleagues8 reported estimated event-free survival of 34% at 2 years, whereas a higher estimated progression-free survival is reported by the other two studies (65%7 and 87%9).
TABLE 20
Definitions of progression-free survival and event-free survival used in high-dose imatinib trials.
TABLE 21
Estimated progression-free survival or event-free survival with high-dose imatinib.
Overall survival
Two studies reported overall survival (Table 22) of individuals in chronic-phase CML,8,9 although the definitions differ. Breccia and colleagues9 defined overall survival as the time from diagnosis to death or date of last follow-up, and reported an estimated 2-year overall survival of 85%. Rajappa and colleagues8 defined overall survival as time from dose escalation to death owing to any cause, and reported an estimated 2-year overall survival of 93%.
TABLE 22
Estimated overall survival with high-dose imatinib.
Adverse events
Haematological AEs were reported by all included studies (Table 23). Breccia and colleagues9 reported a low proportion of participants experiencing anaemia and neutropenia (grade not reported). The other three studies reported grades 3–4 haematological AEs, with anaemia occurring in 8%7 to 30%8 of participants, neutropenia in 18%10 to 39%7,8 of participants, leucopenia in 16%7 to 31%8 of participants, and thrombocytopenia in 0%10 to 21%8 of participants.
TABLE 23
Haematological AEs (%).
The most commonly reported AEs of any grade were anorexia, diarrhoea, fatigue, muscle spasms, musculoskeletal pain, nausea, superficial or peripheral oedema and rash (Table 24); however, the reported proportions varied between the studies. Grades 3 and 4 AEs appeared to be fairly rare, with none occurring in more than 4% of the cohort in the two studies reporting this outcome.7,10
TABLE 24
Non-haematological AEs (%).
Treatment discontinuation due to AEs was reported by three6–8,10 of the four studies and ranged from 0%10 to 20.4%7 (Table 25).
TABLE 25
Discontinuations due to AEs.
Summary of effectiveness of high-dose imatinib
- Four studies (one RCT and three single-arm cohort studies) provided data on the effectiveness of high-dose imatinib.
- The RCT had serious methodological flaws. A high proportion (80%) of participants in the high-dose imatinib arm of the RCT crossed over to the alternative treatment after a median duration of 13 weeks. As such, the individual treatment arms were considered as non-comparative evidence. This is in line with the approach taken in the PenTAG AR.2
- The single-arm studies appear to be at high risk of bias and may be of limited relevance to CML patients in a UK setting.
- Complete cytogenetic response was achieved by 18–36% of individuals.
- Major cytogenetic response was achieved by 33–64% of individuals.
- One study reported that around three-quarters of individuals maintained their major cytogenetic response at 18 months.
- Complete haematological response was achieved by 56–92% of individuals.
- Event-free survival of 2 years or more occurred in only 34% of individuals in one study. progression-free survival was estimated as 65–87% in two other studies.
- Overall survival was reported by two studies, which found that 85–93% of people are expected to survive 2 years or more.
- Haematological AEs (grades 3–4) occurred in up to 40% of individuals.
- Non-haematological events also occurred, with anorexia, diarrhoea, fatigue, muscle spasms, musculoskeletal pain, superficial oedema and rash reported in varying proportions.
- Grades 3–4 non-haematological AEs were fairly rare, with none occurring in more than 5% of individuals.
- Between 0% and 20% of study participants discontinued high-dose imatinib owing to AEs.
- These results should be interpreted with caution owing to the methodological limitations of the included studies.
- Clinical effectiveness - Dasatinib, High-Dose Imatinib and Nilotinib for the Tre...Clinical effectiveness - Dasatinib, High-Dose Imatinib and Nilotinib for the Treatment of Imatinib-Resistant Chronic Myeloid Leukaemia: A Systematic Review and Economic Evaluation
- List of abbreviations - Diagnostic accuracy of the Thessaly test, standardised c...List of abbreviations - Diagnostic accuracy of the Thessaly test, standardised clinical history and other clinical examination tests (Apley’s, McMurray’s and joint line tenderness) for meniscal tears in comparison with magnetic resonance imaging diagnosis
- Objective - KRAS mutation testing of tumours in adults with metastatic colorecta...Objective - KRAS mutation testing of tumours in adults with metastatic colorectal cancer: a systematic review and cost-effectiveness analysis
- Data collection - The Asymptomatic Carotid Surgery Trial-2 (ACST-2): an ongoing ...Data collection - The Asymptomatic Carotid Surgery Trial-2 (ACST-2): an ongoing randomised controlled trial comparing carotid endarterectomy with carotid artery stenting to prevent stroke
- Standardisation of provider and contract referral - The relative clinical effect...Standardisation of provider and contract referral - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
Your browsing activity is empty.
Activity recording is turned off.
See more...