Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Thangaratinam S, Rogozińska E, Jolly K, et al. Interventions to Reduce or Prevent Obesity in Pregnant Women: A Systematic Review. Southampton (UK): NIHR Journals Library; 2012 Jul. (Health Technology Assessment, No. 16.31.)

Interventions to Reduce or Prevent Obesity in Pregnant Women: A Systematic Review.
Show details1. Existing reviews
In preparing this proposal, we have conducted a scoping search in the major electronic databases MEDLINE, EMBASE and The Cochrane library to collate citations of individual research studies and systematic reviews on effectiveness and harm of various dietary interventions on weight change in pregnancy. Although there are 3 reviews in this area they have not included all the relevant studies on effectiveness and harm of dietary interventions. The existing Cochrane review on the adverse effect of weight loss or dietary intervention on mother and fetus provides some data but has not included all relevant studies. The review needs updating and quality assessment of included studies to generate firm inferences. This scoping exercise has identified the following reviews in Table 1 which are not up to date or have limitations in quality. Furthermore the reviews on harm are infrequent. Thus there is a need for new reviews.
TABLE 1
Reviews and primary studies on dietary interventions to reduce or prevent obesity in pregnant women: Scoping literature search.
2. Objectives
Our project will follow the key steps involved in health technology assessment of treatment and will meet the commissioned brief by fulfilling the following objectives:
- Effectiveness of dietary interventions on maternal and fetal outcomes: To determine the effectiveness of various dietary interventions that prevent or treat obesity on
- –
maternal outcomes in pregnancy, puerperium and long term
- –
fetal, neonatal and long term outcome in children
- Effectiveness of dietary interventions in pregnancy on maternal weight: To determine the effectiveness of various dietary interventions in pregnant women on
- –
weight change in pregnancy and afterwards in obese (BMI 30 or more) and overweight (BMI 25 to 29.9) pregnant women
- –
prevention of excessive weight gain in pregnancy and afterwards in women with normal weight (BMI 18.5 to 24.9)
- Harm of dietary interventions in pregnancy: To evaluate the potential short term and long term adverse effects in mother and baby due to
- –
weight change in pregnancy in a) obese and overweight women b) normal weight women
- –
the type of dietary intervention in a) obese and overweight women b) normal weight women.
3. Research Methods
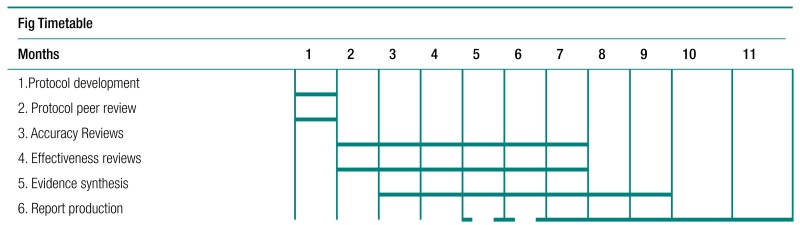
Systematic reviews of effectiveness and harm of interventions will be carried out using review methodology that has been used by the applicants in their previous systematic reviews. It is in line with the recommendations of the NHS Centre for Reviews and Dissemination and the Cochrane Collaboration including those of the Cochrane Adverse Methods Subgroup. The investigation will be carried out simultaneously executing the systematic reviews of effectiveness and harm. Our strategy for these will be based on a prospective protocol, which is briefly outlined below. We will carry out: review of existing reviews; update of out-of-date review; and reviews of topics not reviewed in the literature.
The GRADE methodology will guide us when assessing the quality of the evidence and summarising the results. We have previously used the GRADE methodology in our reviews. The mission of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) group is to help resolve the confusion among the different systems of rating evidence and recommendations and increase transparency within individual evidence syntheses. While the GRADE system has originally been developed for making recommendations, it is now also used for only assessing the quality of the evidence and the outcomes for patients. In that sense, the Cochrane collaboration has now adopted the GRADE-methodology by adding summary of finding tables to its Cochrane reviews.
We plan to explore the need for a health economic evaluation, including decision analytical modelling, of the various dietary and lifestyle interventions on various clinically relevant outcomes. The outputs of our reviews would help us populate a decision-tree, which may be necessary to examine the competitive merits of various strategies.
We will address the following structured question in our project defining population, interventions and comparison and study designs as shown in Table 2.
TABLE 2
Structured questions for systematic review of interventions for preventing or reducing obesity in pregnancy.
The major maternal and fetal outcomes to be reviewed have been standardised through the GLOBE project. We shall identify evidence on additional relevant outcomes for mother and fetus/child and rank them according to their importance for decision making: critical for decision making, important (but not critical) for decision making and not important for decision making. The ranking will be done by Delphi methodology.This step is crucial in order to potentially identify knowledge gaps on critical / important outcomes that have not been investigated so far.
4. Systematic review of effectiveness of interventions
Study identification and selection
For this HTA project, a database of published and unpublished literature will be assembled from searches using a comprehensive search strategy, as well as hand searching, contacting commercial weight management organisations and consultation with experts in the area. We will communicate with major centres of obesity research and the first author of each selected study published in the last five years, with enquiry for any published or unpublished relevant studies not included on our list. Language restrictions will not be applied to electronic searches.
The following databases will be searched: MEDLINE, EMBASE, BIOSIS, LILACS, Pascal, Science Citation Index, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment Database (HTA). In addition, information on studies in progress, from commercial providers like Weight Watchers, Slimming world and unpublished research or research reported in the grey literature will be sought by searching a range of relevant databases including the Inside Conferences, Systems for Information in Grey Literature (SIGLE), Dissertation Abstracts and Clinical Trials.gov. Internet searches will also be carried out using specialist search gateways (such as OMNI: http://www.omni.ac.uk/), general search engines (such as Google: http://www.google.co.uk/) and meta-search engines (such as Copernic: http://www.copernic.com/). Citations identified by the search will be selected for inclusion in the review in a two-stage process using predefined and explicit criteria regarding populations, interventions, outcomes and study design. First, a master database of the literature searches will be constructed by amalgamation of all the citations from various database sources. The citation will be scrutinised by two reviewers. Copies of full manuscripts of all citations that are likely to meet the selection criteria will be obtained. Two reviewers will then independently select the studies, which meet the predefined criteria. These criteria will be pilot tested using a sample of papers and agreement between reviewers will be measured. Disagreements will be resolved by consensus and/or arbitration involving a third reviewer.
Study quality assessment and data extraction
The quality of the selected primary randomised controlled trials (RCT's) and observational studies will be assessed based on accepted contemporary standard. Following the GRADE methodology, the quality assessment and reporting of results will be done separately for each outcome, since even within one review the quality of the evidence can vary between outcomes. We define quality of evidence as ‘the extent of confidence that an estimate of effect is correct’. The GRADE system classifies quality of evidence into one of four levels: high, moderate, low and very low (Table 3).
TABLE 3
Quality of evidence and definitions.
To assess the quality, we consider first of all risk of bias (internal validity), i.e. the extent to which design, methods, execution and analysis did not control for bias in assessment of effectiveness (Table 4). Furthermore, we explore the (in-) consistency of results (heterogeneity), (in-) directness of the evidence (to the question under consideration, including surrogate parameters), (im-) precision of the results and publication bias. Deficiencies on those criteria in the body evidence from RCTs will lower the quality of the evidence from high to moderate or low, perhaps even very low. Deficiencies in the body of evidence from non-RCTs will lower the quality of evidence from low to very low.
TABLE 4
Criteria for assessing risk of bias.
Individual studies will be described by study type, intervention, numbers taking part, population denominator (eg pregnant women or fetuses) and study quality. In addition to using study quality as possible explanations for differences in results (heterogeneity), the extent to which primary research met methodological standards is important per se for assessing the strength of any conclusions that are reached. Studies' findings will be extracted in duplicate using pre-designed and piloted data extraction forms, which we have already developed and used in our previously completed reviews. Any disagreements will be resolved by consensus and/or arbitration involving a third reviewer. Missing information will be obtained from investigators if it is crucial to subsequent analysis. To avoid introducing bias, unpublished information will be coded in the same fashion as published information. In addition to using multiple coders to insure the reproducibility of the overview, sensitivity analyses around important or questionable judgements regarding the inclusion or exclusion of studies, the validity assessments and data extraction will be performed.
Data synthesis
We will use RevMan and Stata softwares to conduct analyses. The former will allow uniformity with Cochrane reviews and the latter will allow the data analytic flexibility that we will need to examine issues not included in the RevMan software. Separate analyses will be performed on randomised and non-randomised data. Any heterogeneity of results between studies will be statistically and graphically assessed, including use of funnel plots. We will explore causes of the heterogeneity and proceed to perform meta-analysis if appropriate. To explore causes of heterogeneity subgroup analyses will be planned a priori to see whether variations in clinical factors e.g. populations, interventions, outcomes or study quality affect the estimation of effects. Individual factors explaining heterogeneity will also be analysed using meta-regression to determine their unique contribution to the heterogeneity. Conclusions regarding the typical estimate of an effect size of the intervention will be interpreted cautiously if there is significant heterogeneity.
5. Review of adverse effect of interventions
In the proposed project addition to the search for relevant reviews and primary studies on effectiveness of interventions including those that were excluded from analysis of benefit, we will evaluate studies that specifically provide details of adverse effects due to the dietary interventions. We will conduct review of harm of interventions based on recommended methods for systematic reviews, particularly those of observational studies and adverse events including those of Cochrane adverse effects subgroup.
Study identification and selection for adverse events
We have purposefully kept the scope of the question of adverse effects of any dietary intervention on pregnant women and their children broad. This will enable us to identify a variety of adverse effects that were previously not known or recognised. The adverse outcomes to be evaluated will be in 3 groups and similar to the outcomes in the effectiveness review, they will be ranked according to their importance: critical for decision-making, important for decision making and not important.
- clinically significant adverse maternal outcomes in pregnancy and later due dietary interventions in (i) overweight or obese women and (ii) women with normal weight
- clinically significant adverse fetal, neonatal, childhood and adult outcomes in the offspring of pregnant women undergoing dietary interventions
- Most common adverse effects that lead to pregnant women discontinuing the intervention
We will design a separate search strategy to identify studies on harm by including adverse effects text words and indexing terms to ensure that they are not missed in the databases previously described. We will use datasets providing counts or proportions attributed to specific interventions or weight change in pregnancy leading to maternal and fetal adverse outcomes, from direct counting or from special surveys. We use the term dataset because some sources are research studies but others are direct counts or other forms of routine data collection (such as vital registration; membership of weight reduction club, web table). We will include only those datasets that represent the target population in the final analysis. In cases of partial data duplication with overlapping datasets, we will select the most recent and largest dataset.
Study quality assessment and data extraction for adverse events
Criteria used to assess study quality will follow the same concept as for assessing study quality for effectiveness: assessing risk of bias, inconsistency of results, indirectness of the evidence, imprecision and publication bias. For assessing the risk of bias in estimating adverse event rates associated with dietary intervention in pregnancy, we will take into account existing checklists for evaluation of randomised and non-randomised studies, including study design and other features associated with outcome (e.g. small for gestational age, pre term delivery etc). For the three possible designs (RCTs, observational studies with a control group, and observational studies without controls (case series)) quality assessment and presentation of results will be done separately. Additionally, information on weight change per se on mother and baby will also be extracted as these could be associated with adverse event rates or severity. The methodological quality of all eligible datasets (‘risk of bias’) will be assessed to investigate internal validity (the extent to which the information is probably free of bias) with the following attributes:
- reporting of adverse maternal and fetal outcome definition to reduce bias in ascertainment of denominator data in the series (any published definition reported Vs no definition)
- adequacy of data source to ascertain a capture of denominator data that is as complete as possible (use of multiple data sources, special surveys, or clinical studies vs routine registration enrolment in weight loss programmes, in which adequate attribution of cause of harm has been shown to be questionable for maternal and fetal outcomes, leading to substantial underreporting)
- use of a robust approach to ascertain that the cause of harm is a representation of the underlying condition that is as true as possible (confidential enquiries, use of multiple sources of outcome vs no special efforts to confirm cause)
- sufficiently high proportion of cases with attributable cause of harm established (< 5% unclassified).
Quality assessment will be done for each outcome. Randomised studies will start as high quality, observational studies with controls will start as low quality, and uncontrolled studies will start as very low quality. The evidence will be downgraded in the presence of methodological weaknesses and uncertainty; it can be upgraded in the presence of large effects, dose–response gradient and remaining plausible confounding which would reduce a demonstrated effect. Based on these criteria, the datasets will be classified into different quality groups.
Data synthesis for adverse events
The number of adverse events reported in pregnant women and children will be obtained for each intervention to compute a percentage of the total number of women and children in whom the occurrence of that particular adverse event or confirmation of its absence was reported. It is inappropriate to calculate adverse events rates from case studies, thus a qualitative summary will be undertaken. Quantitative adverse events rates calculations will be restricted to series of women undergoing dietary interventions and weight change as identified from RCTs and observational studies, with and without controls (case series). We shall quantify the adverse events as relative risks and 95% confidence intervals. The point estimates of proportions and their 95% CIs will be represented in forest plots to explore heterogeneity and the possibility of the differences being due to chance assessed statistically by Cochran Q test. To explore the presence of heterogeneity and its causes, regression models will be adjusted to the proportions attributed to every individual cause of maternal and fetal complications. The proportions will be transformed with the logit transformation. Explanatory variables considered in these models are: type of intervention and dataset methodological quality items.
6. Evidence Synthesis using the GRADE methodology
Once the systematic reviews for effectiveness and harm of dietary interventions have been undertaken, we shall prepare standardised evidence profiles using the GRADE profiling software GRADEPro. Profiles will be done for both groups (obese or overweight women and normal weight women at risk of excessive weight gain), with a separate quality assessment and summary of findings for each critical and important outcome that will allow a quick and informative summary of the evidence.
The following steps will be undertaken to come to an overall judgement: having assessed the quality of evidence for each maternal and fetal outcome, and having decided on the relative importance of the outcomes (critical or important to a decision), we will come up with a judgement on the overall quality of evidence across the most important outcomes, balancing net benefits and harms.
- Review protocol - Interventions to Reduce or Prevent Obesity in Pregnant Women: ...Review protocol - Interventions to Reduce or Prevent Obesity in Pregnant Women: A Systematic Review
- Plain English summary - The second Randomised Evaluation of the Effectiveness, c...Plain English summary - The second Randomised Evaluation of the Effectiveness, cost-effectiveness and Acceptability of Computerised Therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? A randomised controlled trial
- Lower-priority studies not included in the systematic review of clinical effecti...Lower-priority studies not included in the systematic review of clinical effectiveness or considered for the network meta-analyses (n = 28) - Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation
- Search strategies for quality-of-life studies - Selective internal radiation the...Search strategies for quality-of-life studies - Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation
- References - Selective internal radiation therapies for unresectable early-, int...References - Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...