Early trial closure
Recruitment rates were closely monitored throughout the study, which highlighted within 8 months of the first patient being registered that additional strategies and additional centres would be required to improve recruitment and achieve the target of 450 patients randomised. Site visits were undertaken from October 2009 and a number of new strategies were developed through discussion with sites (see Appendix 3). A funding extension application was submitted to the NIHR HTA programme in February 2010 requesting an extension of recruitment time together with funding for new sites. One of the differences noted about recruitment centres that were more successful than others was how well integration had occurred between staff within the newly formed Medicines for Children Research Network (MCRN), Primary Care Research Network (PCRN) and Comprehensive Local Research Network (CLRN). If the study was to succeed it would also have been necessary to open other recruitment centres and these had been carefully selected based on the knowledge we had accrued through discussions and particularly the ability to liaise and work effectively between secondary and primary care. It was recognised that some of the initial recruitment sites would have to be closed. Funding was not granted, however, and the study was closed down prematurely on 24 June 2010. For those patients who were already randomised, follow-up was to continue to T48 or the end of January 2011, whichever was the earliest, as January 2011 was the date that the medications expired. Data cleaning and site close-down visits took place between February and July 2011, with final analyses undertaken during August and September 2011.
Participant flow and recruitment
The first patient was registered on 27 January 2009, the first patient was randomised on 19 May 2009, the last patient was registered on 25 June 2010 and the last patient was randomised on 24 June 2010. Table 3 shows all 13 recruiting centres, the date each site was initiated, the original target recruitment figure, the number of participants registered, the number of participants randomised and the dates of the first and last randomisation. All centres registered at least one patient and 12 centres randomised at least one patient.
TABLE 3
Recruitment by centre
A total of 166 patients were registered at T–4 and 63 (38%) of these were eligible and consented to be randomised at T0. The percentage randomised at each centre ranged between 20% and 58% for those that randomised at least one patient.
Figure 1 provides a CONSORT flow diagram showing the numbers of participants recruited and randomly assigned to the three trial arms.

FIGURE 1
CONSORT flow diagram. SABA, short-acting beta-2 agonist. All patients who did not reach the minimum of 43 weeks because of early closure of the trial have primary outcome data up to at least T24.
Baseline comparability of randomised groups
A summary of the baseline characteristics for all randomised participants is given in Table 4. Overall there was a preponderance of males with the major comorbid condition being eczema, neither of which was unexpected. The male-to-female ratio in children with asthma is approximately 2 : 1 and there is a strong association between eczema and asthma in children. The age of the children ranged from 6.50 to 14.67 years (average 10.39 years).
TABLE 4
Baseline characteristics for all randomised participants
The distribution of characteristics is similar across treatment groups but a higher percentage of males were randomised to fluticasone alone and a higher percentage of children with respiratory and dermatological abnormalities were randomised to fluticasone plus salmeterol. Because of the small number of children recruited this probably occurred by chance but could suggest that patients randomised to fluticasone plus salmeterol had more severe asthma.
No ineligible patients were randomised.
Unblinding of randomised treatments
No patient required unblinding from randomised treatment other than at the end of the study. The process was described in Chapter 2, Randomisation and blinding.
Protocol deviations
Protocol deviations were classified as major or minor according to a preplanned classification system outlined in the SAP (see Appendix 2). There were 18 minor protocol deviations reported for 17 patients (three randomised to fluticasone, five randomised to fluticasone plus salmeterol, two randomised to fluticasone plus montelukast and seven who were not randomised) (Table 5). There were no major protocol deviations. Eleven of the protocol deviations were due to visits that occurred outside the predefined time interval, one of which led to missing data. One protocol deviation occurred because two different parents completed the quality of life questionnaire at two different visits. Six protocol deviations were related to the spirometry test undertaken at T0 or T48.
TABLE 5
Protocol deviations
Efficacy outcomes
Primary outcome
Number of asthma exacerbations requiring treatment with oral corticosteroids
In total, 38 (60%) randomised patients completed their 48-week visit and 54 (86%) patients completed their 24-week visit and could be included in the ITT analyses (Tables 6 and 7 respectively). Reasons for exclusions are provided in Appendix 6. For the primary analysis (ITT at 48 weeks), seven (18%) patients had at least one exacerbation requiring oral steroids, with most having only one episode. The effect of treatment overall (p = 0.98) and for each pair-wise comparison was not significant (Table 8). Because of limited data, the CI around the RR for each pair-wise comparison is extremely wide and includes values of RR that could indicate beneficial, harmful or equivalent treatment effects (see Table 8). The secondary ITT analysis at 24 weeks includes data for 54 patients (see Table 7) of whom 10 (18.5%) had experienced at least one exacerbation. Similarly to the 48-week analysis, all treatment effects are not significant with very wide CIs (see Table 8). The per-protocol analyses show similar results and uncertainty to the ITT analyses (see Table 8). Because of the limited number of patients and events available for parameter estimation, secondary analyses adjusting for prognostic factors were not undertaken as planned in the SAP.
TABLE 6
Exacerbations requiring course of oral steroids over 48 weeks (ITT analysis)
TABLE 7
Exacerbations requiring course of oral steroids over 24 weeks (ITT analysis)
TABLE 8
Relative treatment effects from Poisson regression model adjusted for overdispersion: number of exacerbations requiring oral corticosteroids
Secondary outcomes
Quality of life of children as measured by the Paediatric Asthma Quality of Life Questionnaire with Standardised Activities
A total of 37 children completed the quality of life questionnaire at baseline and at 48 weeks' follow-up. The results show an improvement in mean scores for activity limitations, symptoms and emotional function across all treatment groups at 48 weeks, with the largest improvement in mean score for fluticasone plus montelukast across all domains (Table 9). However, the pair-wise treatment comparison results from ANCOVA show that none of the mean differences adjusted for baseline score is statistically significant. The 24-week analysis results based on 50 patients show an improvement in mean scores for activity limitations, symptoms and emotional function across all treatment groups (Table 10). The largest improvement was seen for fluticasone plus salmeterol across all domains but none of the mean differences adjusted for baseline score is statistically significant.
TABLE 9
Quality of life of children measured using the PAQLQ(S) over 48 weeks
TABLE 10
Quality of life of children measured using the PAQLQ(S) over 24 weeks
Quality of life of caregivers as measured by the Paediatric Asthma Caregiver's Quality of Life Questionnaire
A total of 38 caregivers completed the quality of life questionnaire at baseline and at 48 weeks. The results show an improvement in mean scores for activity limitations and emotional function across all treatment groups at 48 weeks (Table 11). In support of the PAQLQ(S) analysis, the largest improvement in mean score at 48 weeks is seen for fluticasone plus montelukast across all domains. The pair-wise treatment comparison results from ANCOVA, however, show that none of the mean differences adjusted for baseline score is statistically significant. Similar results were obtained using data up to 24 weeks (Table 12).
TABLE 11
Quality of life of caregivers measured using the PACQLQ over 48 weeks
TABLE 12
Quality of life of caregivers measured using the PACQLQ over 24 weeks
Time from randomisation to first exacerbation requiring treatment with a short course of oral corticosteroids
In total, 63 randomised patients could be included in the analysis of time to first exacerbation (Table 13) [median length of follow-up 47 weeks (range 0 to 57 weeks, IQR 33 to 49 weeks) for the 49 censored patients]. Overall, there were 14 (22.2%) patients who had at least one exacerbation event, with most events in the fluticasone plus salmeterol group [seven events (30.4%)] and least events in the fluticasone plus montelukast group [three events (14.3%)]. No statistically significant difference in time to first exacerbation could be detected between the three treatment groups with the log-rank test (Figure 2, χ2 = 1.90, 2df, p = 0.39). Because of the limited data, wide CIs for pair-wise HRs (Table 14) make it impossible to draw any meaningful conclusions.
TABLE 13
Time to first exacerbation

FIGURE 2
Kaplan–Meier survival curves for time to first exacerbation by treatment.
TABLE 14
Hazard ratios for time to first exacerbation
Number of school days missed due to respiratory problems
A total of 37 children with data available up to 48 weeks were included in the ITT analysis. Fewer children missed at least 1 day of school over 48 weeks on fluticasone plus montelukast (18.2%) than on fluticasone (63.6%) and fluticasone plus salmeterol (60%) (Table 15); however, this pattern is not supported by the 24-week data, based on 54 patients, which show that a similar percentage of patients missed at least 1 day across treatment groups (Table 16). The RRs for number of school days missed, estimated from Poisson regression, are not statistically significant (Table 17) and wide CIs prevent useful conclusions for the 48-week and 24-week analyses.
TABLE 15
Number of school days missed over 48 weeks (ITT analysis)
TABLE 16
Number of school days missed over 24 weeks (ITT analysis)
TABLE 17
Relative treatment effects from Poisson regression model adjusted for overdispersion: number of school days missed
Number of hospital admissions due to respiratory problems
Tables 18 and 19 show the number of hospital admissions over 48 weeks and 24 weeks respectively. Two hospital admissions occurred in two patients on fluticasone plus salmeterol over 48 weeks (see Table 18). Analysis of the number of admissions with Poisson regression was not possible because of sparse data and no conclusions can be drawn (Table 20). For the patients with data up to 24 weeks, four (7.4%) patients required at least one hospital admission, with a similar frequency across treatment groups (see Table 19). The CIs for the 24-week RRs estimated from Poisson regression (see Table 20) are very wide and include relative effects in both directions.
TABLE 18
Number of hospital admissions over 48 weeks (ITT analysis)
TABLE 19
Number of hospital admissions over 24 weeks (ITT analysis)
TABLE 20
Relative treatment effects from Poisson regression model adjusted for overdispersion: number of hospital admissions
Amount of rescue beta-2 agonist therapy prescribed for asthma symptoms
A total of 23 out of 37 (62.2%) children were prescribed at least one beta-2 agonist over 48 weeks of follow-up (Table 21), with a median total dose per patient of 24,000 μg. In total, 28 out of 53 (52.8%) children were prescribed at least one beta-2 agonist over 24 weeks of follow-up (Table 22), with a median total dose per patient of 20,000 μg. There was no significant difference in the mean amount prescribed between the three groups from ANOVA at 48 weeks (F-value = 0.96, 2df, p = 0.39) or 24 weeks (F-value = 1.2, 2df, p = 0.31) (Table 23).
TABLE 21
Number of beta-2 agonists prescribed over 48 weeks
TABLE 22
Number of beta-2 agonists prescribed over 24 weeks
TABLE 23
Difference in mean amount of beta-2 agonists prescribed (ANOVA)
Time from randomisation to treatment withdrawal (because of lack of efficacy or side effects)
Although 13 patients were withdrawn from treatment or the study, only one (on fluticasone) withdrew from treatment because of poor asthma control (patient also recorded intercurrent illness as reason) during follow-up (see Table 34). The planned analysis of time to treatment withdrawal was therefore not possible.
TABLE 34
Withdrawals from the study and treatment
Lung function at 48 weeks assessed by spirometry
A total of 30 children had lung function assessed by spirometry at baseline and at 48 weeks and could be included in ANCOVA (Table 24). There were no statistically significant adjusted mean differences between the three treatment groups, with wide CIs that include clinically significant differences in both directions.
TABLE 24
Lung function over 48 weeks assessed by spirome
Safety outcomes
Adverse events were assessed at each visit between randomisation and the final visit. Adverse events were classified according to the Medical Dictionary for Regulatory Activities (MedRA) (www.meddramsso.com) System Organ Class by the MCRN CTU Data Manager, with all classifications approved by the chief investigator after discussion with the trial working group.
The number (and percentage) of patients experiencing each adverse event is shown for each treatment in Table 25; this is categorised further by severity in Table 26. For each patient who experienced more than one episode of the same adverse event, the maximum severity is shown. No formal statistical testing was undertaken on these data.
TABLE 25
All adverse events
TABLE 26
All adverse events according to severity
In total, 53 out of 63 (84%) randomised patients experienced at least one adverse event between randomisation and last follow-up, with a similar percentage of patients across treatment groups [fluticasone 17 (89%); fluticasone plus salmeterol 18 (78%); fluticasone plus montelukast 18 (86%)]. The most common events were respiratory and nervous system disorders, infections and infestations, and general and gastrointestinal disorders. The percentage of patients reporting each adverse event was similar across treatment groups (see Table 25) except for nervous system disorders. Fewer patients reported nervous system disorders on fluticasone plus salmeterol [one patient (4.3%)] than on fluticasone plus montelukast [seven patients (33.3%)] and fluticasone [five patients (26.3%)], with a much greater number of events on fluticasone plus montelukast (37 events compared with 13 on fluticasone and two on fluticasone plus salmeterol) because of one patient experiencing 20 events. All adverse events were graded as mild or moderate (see Table 26) with a similar distribution of severity across treatment groups except for the nervous system disorders described previously.
Serious adverse events and suspected unexpected serious adverse reactions
Between randomisation and trial closure, six patients reported a total of seven serious adverse events (SAEs) (Table 27), with similar frequency across treatment groups (two patients reported three events on fluticasone; three patients reported three events on fluticasone plus salmeterol; one patient reported one event on fluticasone plus montelukast). All SAEs were mild or moderate in severity and all were classified as unrelated to treatment. There were no suspected unexpected serious adverse reactions (SUSARs) and no deaths reported during the trial.
TABLE 27
All SAEs from randomisation
Health economics
The sample size is extremely small in each group; therefore, although every endeavour has been made to fully analyse and report the economic results and to take necessary statistical safeguards, the results should be interpreted with extreme caution.
It can be seen from Table 28 that there was some loss of patients completing the health economics diary over each time period but completion of the health economics booklet was very similar across the groups. This included patients whose 48-week visit took place within 48 weeks because of the early closure of the trial. We removed patients from the 48-week visit analysis when the final visit took place inside 43 weeks from baseline.
TABLE 28
Completion of the health economics booklet at each time period
Table 29 indicates the total events of care (resource use) for each of the three treatment groups during the study period.
TABLE 29
Total resource use (events of care)
Table 30 shows the main cost drivers in the treatment of childhood asthma. The main cost driver, not surprisingly, is the cost of the intervention itself, followed by the costs of GP visits, prescribed inhalers and prescribed medications. With larger patient numbers these results could change.
TABLE 30
Total resource costs
Given that the cost of the trial intervention is the greatest cost driver it is not surprising that the fluticasone plus salmeterol group and the fluticasone plus montelukast group have the greatest overall costs. That said, even though the intervention cost is higher in the fluticasone plus montelukast group, once the overall costs have been factored in it is the fluticasone plus salmeterol group that has the greatest overall cost, although the difference is small. This shows the importance of calculating the full economic cost of an intervention, not just the cost of the intervention itself.
Outcome
The QALY gains shown in Table 31 are based upon independent bootstrapped samples of n = 2000.
TABLE 31
Quality-adjusted life-year gains
Bootstrapped t-tests and Wilcoxon rank-sum tests did not reveal any statistically significant differences in mean QALYs between baseline and 48 weeks when comparing any of the treatment groups in two-way tests. Figure 3 provides a graphical representation of quality of life over time.
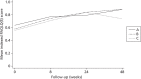
FIGURE 3
Quality-adjusted life-years over time in the trial. A, fluticasone; B, fluticasone plus salmeterol; C, fluticasone plus montelukast.
Cost-effectiveness analysis
In terms of economics the variables of interest between the groups are the differences in costs and effects. Table 32 shows the mean differences in incremental costs and QALY gains between the three groups.
TABLE 32
Mean differences in costs and QALYs by group using bootstrapped data
The measure of interest from these data is incremental cost-effectiveness, captured by the ICER, which shows the difference in the sum of the costs over effects for each individual patient between each of the treatment arms. Table 31 shows that there is very little difference in QALYs before and after the interventions. Similarly, cost differences are not great between the groups, with fluticasone plus salmeterol and fluticasone plus montelukast incurring approximately three times the cost per person as fluticasone. The difference in outcomes was greater for fluticasone plus salmeterol and fluticasone plus montelukast even though the cost was greater; hence, it can be seen that a positive ICER is generated for fluticasone plus salmeterol compared with fluticasone and fluticasone plus montelukast compared with fluticasone (Table 33).
TABLE 33
Incremental cost-effectiveness ratios
It can be seen from the ICER results that the treatment of choice is inhaled fluticasone propionate 100 μg twice daily plus montelukast 5-mg tablet once daily, as this produces 1 additional QALY for £6827, whereas it would cost £12,054 to produce an additional QALY from inhaled fluticasone propionate 100 μg and salmeterol 50 μg twice daily (combination inhaler) plus placebo tablet once daily. Both are by comparison with inhaled fluticasone propionate 100 μg. To further aid interpretation of the results the CEACs are presented for fluticasone plus salmeterol and fluticasone plus montelukast compared with fluticasone, and fluticasone plus montelukast compared with fluticasone plus salmeterol (Figure 4).

FIGURE 4
Cost-effectiveness acceptability curves. A, futicasone; B, futicasone plus salmeterol; C, futicasone plus montelukast.
Figure 4 shows the probabilities that fluticasone plus salmeterol and fluticasone plus montelukast are under the £30,000 per QALY cost-effectiveness threshold as required by NICE. The probability of cost-effectiveness at this level is around 80% for fluticasone plus montelukast and 60% for fluticasone plus salmeterol. Moreover, the comparison between fluticasone plus montelukast and fluticasone plus salmeterol shows that fluticasone plus montelukast dominates fluticasone plus salmeterol; however, the CEAC for fluticasone plus montelukast compared with fluticasone plus salmeterol declines after the threshold ICER because of increasing uncertainty, reflecting the lack of statistical power in the incremental QALYs. In fact, there is very little evidence supporting fluticasone plus salmeterol or fluticasone plus montelukast over the other.
Figure 5 illustrates the relative spread of incremental costs and QALYs for fluticasone plus salmeterol and fluticasone plus montelukast compared with fluticasone on a cost-effectiveness plane. This is useful because it shows that, although fluticasone plus montelukast has a lower average incremental cost and higher average incremental QALY gain, it also has greater uncertainty, which is why fluticasone plus salmeterol and fluticasone plus montelukast cannot easily be compared directly.
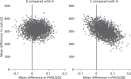
FIGURE 5
The cost-effectiveness plane.
Withdrawals
In total, 13 (20.6%) patients withdrew from the study before the 48-week visit or before early study closure. The numbers of withdrawals and reasons for withdrawal are similar across the three treatment groups (Table 34). Six of the 13 patients had indicated a withdrawal from treatment but no further follow-up data were available. There were no crossovers to another treatment arm.
Compliance
The number of doses missed (inhaler and tablets) since the previous visit was captured on the follow-up CRF. The expected number of tablet doses for each visit was calculated as the number of days since the last visit (i.e. one per day) and the expected number of inhaler doses was calculated as twice the number of days since the last visit (i.e. two per day).
For each visit, each patient had the proportion of doses missed (number of doses missed/number of expected doses) calculated separately for inhalers and tablets. These proportions were averaged over all patients per treatment group separately for inhalers and tablets and are displayed as summary measures in Table 35. The proportion of treatments missed is similar across the groups and time points, except for fluticasone plus montelukast at the 48-week visit.
TABLE 35
Summary of adherence to therapy
Outcome of non-randomised patients
The GPs of all 103 children who were registered at T–4 but who were not randomised at T0 were contacted towards the end of the study and asked to complete a questionnaire about each of their patients, as described in the protocol. As the exposure time since date of registration varied, data are summarised according to the time intervals for which the data were collected. The data presented for ‘up to 1 year’ are likely to be an underestimate of events because only the cumulative data over the period from registration until completion of the form were collected. Some patients with data reported over periods > 1 year may have had a number of their events during the first year from registration.
The questionnaire was kept very simple and short to try and maximise response. Nine (14.5%) children had at least one exacerbation that required a course of oral corticosteroids (Table 36). A total of 50 (80.6%) required at least one prescription of beta2 agonist after registration (Table 37). Unfortunately it was not possible to summarise the amount of beta2 agonist prescribed as the reporting of type of inhaler and amount prescribed was inconsistent and incomplete. In total, 18 (29.0%) patients were prescribed at least one additional treatment for asthma control (Table 38), with the majority (61%) of these being combination therapy (ICS plus long-acting beta2 agonist). Four (6.5%) children required an A&E visit (Table 39) and one (1.6%) child needed a hospital admission for their asthma (Table 40).
TABLE 36
Number of exacerbations requiring a course of oral corticosteroids since T–4 and time to first exacerbation
TABLE 37
Number of beta-2 agonists prescribed since T–4
TABLE 38
Prescriptions since T–4
TABLE 39
Number of A&E admissions due to respiratory problems since T–4
TABLE 40
Number of inpatient admissions due to respiratory problems since T–4
Publication Details
Copyright
Included under terms of UK Non-commercial Government License.
Publisher
NIHR Journals Library, Southampton (UK)
NLM Citation
Lenney W, McKay AJ, Tudur Smith C, et al.; MASCOT Study Group. Management of Asthma in School age Children On Therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety. Southampton (UK): NIHR Journals Library; 2013 Feb. (Health Technology Assessment, No. 17.4.) Chapter 3, Results.




