Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Westwood M, Al M, Burgers L, et al. A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD. Southampton (UK): NIHR Journals Library; 2013 Mar. (Health Technology Assessment, No. 17.9.)

A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD.
Show detailsA systematic review was conducted to summarise the evidence on the clinical effectiveness of NGCCT, for the diagnosis of clinically significant coronary artery stenosis in difficult-to-image patient groups with known or suspected CAD, and for treatment planning in patients with complex congenital heart disease. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care and the NICE Diagnostic Assessment Programme interim methods statement.18,19
Inclusion and exclusion criteria
Participants
Study populations eligible for inclusion were:
- Adults (≥ 18 years) with known (previously diagnosed who have symptoms that are no longer controlled by drug treatment and/or who are being considered for revascularisation) or suspected (chest pain or other suggestive symptoms) CAD, who are difficult to image (not currently candidates for CT imaging). Difficult-to-image patient groups defined a priori were:
- obesity [body mass index (BMI) of ≥ 30 kg/m2]
- high levels of coronary calcium (calcium score > 400)
- arrhythmias [including, but not limited to, atrial fibrillation (AF)]
- high heart rate (HHR) (> 65 b.p.m.)
- intolerance of beta-blockers
- previous stent implantation
- previous bypass graft(s).
[Difficult-to-image patients were not limited to these patient groups, but no other groups were identified during the review process. Following consultation with clinical experts, the definition of HHR (> 70 b.p.m.) specified in the protocol was broadened to avoid potential loss of relevant data, as identified studies frequently defined HHR as > 65 b.p.m.]
- Infants, children and adults diagnosed with complex congenital heart disease, including but not limited to:
- pulmonary atresia with MAPCA
- variants of anomalous pulmonary venous drainage (TAPVD, scimitar syndrome, etc.)
- aortic arch abnormalities (double aortic arch, vascular ring, etc.)
- lesions with both a vascular and airway component (pulmonary artery sling, tracheal stenosis, right aortic arch with aberrant subclavian artery, etc.)
- previously treated lesions where stents or pacemakers make MRI an unsuitable imaging strategy.
Setting
Relevant settings were secondary or tertiary care.
Interventions
Included interventions, described as ‘NGCCT’ throughout, were the following CT scanners:
- Discovery CT750 (GE Healthcare)
- Brilliance iCT (Philips Healthcare)
- Somatom Definition Flash (Siemens Healthcare)
- Aquilion ONE (Toshiba Medical systems).
No additional equivalent technologies were identified during the review process.
Comparators
The only relevant comparator for the assessment of difficult-to-image patients with CAD was ICA.
Relevant comparators, for the assessment of complex congenital heart disease, were 64-slice CT and conventional imaging (without CT).
Reference standard
Studies reporting the diagnostic accuracy of NGCCT for the detection of significant CAD were required to use ICA as the reference standard. Diagnostic accuracy was not considered a relevant outcome for studies of congenital heart disease.
Outcomes
Studies reporting the following outcomes were considered relevant for both clinical applications (CAD and congenital heart disease):
- impact of testing on treatment plan (e.g. surgical or medical management), where information on the appropriateness of the final treatment plan was also reported
- impact of testing on clinical outcome (e.g. angina, MI, CV mortality).
Studies reporting the following outcomes were considered relevant only for difficult-to-image patients with CAD:
- test accuracy
- indeterminacy (the number of patients in whom imaging failed to provide diagnostic information).
For included studies reporting any of the above outcome measures, the following outcomes were also recorded, if reported:
- acceptability of tests to patients
- adverse events associated with testing
- radiation dose associated with imaging.
Study design
The following study designs were eligible for inclusion:
- randomised or non-randomised controlled trials, in which participants were assigned to the intervention or comparator tests, for treatment planning, and outcomes were compared at follow-up
- randomised or non-randomised controlled trials in which participants were assigned to conventional imaging only, or conventional imaging plus high definition or 64-slice CT (congenital heart disease only).
No randomised or non-randomised controlled trials were identified. Therefore, the following observational study types were considered eligible for inclusion:
- cross-sectional test accuracy studies, where the intervention was compared with the reference standard (CAD only)
- observational studies reporting change to treatment plan or clinical outcome subsequent to high-definition CT (CAD and congenital heart disease) or 64-slice CT (congenital heart disease only).
Cross-sectional test accuracy studies were required to report the absolute numbers of true-positive (TP), false-negative (FN), false-positive (FP) and true-negative (TN) test results, or sufficient information to allow their calculation.
The following study/publication types were excluded:
- pre-clinical, animal and phantom studies
- reviews, editorials, and opinion pieces
- case reports
- studies reporting only technical aspects of the test, or image quality
- studies with < 10 participants.
Search strategy
Search strategies were based on target condition and intervention, as recommended in the CRD guidance for undertaking reviews in health care and the Cochrane handbook for diagnostic test accuracy reviews.18,20,21
The following databases were searched for relevant studies from 1 January 2000 to 9 March 2011:
- MEDLINE (2000 to February week 2 2011) (OvidSP)
- MEDLINE In-Process and Other Non-Indexed Citations and Daily Update (2000 to 16 February 2011) (OvidSP)
- EMBASE (2000 to week 6 2011) (OvidSP)
- Cochrane Database of Systematic Reviews (CDSR) (The Cochrane Library Issue 1 : 2011) (Wiley)
- Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1 : 2011) (Wiley)
- Database of Abstracts of Reviews of Effects (DARE) (2000 to 9 March 2011) (CRD website)
- NHS Economic Evaluation Database (NHS EED) (2000 to 9 March 2011) (CRD website)
- Health Technology Assessment database (HTA) (2000 to 9 March 2011) (CRD website)
- Science Citation Index (SCI) (2000 to 5 March 2011) (Web of Science).
Supplementary searches were undertaken on the following resources to identify grey literature, completed and ongoing trials:
- National Institutes of Health Clinicaltrials.gov (2000 to 9 March 2011) (Internet): www.clinicaltrials.gov/
- Current Controlled Trials (2000 to 9 March 2011) (Internet): www.controlled-trials.com/
- World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (2000 to 9 March 2011) (Internet): www.who.int/ictrp/en/
Searches were undertaken to identify studies of NGCCT in the diagnosis of CAD and assessment of congenital heart disease. Search strategies were developed specifically for each database and the keywords associated with CAD and congenital heart defects were adapted according to the configuration of each database. Searches took into account generic and other product names for the intervention. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. Full search strategies are reported in Appendix 1.
Electronic searches were undertaken for the following conference abstracts:
- American College of Cardiology (ACC) (2006–10) (Internet): www.cardiosource.org/Meetings/Previous-Meetings-OLD.aspx
- Society of Cardiovascular Computed Tomography (SCCT) (2006–10) (Internet): www.scct.org/annualmeeting/2010/index.cfm
- European Society of Cardiology (ESC) (2006–10) (Internet): www.escardio.org/congresses/past_congresses/Pages/past-ESC-congresses.aspx
- American Heart Association (AHA) (2007–10) (Internet):
Identified references were downloaded in EndNote X4 software (Thomson Reuters, CA, USA) for further assessment and handling.
References in retrieved articles were checked for additional studies.
Inclusion screening and data extraction
Two reviewers (MW and HR) independently screened the titles and abstracts of all reports identified by searches and any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant, after discussion, were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full-paper-screening stage are presented in Appendix 5.
Studies listed in submissions from the manufacturers of NGCCT were first checked against the project reference database, in EndNote X4; any studies not already identified by our searches were screened for inclusion following the process described above. Studies referenced by manufacturers and excluded at the full-paper-screening stage are noted in Appendix 5. Appendix 5 also includes a list of studies, referenced by manufacturers, which were excluded at title and abstract screening.
Where there was uncertainty regarding possible overlap between study populations, authors were contacted for clarification.
Data were extracted on study details (study design, participant recruitment, setting, funding, stated objective, and categories of participants relevant to this assessment for whom data were reported); study participants (total number of participants, number of participants in each relevant group, study inclusion criteria, study exclusion criteria, and participant characteristics relevant to CV risk for the relevant participant groups or the whole study population); assessed technology and reference standard (technical details of the test, any use of beta-blockers prior to scanning, details of who interpreted tests and how, threshold used to define a positive test); and study results. All studies included in the review were diagnostic accuracy studies and the results extracted were unit of analysis (patient, artery or arterial segment); numbers of TP, FN, FP and TN test results; numbers of patients, arteries or segments classified as non-diagnostic by NGCCT; and radiation exposure associated with imaging. All data were extracted by one reviewer, using a piloted, standard data extraction form and checked by a second; any disagreements were resolved by consensus. Full data extraction tables are provided in Appendix 4.
Quality assessment
All studies included in the systematic review were test accuracy studies. The QUADAS tool,22 is recommended for assessing the methodological quality of test accuracy studies.18,20 However, a revised version of QUADAS (QUADAS-2) has recently been published.23 QUADAS-2 more closely resembles the approach and structure of the Cochrane risk of bias tool. It is structured into four key domains covering participant selection, index test, reference standard, and the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear) and the tool provides signalling questions, in each domain, to aid reviewers in reaching a judgement. The participant selection, index test and reference standard domains are also, separately, rated for concerns regarding the applicability of the study to the review question (low, high or unclear). Thus, QUADAS-2 separates bias from external validity (applicability) and does not include any items which assess only reporting quality. Guidance for the use of QUADAS-2 will emphasise the need to tailor the tool to specific projects and the need to avoid the use of summary quality scores. Further information on QUADAS-2 is available at the QUADAS website: www.bris.ac.uk/quadas/quadas-2.
Review-specific guidance was produced for the use of QUADAS-2 in this assessment and is reported in Appendix 2. The version of QUADAS-2 used in this assessment included only the risk of bias components, as it was considered that the inclusion criteria matched the review question and that questions of applicability were, therefore, not relevant.
The results of the quality assessment are summarised and presented in tables and graphs in the results of the systematic review (see Chapter 3, Results) and are presented in full, by study, in Appendix 3. No diagnostic accuracy data set included in this assessment was of sufficient size to allow statistical exploration of between-study heterogeneity based on aspects of risk of bias. The findings of the quality assessment were also used to inform recommendations for future research.
Methods of analysis/synthesis
All studies included in the systematic review were test accuracy studies in difficult-to-image patients with CAD. Results were summarised by patient group (e.g. obese, HHR, high coronary calcium score, etc.) and further stratified by unit of analysis (patient, artery or arterial segment). For all included studies, the absolute numbers of TP, FN, FP and TN test results, as well as sensitivity and specificity values, with 95% confidence intervals (CIs), were presented in results tables, for each patient group reported. Data on the numbers of non-diagnostic tests and radiation exposure were also included in the results tables and described in text summaries.
Where groups of similar studies (same patient group and unit of analysis) included four or more data sets, summary receiver operating characteristic (SROC) curves and summary estimates of sensitivity and specificity, with 95% CIs, were calculated using the bivariate modelling approach;24,25 four data sets is the minimum requirement to fit models of this type. Analyses were conducted in Stata 10 (StataCorp LP, College Station, TX, USA), using the ‘metandi’ function.26 In two cases, a bivariate model could not be fitted because the number of studies was small (four), 2 × 2 data contained one or more zero values, and between-study heterogeneity was low. In these cases, pooled estimates of sensitivity and specificity, with 95% CIs, were calculated using a random-effects model; these analyses were conducted using Meta-DiSc 1.4 (Hospital Ramon y Cajal and Universidad, Madrid, Spain)27 and forest plots were constructed, showing the sensitivity and specificity estimates from each study together with pooled estimates. No distinction was made between patients with known or suspected CAD as per-patient data sets were generally small, with low to moderate between-study heterogeneity. In addition, ‘known’ and ‘suspected’ CAD were often poorly defined by the included studies.
Between-study heterogeneity was assessed using the chi-squared test and inconsistency was quantified using the I2-statistic.28 There were no data sets of sufficient size (minimum 10) to allow statistical exploration of sources of heterogeneity by including additional co-variables in the SROC model.
Where meta-analysis was considered unsuitable for the data identified (e.g. because of the heterogeneity and/or small numbers of studies), studies were summarised using a narrative synthesis. Text and tables were stratified by patient group.
No data were identified on the effects of NGCCT on treatment planning and/or clinical outcome, adverse events associated with testing, or acceptability of tests to patients.
Results
The literature searches of bibliographic databases identified 3986 references. After initial screening of titles and abstracts, 119 were considered to be potentially relevant and ordered for full-paper screening. A further 11 papers were ordered based on screening of submissions from industry and two studies cited in trials registry entries were also obtained. Of the total of 132 publications considered potentially relevant, five29–33 could not be obtained within the timescale of this assessment; these were held in British Library stacks, which are currently closed for asbestos removal or they were not held by the British Library. Figure 1 shows the flow of studies through the review process, and Appendix 5 provides details, with reasons for exclusions, of all publications excluded at the full-paper-screening stage.
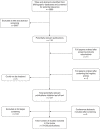
FIGURE 1
Flow of studies through the review process.
Based on the searches and inclusion screening described above, 23 publications of 21 studies were included in the review. Hand-searching of conference proceedings resulted in the inclusion of a further three studies, which were published in abstract form only (see Figure 1).34–36 A total of 24 studies in 26 publications were, therefore, included in the review (see Table 1).
TABLE 1
Included studies
All included studies were test accuracy studies conducted in patients with known or suspected CAD. No study reported data on changes to patient management or outcomes, test-related adverse events or patient preferences. No studies were identified, of patients with congenital heart disease, which met the inclusion criteria of the review.
Nineteen of the 24 included studies reported using Somatom Definition (a similar previous model of Somatom Definition Flash), and one study used Somatom Definition Flash.34 Three studies did not specify the instrument used;36–38 the authors of one of these37 had used Somatom Definition in an earlier study, which was also included in this review,39 and another study was later confirmed by the manufacturer to have used Discovery CT750 HD.38 The remaining study used Aquilion ONE.40 This study assessed patients who had previous stent implantation for in-stent restenosis.40
All included studies were published in 2006 or later.
Eleven38,39,41–46,48,55,59 of the 21 included studies reported data on difficult-to-image patients as subgroup analyses. Six of these studies39,41–45 reported sufficient information to allow calculation of the proportion of the total participants who had one or more difficult-to-image criteria; the mean percentage was 41.5% (range 28–51%). Table 1 shows the details of included studies and the specific difficult-to-image patient groups for which each publication reported data. Further details of the characteristics of study participants and the technical details of the conduct of the index test (NGCCT) and reference standard and their interpretation are reported in the data extraction tables presented in Appendix 4.
Accuracy of new-generation cardiac computed tomography for the detection of coronary artery disease in obese patients
One study42 assessed the performance of NGCCT for the detection of significant stenosis (defined as ≥ 50% vessel narrowing) in obese patients with suspected CAD or suspected progression of known CAD; obese patients were defined as those with a BMI of ≥ 30 kg/m2. This study reported high sensitivity and specificity values; however, data were only reported per arterial segment; 543 data points (segments) were derived from 44 patients; data of this type are potentially problematic in that they assume independence of data sets derived from the same patient, which is unlikely to be true in practice, and may thus result in underestimation of variance. Some patients with additional characteristics which may contribute to difficulty in imaging [13 patients who had previous bypass graft(s) were excluded from this study, but it was not clear how many, if any, of these patients were also obese]. Therefore, the potential for biased accuracy assessments due to inappropriate exclusions could not be judged. Eleven (2%) of the arterial segments assessed in this study were classified as non-diagnostic and, although these segments appear to have been included in the analysis, it was unclear how they were classified. Table 2 summarises the QUADAS-2 assessment and the results of this study are summarised in Table 3.
TABLE 2
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in obese patients
TABLE 3
Accuracy of NGCCT for the detection of CAD in obese patients
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with high calcium score
For the purpose of this assessment, levels of coronary calcium likely to result in a patient being difficult to image were classified as a high calcium score (HCS) > 400. Four studies46,48,55,59 reported 10 data sets describing the accuracy of NGCCT for the detection of CAD in patients with HCS. Three46,48,55 of the four studies reported only per-segment or per-artery accuracy data; data of this type are potentially problematic in that they assume independence of data sets derived from the same patient, which is unlikely to be true in practice, and may thus result in underestimation of variance. All studies excluded some patients with additional characteristics which may contribute to difficulty in imaging [e.g. previous bypass surgery (four studies46,48,55,59), previous stent implantation (three studies48,55,59)]. However, no study reported the numbers of excluded patients who also had HCS. Therefore, the potential for biased accuracy assessments due to inappropriate exclusions could not be judged. One study48 excluded non-diagnostic segments from its analysis; however, even if all of these segments were in the HCS patient group considered in this section, they would represent a maximum of 7% of the segments analysed; the effect of their exclusion on the reported accuracy estimates is, therefore, likely to be minimal. Table 4 summarises the QUADAS-2 assessments for these studies and Table 5 summarises individual study results.
TABLE 4
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in patients with HCS
TABLE 5
Accuracy of NGCCT for the detection of CAD in patients with HCS
All four studies reported per-segment data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 92.7% (95% CI 88.3% to 95.6%) and 90.6% (95% CI 80.6% to 95.8%), respectively. The I2-statistic indicated moderate between-study heterogeneity in the estimates of sensitivity (I2 = 54.2%) and high between-study heterogeneity in the estimates of specificity (I2 = 92.2%). Figure 2 shows the associated SROC curve for per-segment data in patients with HCS; the open circles, representing individual study results, are scaled to indicate relative sample size. In contradiction with the I2-values, this plot indicates a lack of between-study heterogeneity, with individual study results ‘clustered’ in the upper left quadrant; this contradiction is indicative of the limited utility of statistic tests for heterogeneity in very small sample sizes.

FIGURE 2
Summary receiver operating characteristic curve for per-segment data in studies of patients with HCS. HSROC, hierarchical summary receiver operating characteristic.
Two studies48,59 also reported accuracy data on a per-artery basis; these results are summarised in Table 5.
Only one study reported per-patient estimates of accuracy and these were of limited value as all 12 included patients were classified as TP using ≥ 50% vessel narrowing as the threshold to define significant stenosis.59 This same study59 also reported data for all three units of analysis (patient, artery and segment) using a threshold of > 75% vessel narrowing to define significant stenosis; sensitivity and specificity estimates were broadly similar to those obtained using the ≥ 50% vessel narrowing threshold and are reported in Table 5. However, using the higher threshold, estimates of per-patient accuracy could be calculated, sensitivity 90.9% (95% CI 58.7% to 99.8%) and specificity 100% (95% CI 25.0% to 100%); the wide CIs reflect the very small number of patients included in the analysis.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with arrhythmias
Five studies35,47,49,54,56 reported 10 data sets describing the accuracy of NGCCT for the detection of CAD in patients with arrhythmias. Three35,49,54 of the five studies reported using no additional (extra to the patient's normal medication) beta-blockers prior to scanning, and beta-blocker use was unclear in a fourth study.56 The fifth study47 used beta-blockers prior to scanning in 40% of patients, and excluded 14% of otherwise eligible patients because they were unresponsive to beta-blockers and had rapid AF (> 100 b.p.m.) at the time of scanning; this study was judged to be at high risk of bias with respect to participant selection. In one study,54 only 31% of eligible patients received the reference standard and were included in the analysis; this study was judged to be at high risk of bias, with respect to the flow of patients through the study, in this case due to partial verification bias. Table 6 summarises the QUADAS-2 assessments for these studies and Table 7 summarises individual study results. All but one of these studies were conducted in patients with AF; the fifth study included patients who were ‘without stable sinus rhythm during scanning’.
TABLE 6
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in patients with arrhythmias
TABLE 7
Accuracy of NGCCT for the detection of CAD in patients with arrhythmias
All four studies35,47,49,54,56 of patients with AF reported per-patient data. The pooled estimates of sensitivity and specificity (derived from these data using a DerSimonian–Laird random-effects model, in which 0.5 was added to all cells to allow for zero values) were 97.7% (95% CI 88.0% to 99.9%) and 81.7% (95% CI 71.6% to 89.4%), respectively. Between-study heterogeneity was low: the I2-values were 1.4% for sensitivity and zero for specificity. No SROC curve was fitted as study results were too similar. Figure 3 illustrates the per-patient sensitivity and specificity values for each study, with pooled estimates. The filled circles, representing individual studies, are scaled to indicate relative sample sizes and the wide CIs reflect the generally small sample sizes involved. One study reported the proportion of patients with AF who had non-diagnostic images (5%).47

FIGURE 3
Forest plot of per-patient sensitivity and specificity of NGCCT for the detection of CAD in patients with AF.
One study also reported per-artery data and these results are described in Table 7.47
Four studies35,49,54,56 reported per-segment data. These data were more heterogeneous than was the case for the per-patient data: the I2-values were 79.6% for sensitivity and 89.5% for specificity. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 87.4% (95% CI 68.3% to 95.7%) and 96.0% (95% CI 91.2% to 98.2%), respectively. Figure 4 shows the associated SROC curve for per-segment data in patients with arrhythmias, with the open circles, representing individual study results, being scaled to indicate relative sample size.

FIGURE 4
Summary receiver operating characteristic (SROC) curve for per-segment data in studies of patients with arrhythmias. HSROC, hierarchical summary receiver operating characteristic.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with high heart rate
Eight studies39,41,44–46,48,55,59 reported 24 data sets describing the accuracy of NGCCT for the detection of CAD in patients with HHRs. The five studies39,41,44,45,55 that reported the heart rates observed in patients classified as HHR reported mean heart rates of between 76 ± 9 and 88.8 ± 8.4 b.p.m. Three studies46,48,55 reported only per-segment or per-artery accuracy data. Data of this type are potentially problematic in that they assume independence of data sets derived from the same patient; this is unlikely to be true in practice, and may thus result in underestimation of variance. With the exception of one study,60 all studies in this group excluded patients with previous revascularisations (previous stent implantation and/or previous bypass graft); one study44 was a retrospective analysis of selected patients who had undergone both CT and ICA and was judged to be at high risk of bias. Two studies39,45 also excluded patients with AF. The first of these39 excluded > 10% of otherwise eligible participants and was, therefore, judged to be at high risk of bias with respect to participant selection. In the second of these studies45 only 48% of patients received the reference standard and were included in the analysis; this study was therefore also judged to be at high risk of bias with respect to the flow of patients through the study, owing to partial verification bias. Table 8 summarises the QUADAS-2 assessments for these studies and Table 9 summarises individual study results. Studies in this group defined HHR as ≥ 66, ≥ 65 or ≥ 70 b.p.m.; for the purposes of meta-analysis, these studies were treated as a single group assessing the accuracy of NGCCT in patients with a HR of ≥ 65 b.p.m. The baseline use of beta-blockers by study participants varied (see Appendix 4, Inclusion/exclusion criteria and participant characteristics of included studies), but all studies in this section reported that no additional beta-blockers were given prior to CT scanning.
TABLE 8
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in patients with HHR
TABLE 9
Accuracy of NGCCT for the detection of CAD in patients with HHRs
Five studies39,41,44,45,59 reported per-patient data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 97.7% (95% CI 93.2% to 99.3%) and 86.3% (95% CI 80.2% to 90.7%), respectively; there was moderate between-study heterogeneity in both the estimates of sensitivity (I2 = 39.0%) and the estimates of specificity (I2 = 49.8%). Figure 5 shows the SROC curve for per-patient data in patients with HHR. One study45 reported per-patient accuracy data for multiple definitions of HHR; these results are summarised in Table 9. One study39 reported the proportion of patients with HHR who had non-diagnostic images (6.8%).

FIGURE 5
Summary receiver operating characteristic curve for per-patient data in studies of patients with HHR. HSROC, hierarchical summary receiver operating characteristic.
Four studies39,44,48,59 reported per-artery data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 93.7% (95% CI 87.8% to 96.9%) and 92.4% (95% CI 83.3% to 96.8%), respectively; between-study heterogeneity was low (zero) for the estimates of sensitivity, but high for estimates of specificity (I2 = 83.7%). Figure 6 shows the SROC curve for per-artery data in patients with HHR.

FIGURE 6
Summary receiver operating characteristic curve for per-artery data in studies of patients with HHR. HSROC, hierarchical summary receiver operating characteristic.
All eight studies reported accuracy data by arterial segment, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 92.7% (95% CI 89.3% to 95.1%) and 95.7% (95% CI 92.8% to 97.4%), respectively; there was high between-study heterogeneity in both the estimates of sensitivity (I2 = 67.1%) and the estimates of specificity (I2 = 92.8%). Figure 7 shows the SROC curve for per-segment data in patients with HHR. One study45 reported per-segment accuracy data for multiple definitions of HHR; these results are summarised in Table 9.

FIGURE 7
Summary receiver operating characteristic curve for per-segment data in studies of patients with HHR. HSROC, hierarchical summary receiver operating characteristic.
One study59 reported additional data for all three units of analysis (patient, artery and segment) using a threshold of > 75% vessel narrowing to define significant stenosis; sensitivity and specificity estimates were broadly similar to those obtained using the ≥ 50% vessel narrowing threshold and are reported in Table 9.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in beta-blocker intolerance
No studies of the accuracy of NGCCT for the detection of CAD in patients who were intolerant to beta-blockers were identified.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in stented patients
Seven studies34,36,38,40,50–52 reported 10 data sets describing the accuracy of NGCCT for the detection of CAD in patients with previous stent(s) implantation. Three studies34,38,52 reported only per-stent or stented-lesion accuracy data; data of this type are potentially problematic in that they assume independence of data sets derived from the same patient, which is unlikely to be true in practice, and may thus result in underestimation of variance. Four studies excluded some patients with additional characteristics that may contribute to difficulty in imaging. These included HHR and intolerance to beta-blockers,40 previous bypass graft36 and irregular heart rhythm/AF.51,52 The last of these studies51 also excluded patients with stents in bypass grafts, resulting in the exclusion of > 10% of otherwise eligible participants and a classification of high risk of bias with respect to participant selection. This same study51 excluded non-diagnostic stents from its analyses; however, as the distribution of these stents between patients was not reported, their potential effect on per-patient accuracy estimates could not be assessed. Table 10 summarises the QUADAS-2 assessments for these studies and Table 11 summarises individual study results. Six34,38,40,50–52 of the seven studies considered only in-stent restenosis and the seventh36 considered both in-stent restenosis and stenosis of native vessels.
TABLE 10
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in patients with previous stent(s)
TABLE 11
Accuracy of NGCCT for the detection of CAD in patients with previous stent(s)
Four studies36,40,50,51 reported per-patient data, using a threshold of ≥ 50% or > 50% vessel narrowing to define significant stenosis. The pooled estimates of sensitivity and specificity, derived from these data using a DerSimonian and Laird random-effects model, where 0.5 was added to all cells to allow for zero values, were 96.0% (95% CI 88.8% to 99.2%) and 81.6% (95% CI 74.7% to 87.3%), respectively. Between-study heterogeneity was low: the I2-values were 19% for sensitivity and zero for specificity. No SROC curve was fitted as study results were too similar. Figure 8 illustrates the per-patient sensitivity and specificity values for each study, with pooled estimates. One study40 reported the proportion of patients with previous stent implantation who had non-diagnostic images (9%).

FIGURE 8
Forest plot of per-patient sensitivity and specificity of NGCCT for the detection of CAD in patients with previous stent(s).
Six studies34,38,40,50–52 reported accuracy data by stent or stented lesion. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 93.6% (95% CI 86.1% to 97.2%) and 91.0% (95% CI 87.3% to 93.7%), respectively; between-study heterogeneity was low (zero) for the estimates of sensitivity, and moderate for estimates of specificity (I2 = 35.1%). Figure 9 shows the SROC curve for per-stent/stented-lesion data in patients with previous stent(s). One study38 reported additional data, using a threshold of ≥ 70% narrowing to define significant in-stent restenosis; sensitivity and specificity estimates were broadly similar to those obtained using the ≥ 50% narrowing threshold and are reported in Table 11.

FIGURE 9
Summary receiver operating characteristic curve for per stent/stented-lesion data in studies of patients with previous stent(s). HSROC, hierarchical summary receiver operating characteristic.
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease in patients with coronary artery bypass graft
Three studies34,37,58 reported six data sets describing the accuracy of NGCCT for the detection of CAD in patients with previous bypass graft(s). Two34,37 of the three studies included in this section were published only as conference abstracts. In these cases, the minimal methodological information reported made it difficult to assess the risk of bias; this is reflected in the high proportion of unclear (?) judgements. The study that was reported as a full paper58 reported only accuracy results per segment. Table 12 summarises the QUADAS-2 assessments for these studies. A variety of different units of analysis were used, including bypass grafts, segments of bypass grafts, segments of native vessels and/or distal run-off, and patients; results are summarised in Table 13. Only one study37 assessed the per-patient accuracy of NGCCT for the detection of any significant stenosis (≥ 50% narrowing) in a bypass graft, distal run-off, or native vessel. The per-patient sensitivity estimated from this study was 96.4% (95% CI 87.5% to 99.6%) and the per-patient specificity was 87.0% (95% CI 66.4% to 97.2%).
TABLE 12
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in patients with previous bypass graft(s)
TABLE 13
Accuracy of NGCCT for the detection of CAD in patients with previous bypass graft(s)
Accuracy of new-generation cardiac computed tomography for detection of coronary artery disease (multiple criteria)
Three studies reported the accuracy of NGCCT in patients with different combinations of difficult-to-image criteria.42,52,58 Two studies52,58 only reported per segment or per lesion accuracy data. The only study58 to report per-patient data excluded non-diagnostic segments and, as it was unclear how these were distributed between patients, it was not possible to assess how their exclusion may have affected per-patient results. Table 14 summarises the QUADAS-2 assessments for these studies and Table 15 summarises individual study results. Units of analysis differed between studies and only one study43 reported per-patient data. The per-patient sensitivity estimated from this study was 91.7% (95% CI 61.5% to 99.8%) and the per-patient specificity was 88.2% (95% CI 72.5% to 96.7%), for patients with HR of > 65 b.p.m. and/or AF.
TABLE 14
QUADAS-2 results for studies of the accuracy of NGCCT for the detection of CAD in patients with combinations of difficult-to-image criteria
TABLE 15
Accuracy of NGCCT for the detection of CAD in patients with combinations of difficult-to-image criteria
Summary
All 24 studies (26 publications, see Table 1) included in the systematic review were diagnostic test accuracy studies that reported data on the performance of NGCCT in difficult-to-image patients with known or suspected CAD. Figure 10 provides a summary of the risk of bias assessments for these studies. The majority of studies were judged to be at low risk of bias with respect to the reference standard domain of QUADAS-2; this reflects the specification, in the inclusion criteria of the review, of a single acceptable reference standard (ICA). Unclear ratings for this domain mainly reflected poor reporting of the interpretation of the reference standard and uncertainty whether or not those interpreting ICA were blinded to the index test results. The judgement of risk of bias with respect to patient selection was problematic and this is reflected in the high proportion of unclear ratings. The unclear rating frequently related to uncertainty regarding the potential impact of inappropriate exclusions. Difficult-to-image patient groups were frequently reported as subgroups within larger studies, with those who had one or more additional criteria, which may contribute further to difficulty in imaging, being excluded from the study (e.g. a study reporting data for general CAD patients and a subgroup of patients with HHR may have excluded patients with previous revascularisations). In addition, the numbers/proportion of patients excluded in this way were frequently not reported. Inclusion of multiple measurements per patient (per-arterial segment, per-artery or per-stent data) was a common problem in the index test domain. Where studies excluded non-diagnostic arterial segments from their analyses, the potential impact of these exclusions was frequently unclear because their distribution between patients was not reported. For example, if a positive test for per-patient data is defined as one or more positive segments, exclusion of a non-diagnostic segment which is actually stenosed may result in misclassification of the whole patient as TN (i.e. a reduced estimate of the number of FN patients).

FIGURE 10
Summary of QUADAS-2 assessments.
Where per-patient estimates of test accuracy were possible, these were generally high. Pooled estimates of sensitivity and specificity are summarised in Table 16. In particular, all per-patient estimates of sensitivity were > 95%, indicating that NGCCT could reliably rule out significant stenosis and thus potentially avoid invasive investigations such as ICA. Furthermore, although there were no data specifically for beta-blocker intolerant patients, it should be noted that no study reporting per-patient data for patients with HHR used additional beta-blockers prior to scanning. It may therefore be inferred that NGCCT could reasonably be used to image patients who are intolerant to beta-blockers who could not otherwise be reliably imaged by 64-slice CT.
TABLE 16
Summary of test accuracy results
With the exception of one small study, data on the accuracy of NGCCT in patients with high coronary calcium scores, previous bypass grafts, or obesity were limited to per arterial segment or per-artery data. Sensitivity estimates remained high (> 90% in all but one study).
Data on the number of difficult-to-image patients in whom NGCCT was non-diagnostic were sparse; where numbers of non-diagnostic images were reported, these were often for the whole study population, rather than the difficult-to-image subgroup. Three studies did report subgroup-specific non-diagnostic image rates in different populations; these were 5% for patients with arrhythmias,47 6.8% for patients with HHR44 and 9% for patients with previous stent implantation.40
- Assessment of clinical effectiveness - A systematic review and economic evaluati...Assessment of clinical effectiveness - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
- Health economic results - Saline in Acute Bronchiolitis RCT and Economic evaluat...Health economic results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review
- Methods - Preoperative intravenous iron for anaemia in elective major open abdom...Methods - Preoperative intravenous iron for anaemia in elective major open abdominal surgery: the PREVENTT RCT
- List of abbreviations - A systematic review and economic evaluation of new-gener...List of abbreviations - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
- Methods - The clinical effectiveness and cost-effectiveness of brief interventio...Methods - The clinical effectiveness and cost-effectiveness of brief intervention for excessive alcohol consumption among people attending sexual health clinics: a randomised controlled trial (SHEAR)
Your browsing activity is empty.
Activity recording is turned off.
See more...