Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Westwood M, Al M, Burgers L, et al. A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD. Southampton (UK): NIHR Journals Library; 2013 Mar. (Health Technology Assessment, No. 17.9.)

A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD.
Show detailsSearch strategy
Searches were undertaken to identify cost-effectiveness studies of NGCCT. As with the clinical effectiveness searching, search strategies were developed specifically for each database and searches took into account generic and other product names for the intervention. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. Full search strategies are reported in Appendix 1.
The following databases were searched for relevant studies from 1 January 2000 to 21 March 2011: MEDLINE (2000 to March week 2 2011) (OvidSP)
- MEDLINE In-Process and Other Non-Indexed Citations and Daily Update (2000 to 17 March 2011) (OvidSP)
- EMBASE (2000 to week 11 2011) (OvidSP)
- NHS EED (2000 to 9 March 2011) (CRD website)
- Health Economic Evaluation Database (HEED) (2000 to 9 March 2011) (Wiley) http://onlinelibrary.wiley.com/book/10.1002/9780470510933
- Paediatric Economic Database Evaluation (PEDE) (2000 to 5 March 2011) (internet) http://pede.ccb.sickkids.ca/pede/search.jsp
Supplementary searches on catheter angiography were undertaken on the following resources to identify guidelines and guidance:
- National Guideline Clearinghouse (NGC) (2005 to 16 March 2011) (Internet) www.guideline.gov/
- International Guideline Library (G-I-N) (2005 to 16 March 2011) www.g-i-n.net
- NICE guidance (up to 16 March 2011) (internet) http://guidance.nice.org.uk/
- Turning Rsearch Into Practice (TRIP) database (2005 to 16 March 2011) (internet) www.tripdatabase.com/
- HTA (2005 to 16 March 2011) (CRD website).
Identified references were downloaded in EndNote X4 software for further assessment and handling. References in retrieved articles were checked for additional studies.
Cost-effectiveness of new-generation cardiac computed tomography in coronary artery disease
Model structure and methodology
In order to assess the cost-effectiveness of NGCCT for difficult-to-image patient groups with CAD a model was developed. This model provides a framework for the synthesis of data from the review of clinical effectiveness of NGCCT (see Chapter 3, Results), which only consisted of accuracy data, and other relevant parameters, such as costs and effects of complications due to procedures, the long-term costs and effects of patients with CAD, and the risk of cancer from radiation exposure, in order to evaluate the potential long-term cost-effectiveness of NGCCT.
The cost-effectiveness of NGCCT for difficult-to-image patient groups is estimated for two CAD populations: the suspected CAD population and the known CAD population. Patients suspected of CAD are patients who have chest pain or other symptoms suggestive of CAD. Patients with known CAD are patients who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or being considered for revascularisation. The use of NGCCT has different purposes in the two CAD populations: for the suspected CAD population the purpose is to diagnose patients with CAD and for the known CAD population the purpose is to aid decision-making regarding a revascularisation.
The overall decision problem for which we aimed to develop a model can be subdivided into separate components. As for most of these components models were already available, we decided to combine five models to estimate the cost-effectiveness of the NGCCT:
- a decision tree that models the diagnostic pathway (see below, Diagnostic model)
- an alive–dead Markov model for ‘healthy’ patients without CAD (see below, Healthy population Markov model)
- a simple stroke model to estimate the impact of test and treatment-related stroke (see below, Stroke model)
- a model for the prognosis of patients with CAD (see below, EUROPA)
- a model constructed by the Centre for Health Economics, University of York to model the impact of imaging due to radiation on cancer morbidity and mortality, hereafter referred to as the York Radiation Model (YRM)61 (see below, York Radiation Model).
The comparator used for the evaluation of suspected or known CAD in difficult-to-image patients was ICA (see Chapter 3). Three strategies were evaluated in this assessment. The first strategy (ICA only) is a strategy through which patients with suspected or known CAD only undergo an ICA. Although ICA is the reference standard test and is assumed to be 100% sensitive and specific, it is associated with a risk of serious complications, including death, non-fatal MI and stroke. NGCCT does not have a sensitivity and specificity of 100% and thus is less accurate than the ICA. The second strategy (NGCCT–ICA) evaluates the combination of cardiac CT using the new-generation technologies and ICA. Cardiac CT is first performed in all patients and patients with a positive CT scan then undergo an ICA.3 This additional test will reveal any patients with a false-positive CT test result but it also provides other information that a CT currently does not.3 The third strategy (NGCCT only) uses only NGCCT to diagnose patients.
The five models used in the analyses are described, in detail, below. The stochastic analyses are based on cohort simulations. To investigate decision uncertainty, second-order uncertainty microsimulations were run. All costs and effects were discounted by 3.5%. The model incorporated a lifetime horizon to estimate outcomes in terms of quality-adjusted life-years (QALYs) and costs from the perspective of the NHS. Only health effects of patients were included.
Diagnostic model
The diagnostic pathway was modelled using a modified version of the CE-MARC model, developed by Walker (University of York, 2011, personal communication) which is based on the CE-MARC study.62 The CE-MARC study62 compared CV MRI with other diagnostic tests. Modification of the original CE-MARC model was necessary because the test strategies considered in this assessment did not correspond with the test strategies used in the original model. Furthermore, they did not include the treatment medication-only option required for our suspected CAD population. Our model identifies patients as TP, TN, FP and FN depending on the diagnostic performance of the test or test strategy and the prior likelihood of the test outcome. Furthermore, it estimates the mortality and morbidity of the tests and the interventions.
Decision trees for this process are shown in Figures 11–13 for patients with suspected CAD and in Figures 16–18 for patients with known CAD. Two versions of the diagnostic model were created because the known (two-treatment model) and suspected CAD (three-treatment model) populations are treated differently after a positive test outcome. The disease progression of the survivors of the tests and revascularisation procedures was modelled with the disease progression model (see EUROPA, below). We assumed that the tests were performed immediately after each other without any time delay.

FIGURE 11
Coronary artery disease suspected population: ICA only.

FIGURE 13
Coronary artery disease suspected population: NGCCT.

FIGURE 16
Known CAD: ICA only.
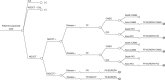
FIGURE 18
Known CAD: NGCCT only.

FIGURE 12
Coronary artery disease suspected population: NGCCT-ICA.
Diagnostic model for patients with suspected coronary artery disease
The purpose of testing patients with suspected CAD (based on clinical symptoms) is to diagnose those patients and give, when necessary, appropriate treatment.
The prior likelihood of having CAD in patients with suspected CAD is assumed to be 10–29%, based on the clinical guideline Chest pain of recent onset.63 This prior likelihood is based on some patient characteristics (age, gender, diabetes, smoking and hyperlipidaemia, and either non-anginal chest pain, atypical angina or typical angina). According to the guideline, in these patients, first a CT calcium scoring is performed and the patients referred for 64-slice CT (i.e. our population) have a score of 1–400. Patients with a higher prior likelihood than 10–29% should be referred for ICA. Some difficult-to-image subgroups could have a higher prior likelihood but how much higher is unknown. Therefore, we performed a scenario analysis where the prior likelihood was set at 30% for all subgroups. Table 17 summarises the prior likelihood of CAD in the known and suspected CAD populations.
TABLE 17
Prevalence in CAD populations
The sensitivity and specificity of ICA was assumed to be 100%, as in Mowatt et al.3 The systematic review performed for this assessment provided the estimates of the sensitivity and specificity for the NGCCT. As described in Chapter 3, Summary, estimates of sensitivity and specificity differed for the different difficult-to-image patient groups. The sensitivity and specificity of the NGCCT in the beta-blocker-intolerant patient group were assumed to be the same as the sensitivity and specificity in patients with a HHR. As beta-blockers are used to lower the heart rate of the patients, it is not the intolerance itself that makes the patient difficult to scan but rather the fact that such a patient may have a heart rate that is too high during the scan; studies reporting per-patient sensitivity and specificity in patients with a HHR did not use beta-blockers prior to scanning. Table 18 shows the sensitivity and specificity estimates for the NGCCT in the different difficult-to-image patient groups.
TABLE 18
New-generation cardiac computed tomography accuracy estimates (subgroup specific)
The result of the test and the presence of the disease determine whether a patient is classified as TP, TN, FP or FN (illustrated in Figure 14). The three strategies (ICA only, NGCCT only and NGCCT–ICA) all have other properties and therefore test outcomes differ by strategy. The four outcomes were calculated using the following formulae: TP, prior likelihood × sensitivity; TN, (1 – prior likelihood) × specificity; FP, (1 – prior likelihood) × (1 – specificity); FN, prior likelihood × (1 – sensitivity). Possible test outcomes are described by strategy.
FIGURE 14
A 2 × 2 table for patients with suspected CAD.
Patients with suspected CAD who have a positive test result are thought to have CAD according to the test and need to be treated with medication only or a revascularisation. A negative test result implies that the patient with suspected CAD does not have the disease and does not need to be treated.
- ICA-only strategy Patients diagnosed with the reference standard ICA can be defined as only TP or TN because ICA is assumed to be 100% accurate and therefore misdiagnosis is not possible.
- NGCCT-only strategy The sensitivity and specificity of the NGCCT are not 100%, and the results of these tests can therefore define patients as TP, TN, FP or FN. For the patients who are diagnosed incorrectly the test result will have consequences. A proportion of the FNs will later be identified as TPs because patients may have persistent symptoms. However, in our model, these patients could have experienced an event [e.g. MI or cardiac arrest (CA)] before the correct diagnosis is established. The FPs may receive unnecessary treatment with its attendant consequences.
- NGCCT–ICA strategy In this strategy, an ICA is performed to confirm a positive NGCCT scan. Therefore, all patients with a FP result for the NGCCT will subsequently be correctly classified by the ICA as TNs. As a result, these patients will not receive any unnecessary treatment. In the model, all of these patients are subsequently considered as TNs for the NGCCT–ICA strategy since the ICA correctly reclassified them. However, an ICA is not performed in patients with a negative NGCCT result. As the sensitivity of the NGCCT is not 100%, it is possible for FN results to arise from this NGCCT–ICA strategy. As with the FNs from the NGCCT-only strategy, a proportion of these FNs will be identified at a later stage.
Diagnostic model for population with known coronary artery disease
The purpose of testing patients with known CAD (defined as those who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or are being considered for revascularisation) is to inform revascularisation decisions.
The prior likelihood of performing a revascularisation in patients with known CAD is assumed to be 39.5%, based on the CE-MARC study (see Table 17).64 The CE-MARC study62 calculated the cost-effectiveness of using CV MRI to determine whether or not a revascularisation is necessary. The purpose of diagnostic testing assessed in the CE-MARC study62 captures the aim of this economic evaluation for the known CAD population and therefore the prior likelihood of the CE-MARC population can be used in the diagnostic model.
The accuracy of the NGCCT for the known CAD population is assumed to be the same as for the suspected CAD population. This assumption was made because for some difficult-to-image patient groups there were no data or just one article for a known CAD population. Details of the reported inclusion criteria, for all studies included in the systematic review, are provided in Appendix 4.
A positive test result for the patient population who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or who are being considered for revascularisation indicates that the patient will benefit from a revascularisation and should undergo a CABG or a PCI. A negative test result for the same population implies that the patient will not benefit from a revascularisation and drug treatment only should be continued.
The same test outcomes apply to the known CAD population as previously described before for the suspected CAD population (Figure 15). Thus the ICA-only strategy will define only TP and TN because ICA is assumed to be 100% accurate. The NGCCT-only strategy gives four possible outcomes: TP, FP, TN and FN. The combined strategy (NGCCT–ICA) defines three outcomes: TP, TN and FN.
FIGURE 15
A 2 × 2 table for patients with known CAD.

FIGURE 17
Known CAD: NGCCT-ICA.
Healthy population Markov model
Patients without the disease (TN and FP from the suspected CAD population; see Table 19) were modelled with a simple alive–dead Markov model (Figure 19) based on UK life tables.65 Based on UK life tables, patients could either die of all causes (including CV, because a negative test result does not mean that patients will never develop CAD) or stay in the ‘alive’ state. Only QALYs but no costs were calculated with this model.
TABLE 19
EUROPA model entry and healthy population model entry: suspected CAD population
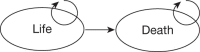
FIGURE 19
Simple alive-dead Markov model.
Of the patients without the disease, only those with a FP test result may undergo unnecessary medical tests and procedures before the absence of CAD is established. The analyses performed in this study included the costs and health outcomes resulting from these tests and procedures in the diagnostic model. However, beyond this, there was no reason to expect any long-term difference in prognosis between patients with a TN test result and those with a FP test result. Long-term costs were therefore not included in the analyses.
Stroke model
As stated previously, ICA and revascularisations are associated with complications and one of these is stroke. The costs and health expectancy of patients who experienced a stroke due to the initial ICA or revascularisation were modelled using a simple alive–dead stroke model. Life expectancy is based on updated UK life tables, combined with a multiplier for age-specific mortality among stroke patients.66 Costs and QALYs for stroke patients were calibrated to correspond with the results of an economic evaluation by Sandercock et al.,66 which estimated the cost-effectiveness of thrombolytic treatment for acute ischaemic stroke compared with standard care for the NHS perspective. In particular, we assumed that stroke patients would receive thrombolytic treatment.67
EUROPA
The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease (EUROPA) trial assessed the ability of the ACE inhibitor perindopril to reduce CV death, MI, and CA in a broad population of patients with stable coronary heart disease and without heart failure or substantial hypertension.68 Based on the patients in this trial, Briggs et al.69 built a Markov model.
Patients with the disease who have not experienced a stroke due to the initial ICA or initial revascularisation, irrespective of the test outcome enter the EUROPA model. The Markov based EUROPA model predicts changes to life expectancy and QALYs for patients with CAD. These changes are calculated based on risk equations which predict the probability of events [CA, (non-)fatal MI] that patients could suffer and the mortality associated with those events. The time cycle used in the EUROPA model is 3 months.
EUROPA model structure
The EUROPA Markov model (Figure 20) consisted of five health states that were defined as absence of primary event in the EUROPA trial: ‘trial entry’, ‘CV death’, ‘non-fatal primary event in current year’, ‘history of non-fatal event (NFE)’ and ‘non-CV death’.70 The 3-monthly transition probabilities between the different states were based on risk equations and on UK life tables on non-CV death. The risk equations consisted of several covariates based on baseline characteristics and previous conditions, such as age, gender, previous MI, diabetes mellitus, etc. The prognosis of the patients was partly dependent on the initial test outcome and treatment decision.
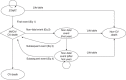
FIGURE 20
EUROPA Markov model. Based on Briggs et al. CA, cardiac arrest; Eq, equation.
All patients with CAD (with the exception of those who experience non-fatal complications from ICA, PCI or CABG) enter the EUROPA model in the ‘Start’ state. A patient can either stay in this state, die from a non-CV cause (and move to the ‘Non-CV death’ state), or experience a CV event and move to the ‘CV death’ state if the event is fatal or to the state ‘non-fatal event (first year)’ if the event is not fatal. The ‘non-CV death’ and the ‘CV death’ states are both mutually absorbing states. Patients can end up in the ‘non-fatal event (first year)’ state in two different ways: by experiencing a non-fatal MI from the initial ICA or revascularisation or by experiencing a non-fatal event at a later time (modelled in the EUROPA model by the risk equations). When a patient is in the ‘non-fatal event (first year)’ state he or she can remain in this state for maximum of 1 year without experiencing a subsequent event. After that, a patient can move to the ‘non-fatal event (after first year)’ state if he or she has stayed in the ‘non-fatal event (first year)’ state for a year without experiencing a new event. Patients in the ‘non-fatal event (first year)’ can also move to the ‘Non-CV death’ state if the patient dies from a non-CV cause; the ‘CV death state’ if the patient experiences a subsequent event which is fatal (‘CV death’ state) or stay in the ‘non-fatal event (first year)’ state if the subsequent event is not fatal. A patient in the ‘non-fatal event (after first year)’ state can stay there, move to the ‘non-fatal event (first year)’ state if the patient experiences a non-fatal subsequent event, move to the ‘CV death’ state if the patient experiences a fatal subsequent event, or move to the ‘non-CV death’ state if the patient dies from a non-CV cause. The risks of events and the mortality associated with events are predicted by the risk equations. Non-CV mortality was based on UK life tables.
EUROPA model entry for population with suspected coronary artery disease
The proportions of patients classified as TP and FN entering the EUROPA model were based on the calculations using prevalence of the disease, sensitivity and specificity of the tests as defined in the diagnostic model. These proportions can vary between the three strategies. Table 19 shows intermediate results of the diagnostic model in two ways. The first part shows how the four test outcomes are represented for each strategy, each difficult-to-image patient group. The second part shows the impact of immediate procedure-related mortality and morbidity on the distribution of the test outcomes. As expected the mortality rates differ considerably between the three strategies. Patients suspected of CAD diagnosed with the ICA alone have the highest overall mortality and morbidity rate. The TN proportion is the lowest in the difficult-to-image arrhythmias group due to the low specificity. The disease progression of the TP and the FN (patients with the disease) was modelled with the EUROPA model. These two outcomes were divided into three treatment possibilities: medication, PCI or CABG. The other two test outcomes (FP and TN) were modelled through a simple alive–dead Markov model (healthy population model) based on life tables, as described above (see Healthy population Markov model).
EUROPA model entry for population with known coronary artery disease
Table 20 presents the intermediate outcomes of the three strategies for the known CAD population. The first part shows how the test outcomes are distributed in each strategy for each difficult-to-image patient group. The second part incorporates also the mortality and morbidity associated with the ICA and revascularisations. The NGCCT–ICA strategy results in the lowest mortality and morbidity rates. The prognosis of patients in all four outcomes (TP, TN, FP and FN) was modelled using the EUROPA model because all patients have CAD.
TABLE 20
EUROPA entry for patients with known coronary artery disease
Every cycle a certain proportion of the FN patients in both populations will be identified as TP based on the Canadian Cardiovascular Society (CCS) angina classification. Identified TPs will be treated and they will have the same prognosis as the TPs who were identified directly by the diagnostic test. The FNs that are still not identified have a higher chance of experiencing an event.
EUROPA model risk equation adjustments
Risk equations to predict the events for patients with CAD were based on the EUROPA trial.68 Using the EUROPA model for the evaluation of the NGCCT in the two CAD populations (suspected and known), and for the different difficult-to-image patient groups, required some adjustment of the EUROPA model. These adjustments were necessary as the baseline characteristics of the EUROPA population were not completely comparable with the subgroups in the known and suspected CAD populations.
As shown in Figure 20, four equations were used to calculate transition probabilities between the states. The first equation based on time-to-event survival analysis estimated the probability of any event that will occur in one cycle of 3 months as a function of the following covariates: age, years older than 65, perindopril usage, smoking, previous MI, existing vascular disease [stroke, transient ischaemic attack (TIA) or peripheral vascular disease], family history of CAD, symptomatic angina or history of heart failure, systolic blood pressure, total cholesterol, obese (BMI of > 30 kg/m2), gender, nitrates usage, calcium channel blockers usage, lipid-lowering treatment, units creatinine clearance below 80 ml/minute and previous revascularisation (PCI or CABG) (Table 21). The second equation of the EUROPA model estimates the odds that the event is fatal, based on age, previous MI and total cholesterol. The third equation estimates the risk of a subsequent event in the first year after a first NFE and is based on the presence of symptomatic angina or history of heart failure. The fourth equation, which predicts the risk of a subsequent event after 1 year, is the same as the first equation except that the covariate previous MI is updated by setting the covariate previous MI at ‘1’.
TABLE 21
Original EUROPA risk equations and mean values: EUROPA population
The risk equations consist of covariates based on the EUROPA trial and therefore baseline characteristics had to be established for the 12 subgroups (seven difficult-to-image patient groups in the known CAD population and five in the suspected CAD population). Means were used in the risk equation, as we used a cohort model. The accuracy of the NGCCT was based on the systematic review reported in Chapter 3, and this review was also used as a source to estimate the baseline characteristics of the different subgroups for use in the risk equations; details of the baseline characteristics of study populations included in the review are reported in Appendix 4. Only subgroup-specific publications were used, thus studies which determined the accuracy of the NGCCT in two or more difficult-to-image patient groups were not used. The baseline characteristics of the EUROPA population were used when information for a specific subgroup and baseline characteristic was not found; this approach assumes that there were no differences between the EUROPA population and the specific subgroup (see Table 21).
Population with suspected coronary artery disease
Baseline characteristics, such as age, gender, family history, diabetes mellitus, obesity, smoking and symptomatic angina, were collected from the articles included in the review that focused on the suspected CAD population. The richness of the information collected from the articles differed between the difficult-to-image patient groups. In all difficult-to-image patient groups except for the ‘intolerant to beta-blockers’ group, a minimum of gender and age data were found. When population specific information regarding risk-related characteristics was not found in the literature, the assumption was made that the difficult-to-image subgroup did not differ from the EUROPA population and therefore the value of the EUROPA population (see Table 21) was taken. ‘Perindopril usage’ was assumed to be 0.23 for the whole suspected CAD population.71 We will assume that the effect of perindopril does apply for any ACE inhibitor. The covariates ‘age’, ‘age > 65 years’, ‘men (y/n)’, ‘smoking (y/n)’, ‘diabetes mellitus (y/n)’, ‘positive family history (y/n)’, ‘obese (y/n)’, ‘symptomatic angina (y/n)’ differed per difficult-to-image subgroup. No subgroup-specific information was collected for the covariates ‘systolic blood pressure’, ‘creatinine clearance’, ‘total cholesterol’ and ‘the usage of lipid-lowering treatment at baseline’. The five other covariates depended on the strategy, treatment and test outcomes. Tables 22 and 23 illustrate how proportions were assigned to the covariates. The proportion that has had an MI was based on the non-fatal complications of ICA and revascularisation. FNs in strategies 2 and 3 have not experienced an MI, revascularisation or vascular disease because they do not undergo an ICA or a revascularisation. The covariate previous revascularisation was set at 1 for the TPs treated with a revascularisation. Nitrates usage was assumed to be ‘0’ for all test outcomes. Usage of calcium channel blockers was assumed to be ‘1’ for TPs who received medical treatment. This is because, although they might actually be prescribed a beta-blocker instead,71 there was only a covariate in the risk equation for calcium channel blocker and not beta-blocker. This assumption can be justified because the efficacy of calcium channel blockers does not differ from that of beta-blockers.71
TABLE 22
Input for the EUROPA risk equations: suspected CAD population
TABLE 23
Subgroup-specific input for the EUROPA risk equations: suspected CAD population
Population with known coronary artery disease
The procedure described above to establish the baseline characteristics for the suspected CAD population was also used for the known CAD population. No information about gender and age was available for the beta-blocker intolerance and high coronary calcium score groups. For the other groups these data were collected from the accuracy studies included in the systemic review. The covariates ‘age’, ‘age > 65 years’, ‘men (y/n)’, ‘smoking (y/n)’, ‘diabetes mellitus (y/n)’, ‘positive family history (y/n)’ and ‘obese (y/n)’ differed per difficult-to-image patient group. No subgroup-specific information was available for the covariates ‘symptomatic angina’, ‘systolic blood pressure’, ‘creatinine clearance’, ‘total cholesterol’ and ‘the usage of lipid-lowering treatment at baseline’. Perindopril intake proportion was set at 0.23, based on published data.70 The proportion of patients experiencing an MI or the proportion where vascular disease is present was based on the EUROPA population. The proportions were not raised with ICA or revascularisation-induced MI. Nitrates usage and calcium channel blockers at baseline were not reported in the studies included in the systematic review and therefore these proportions were based on the EUROPA population (see Table 21). The proportion for previous revascularisation was set at ‘1’ for the TPs in all strategies, for the FPs in strategy 3, and for the subgroups' previous PCI and previous CABG this was set at ‘1’ for all test outcomes. The remaining proportions were set as for the EUROPA population (Tables 25 and 26).
TABLE 25
Subgroup-specific input for the EUROPA risk equations: known CAD population
TABLE 26
Input for the EUROPA risk equations: known CAD population
Difficult-to-image patient group-specific data
In addition to CAD population-specific adjustments of the EUROPA risk equations, adjustments were necessary for each specific difficult-to-image patient group. It is likely that some of the reasons why patients are difficult to scan may also lead to a higher probability of a CV event.
In the obese patient group, the increased risk of events was already captured in the risk equation, as it contains a covariate for obesity. For the obese group, the covariate obesity was set at ‘1’ for all test outcomes, strategies and CAD populations.
For simplicity, we treated the difficult-to-image subgroup with a previous CABG the same as the difficult-to-image subgroup with a previous PCI.72 The covariate ‘previous revascularisation’ is present in the first and fourth risk equations of the EUROPA model; thus, the risk of having a primary or subsequent event for these specific patient groups was captured.
For the difficult-to-image groups arrhythmias and high coronary calcium level, a relative risk (Table 24), compared with the EUROPA population, was used to adjust the risk of events. For the HCS patient group, data from an unpublished study73 were used to estimate the relative risk without correcting for other factors of experiencing primary events in patients with a coronary calcium score > 400 compared with patients without a coronary calcium score of > 400. The proportion with a coronary calcium score of > 400 in the EUROPA population was not reported and therefore the study of Shemesh et al.74 was used to estimate a proportion assuming that the populations are comparable. We assumed that this relative risk also applies for the risk of having a subsequent event.
TABLE 24
Relative risks of CV events compared with EUROPA population for arrhythmias and high coronary calcium level subgroups for the known and suspected CAD population
A relative risk, compared with the EUROPA population, was also used to estimate the risk of experiencing events for the patient group with arrhythmias. The term ‘arrhythmias’ encompasses several different conditions, with AF being the most common. A relative risk was calculated, controlling for other factors for patients with arrhythmias, based on the relative risk found in the QRISK study, which investigated the relative risk of experiencing events for patients with AF against patients without AF.75 The proportion of the patients with AF was not reported by the EUROPA study and therefore we assumed that the proportion AF in patients with CAD is 19% based on Banasiak et al.76
No adjustments to the risk equations were necessary for the intolerant to beta-blockers patient group because it was assumed that intolerance of beta-blockers does not lead to an increased risk of experiencing events; patients undergoing a cardiac CT receive beta-blockers to lower their heart rate in order to produce images of adequate quality, not in order to prevent events. Patients with CAD will often be treated with beta-blockers but these can be replaced with calcium channel blockers and/or ACE inhibitors and therefore intolerance to beta-blockers will probably not affect prognosis.
For the patient group with HHR the risk equations were not adjusted because it was assumed that HHRs affect only the quality of CT imaging. The patient groups with HHR and intolerance to beta-blockers were modelled with the original risk equations based on the EUROPA population.
York Radiation Model
The impact of imaging-associated radiation on cancer rates and outcomes was not estimated with the EUROPA model but was with the YRM.61 The EUROPA model takes into account only mortality and not the QALYs and costs of treatment of radiation-induced cancer. The YRM is a radiation impact model recently developed by the Technology Assessment Group of the University of York to assess the health impact of a reduction in radiation when using a new X-ray imaging system for diagnostic purposes.61
Biological effects of radiation
The dose of ionised radiation absorbed by a body is measured in grays (Gy). However, the health-relevant (and harmful) energy absorbed depends on the tissue and type of radiation and is expressed in sieverts (Sv). Because of the small doses of imaging radiation, more often millisieverts (mSv) are used (1000 mSv = 1 Sv). Also, 1 Sv = 1 Gy × a weighting factor (e.g. for a breast scan the weighting factor is 0.05).
Exposure to ionised radiation has mainly three biological adverse effects.77 First, radiation has a harmful effect on developing embryos when the expecting mother is exposed to radiation. This is not relevant in our application. Second, radiation exposure might affect reproductive health, i.e. radiation exposure may lead to adverse congenital health outcomes of later offspring. There is, however, no convincing evidence for this effect in humans, only in animal experiments. The third, most harmful, effect is an increased lifetime risk of cancer incidence. For low doses, sparse clinical evidence exists. A prominent source is a cohort study of Japanese atomic bomb survivors who were exposed to radiation. These data provide strong evidence of an increased cancer mortality risk at equivalent doses of > 100 mSv, good evidence of an increased risk for doses between 50 and 100 mSv, and reasonable evidence for an increased risk for doses between 10 and 50 mSv.78
The standard epidemiological risk models use a linear relationship between radiation exposure and lifetime probability of solid cancer without assuming a threshold, i.e. even a minimal exposure is assumed to increase the lifetime risk of cancer incidence. The younger the age at exposure, the higher is the lifetime probability of cancer incidence for a given amount of radiation, partly because children have on average more life-years remaining to develop cancer. The cumulative lifetime risk of an individual for repeated exposure to radiation is calculated by summing the probabilities for lifetime cancer incidence over each exposure.
In a recent report, the Centre for Radiation, Chemical and Environmental Hazards (CRCE), formerly the National Radiological Protection Board (NRPB), of the Health Protection Agency (HPA), has calculated lifetime risks for cancer incidence by age and sex for different levels of radiation.79 Those calculations are based on a 2007 publication of the International Commission on Radiological Protection (ICRP).80
Structure of York Radiation Model
The calculations for health consequences of radiation exposure are based on an adjusted version of the YRM (Figure 21). The YRM consists mainly of four elements: a radiation module, a cancer module, a utility module and a main module combining all intermediate calculations.
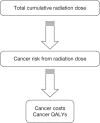
FIGURE 21
Stylised overview of YRM.
In the radiation module, the YRM estimates the lifetime probability of an individual, given the timing and the amount of radiation exposure. To translate the cumulative radiation dose into the probability of lifetime cancer incidence the HPA model is used (see Table 47).80
TABLE 47
Lifetime risks of cancer incidence for all cancers by age and sex at exposure based on HPA data
The cancer module is based on prior research.61 In the absence of cancer models for all types of cancer, four common cancers are modelled: lung and colorectal cancer for both sexes, breast cancer only for females, and prostate cancer only for males. For each cancer, the module contains the further expected QALYs and disease costs for patients with cancer at the average age at diagnosis (see Table 46). For each sex, these values are then combined and weighted according the relative incidence of radiation-induced cancer. For males, the weights are approximately 46% colorectal, 42% lung and 12% prostate, whereas for females the weights are 16% colorectal, 50% lung and 34% breast.
TABLE 46
Total costs and QALYs lost due to cancer, discounted at 3.5% per annum to age at cancer diagnosis (SD in parentheses)
The utility module is based on data for the general UK population (see Table 49).61 For patients who do not get cancer, the remaining lifetime QALYs from the age at first radiation exposure are calculated. For patients who do get cancer, the utility module calculates the QALYs until the age at diagnosis of cancer, i.e. the timespan without cancer.
TABLE 49
Age-specific utilities based on underlying health of the general UK population
The main module combines the outcome of the three prior modules. So for a given age at first exposure, the share of patients who get radiation-induced cancer during their lifetime is calculated. For those patients, their QALYs until age at cancer diagnosis equal the general UK population and after that the remaining QALYs and the (additional) disease costs owing to cancer are taken from the cancer module. For the rest of the patients, just the remaining QALYs based on the general UK population are calculated. These values are combined and weighted by the sex ratio of the patient population. Both QALYs and disease costs are discounted to the age at first exposure to radiation. The intervention, i.e. the reduction in radiation exposure through the comparator technology, is modelled via the reduction in the probability of lifetime radiation-induced cancer. The YRM allows to conduct a probabilistic sensitivity analysis (PSA) accounting for the uncertainties in age at cancer incidence, cancer costs and QALYs lost due to cancer.
Radiation dose and patient populations
Computer tomography is a relatively high-dose X-ray imaging technique. The effective dose, i.e. absorbed radiation dose by a patient measured in sieverts, depends on a number of factors such as age of patient, the region of the body scanned, tissue type involved, precise type of CT, and scanning protocol for the particular diagnosis in question. Furthermore, CTs are an evolving technology in which the radiation doses vary with CT generation and by manufacturer. Moreover, scanning protocols themselves change over time. In particular, multislice CTs allow for increasingly rapid scans and lower radiation doses. Although 64-slice scanners have increasingly become the standard, earlier-generation CTs are still in use.
The broad range of CT types and CT applications compels studies which aim to quantify the radiation burden attributable to CTs in the general population to measure the radiation dose by scan for a particular body region/diagnosis type, for example head or full chest, roughly differentiating only by CT type (mostly single slice vs multislice). To account for the particular diagnostic needs of the disease assessed, we conducted expert surveys to obtain the relevant dosages by scanning strategy. The results are shown in Table 52 (for patients with CAD) and Table 66 (for patients with congenital heart disease).
TABLE 52
Radiation dose (in millisieverts) of scanning strategies for CAD patients based on a disease-specific expert survey
TABLE 66
Scenario analysis: prior likelihood, suspected population 0.3
The results of our expert surveys are in line with the literature that focuses on general chest CTs (Table 27). A study by the NRPB for the UK, conducted in 2003, shows slightly higher results than our expert survey, as its results were mostly based on single-slice and four-slice technology,81 which usually have higher radiation doses than 64-slice technology. More recent studies, such as the UNSCEAR 2008 report, assessing the trends in worldwide radiation exposure,82 and a review article focusing on children's exposure and based on German data,83 support the overall lower radiation dose for CT64 indicated by our expert survey. Note that in Table 27 values are also presented for younger age groups, as those values are required for the analysis presented below (see Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease).
TABLE 27
Comparative radiation dose by age at exposure from diagnostic examination of ‘chest’ with a CT (in millisieverts)
The YRM was used for the two patient populations under assessment, the patients with CAD (this section) and the congenital heart disease patients (see Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease). The adjusted version of the YRM does not model benefits of the different CT strategies, but only the harmful consequences of radiation exposure. Hence, it can be used for both patient populations without further modifications; only the key parameter age at exposure, radiation dose (dependent on type and number of scans) and sex are used. In the case of the patients with CAD, the YRM output was used for further analysis. For an overview of the radiation doses in the patient populations for the different strategies under assessment see Tables 52 and 69.
TABLE 69
Radiations dose (baseline and range) for diagnosis in patients with congenital heart disease with a CT scan based on disease-specific expert reply (in millisieverts)
Overview of the models used
Table 28 provides an overview of which models were used for each difficult-to-image patient group within each CAD population (suspected or known). The diagnostic model was used for each subgroup and modelled separately for 100% of the patients. To estimate the extra costs and QALY loss due to radiation, the YRM was used for each subgroup for the entire population. The healthy population model was used only for the suspected CAD population to model the patients who do not have CAD (TN and FP). The known and suspected CAD populations with CAD were modelled separately using two versions of the EUROPA model. The suspected CAD population had three treatment options (PCI, CABG and medication), whereas the known CAD population could undergo only a CABG or a PCI. The difficult-to-image patient groups ‘previous CABG’ and ‘previous stent implantation’ were treated as one subgroup in the EUROPA model because Deckers et al.72 and Briggs et al.69 use only one coefficient in the risk equation, namely previous revascularisation.69,72 Cost and QALYs for patients who have experienced a stroke due to the initial ICA or initial revascularisation are based on a previously conducted study by Sandercock et al.66 Subgroup-specific costs and QALYs obtained in the stroke model were calculated by using the subgroups ‘specific age’ and ‘proportion men’.
TABLE 28
Overview model runs for subpopulations
Model parameters
This section describes the parameters used in the diagnostic model, the EUROPA model, the healthy population model, the YRM and the stroke model. Distributions of the parameters are presented in Table 61 and described below (see Results, Sensitivity analyses). The last section describes how the difficult-to-image patient groups were combined to get overall incremental cost-effectiveness ratio (ICER) estimates for each CAD population (suspected and known).
TABLE 61
Parameters distributions
Diagnostic model
The diagnostic model estimates the initial costs of diagnosis and initial treatment. Mortality and morbidity associated with the treatments and the diagnostic tests were also modelled and have an impact on the effectiveness of the three strategies. The events occur at one moment in time; the diagnostic model is time independent.
Costs
The costs included in the diagnostic model were the costs for the diagnostic tests and the costs of the two revascularisation procedures (Table 31). Medication-induced costs were modelled as part of the background costs in the disease progression model. The average cost prices for the revascularisation procedures and the ICA were calculated based on the NHS reference prices 2010–11.84 An average cost price is calculated by multiplying the number of admissions with the costs for each different specific procedure. An ICA was estimated as costing on average £1003. A CABG would cost £8280 per procedure, and £9242 in combination with a ICA. A PCI in combination with an ICA would cost £4196, and a PCI without an ICA would cost £3633 per procedure.
TABLE 31
Costs of diagnostic tests and treatment
Given that the cost of ICA (invasive CA) was estimated using the NICE reference cost, for comparability a reference cost would have been useful for each of the different types of scan – both standard 64-slice and the NGCT. However, the only data available were for any CT, i.e. not specifically for CTCA (Table 29).
TABLE 29
Costs for any CT
Therefore, a bottom-up costing was performed, which attempted to use the categories that the reference cost would be composed of, which are shown below (Table 30).
TABLE 30
Estimated costs for any cardiac CT
The final costs of 64-slice and NGCCT are calculated to be £132.62 and £169.26, respectively. The estimated costs of 64-slice CT are higher than the reference costs. However, this is plausible given that much of the capital cost of existing scanners is probably not included in the reference costs. This is because many scanners are actually purchased using non-NHS money, i.e. by private donations (Valerie Fone, Trust Imaging Services Manager, Royal Brompton and Harefield NHS Foundation Trust, personal communication; see Appendix 7). Also, the staff costs for CTCA are higher given the considerable use of consultant as opposed to more junior or no radiologist time. Scenario analyses will be performed for 4160 scans per year (cost price NGCCT: £150) and 2080 scans per year (cost price NGCCT: £207).
Prior likelihood
The prior likelihood for the suspected and known CAD populations is presented above (see Model structure and methodology, Diagnostic model).
Initial treatment decision
Diagnostic tests, using the NGCCT, are performed to determine if treatment is necessary for a difficult-to-image patient. The cost-effectiveness of the NGCCT was estimated for two CAD populations which are treated differently. For the assumptions concerning the treatment options for the suspected CAD population expert opinion was used.
Suspected coronary artery disease population
Patients with suspected CAD and a positive cardiac CT or ICA test result can be treated with drug therapy alone, a CABG or a PCI. The proportions undergoing either revascularisation (18.1%) or medication (81.9%) after a positive test result were based on expert opinion (Hofstra, 2011, personal communication; which was based on an unpublished study conducted in the Netherlands73). The proportion of PCI compared with CABG in patients requiring revascularisation was based on UK procedure figures, which showed a 70%:30% proportion for PCI compared with CABG.88
Patients treated with medication only are treated with beta-blockers or calcium channel blockers.12 When the symptoms are not controlled with one of the two drugs, then a combination can be given or a nitrate can be prescribed. A revascularisation is then considered if symptoms of patients are still uncontrolled by drug treatment alone. The proportions undergoing revascularisation or medication treatment is comparable with a previously published article based on the Euro Heart Survey, which reported a revascularisation rate of 13%.70 Furthermore, expert opinion indicated that the results of this study were also appropriate for the difficult-to-image patient groups considered in this assessment.
Population with known coronary artery disease
Given a positive CT or ICA test for patients with known CAD, two treatment options are considered: either PCI or CABG. The proportions undergoing PCI or CABG in patients with known CAD were also assumed to be 70%:30%, based on the same expert opinion used for the suspected CAD population.
Procedure-related mortality and morbidity
Invasive coronary angiography and revascularisation are accompanied by a risk of serious complications, including stroke, non-fatal MI and death (Table 32). The mortality rates are important for the impact on QALYs of the three strategies. The strategy in which all patients will undergo an ICA has the highest test-related mortality rate and this mortality rate influences the cost-effectiveness ratio by lowering the expected QALYs.
TABLE 32
Complications of ICA and revascularisations
The complication rate used in this model is based on published data.89 A literature search for UK guidelines for performing coronary angiography was conducted to identify a study that provided primary data on complications caused by diagnostic ICA. Seventeen UK guidelines were found and these were checked for studies presenting primary data; 17 potentially relevant studies were found. A further four primary studies89–92 were identified after checking the references of the initial 17 studies and performing a citation search. Two studies90,91 did not present a complication rate based on the UK population but were conducted in Turkey and Canada, respectively. One study92 reported a complication rate for a UK population but was based on a single centre. A multicentre study89 on diagnostic angiography in the UK (and the most recently performed study) was considered to be the most appropriate study to inform the model. This study reported a complication rate of 7.4 (95% CI 7.0 to 7.7) and a mortality rate of 0.7 (95% CI 0.6 to 0.9) per 1000 patients, based on 219,227 procedures undertaken between 1991 and 1999. The mortality rate and the cerebrovascular accident rate presented in this study were comparable with data from another of the identified studies.90 The overall complication rate and the MI rate presented were considerably lower than those presented in the other studies. We assumed that the complication rate of coronary angiography presented by the selected study is applicable regardless of the underlying risk of CV events particularly in difficult-to-image patient groups.
Both revascularisation procedures, CABG and PCI, are associated with complications including stroke, non-fatal MI and death. These complications are included in the diagnostic model. The mortality rate (0.018) of a CABG is based on Bridgewater et al.93 CABG-related stroke was taken from the study.93 As there were no studies that reported CABG-related MI, we used the study by Serruys et al.95 to give an estimate of CABG-related MI. A survival curve (patients without MI and stroke) presented in the Serruys study95 was used: at 30 days, the survival was 96%; thus, 4% experienced a stroke or a MI. As we found a stroke rate of 1.6%94 related to the procedure we used 2.4% as an estimate for CABG-related MI assuming that within 30 days after the procedure it is still related to the procedure. This could lead to an overestimation of the MI rate, because the 4% reported by Serruys et al.95 is not related to the procedure per se.
The complication rates induced by PCI were based on the study of Rajani et al.;96 mortality due to a PCI is 0.0029, to a MI 0.0005 and stroke due to PCI 0.0005.
Finally, it has also been suggested that the intravenous contrast used in ICA, PCI and the NGCCT may carry a small risk of contrast-induced renal failure, dialysis and mortality.97 However, a paper reviewing this risk in CT scans showed a negligible risk. In a total of six studies in patients receiving contrast fluid for a CT, no patients needed dialysis or died out of 1175 patients.98 Thus, we have added no complications for the NGCCT. Contrast-related mortality may be assumed to be part of overall mortality due to ICA and PCI discussed earlier. Thus, the only remaining issue is a potential underestimation of the complications of these invasive procedures. As the complication rate can be greatly influenced by taking prophylactic measures in patients who are more at risk, this additional risk is here considered to be negligible.99
Healthy population model
The healthy population model applies only for the suspected CAD population because all patients with known CAD have a different prognosis than patients without CAD; this was modelled using the EUROPA model. The TN and the FP patients in the suspected CAD population do not have CAD and therefore modelling their ‘future’ with the EUROPA model is not appropriate. Life tables were used to predict mortality for those groups of patients assuming that these patients do not differ from the average UK population. Costs are not assigned to this Markov model.
Survival
Three-monthly, age-dependent transition probabilities were used to model mortality for TN and FP patients in the suspected CAD population. The transition probabilities were based on UK life tables for all-cause mortality (Table 33).65 All-cause mortality life tables were used, as these patients can still develop and die from CAD in the future.
TABLE 33
Quarterly mortality rates (all causes)
Utility for patients without coronary artery disease
Patients from the suspected CAD population with a TN or FP test outcome are patients without CAD and it is therefore assumed that the health-related quality of life (HRQoL) for these patients would be equal to the population norms by gender and age (Table 34).100 Of course, when patients presented they must have had similar symptoms to those who actually have CAD. However, we have assumed that these symptoms resolve over time, either through spontaneous improvement or through appropriate treatment. Additionally, it should be realised that the general population utility already is based on the presence of some illness, which implies that the difference between the utility of suspected CAD population who do not have CAD and the general population may be expected to be small. QALYs are discounted with 3.5%.97
TABLE 34
Population norm by European Quality of Life-5 Dimensions (EQ-5D) (Kind et al.)
EUROPA model
The EUROPA model models the progression of stable CAD by predicting CV events and mortality. Health-care costs were evaluated by Briggs et al.69 from resource items collected as part of the EUROPA study68 and these are grouped, for our analysis, into three categories: background costs, NFE costs and fatal event costs. More details can be found in the technical appendix of Briggs et al.69 During the EUROPA trial a cost data set was constructed by recording, for each patient, the costs for each year in the trial. Covariates were then defined that related to the states of the model. A linear regression model (controlling for clustering by individual) was then used to estimate the cost associated with each of the model states, together with the potential effects of other covariates.69Table 35 shows the results of the cost regression.
TABLE 35
EUROPA costs
The original cost prices of the EUROPA trial 2003–4 were updated with a price correction based on the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2010 (PSSRU 2010). Inflation correction is 1.2077402 and costs are discounted at an annual rate of 3.5%.101
Background costs
Background costs are costs which are applied to the trial entry state and the NFE states. The background costs are based on age, the existence of vascular diseases or diabetes mellitus, medication usage, creatinine clearance and symptomatic disease. For each combination of difficult-to-image patient group, strategy, treatment decision, test outcome and known or suspected CAD population background costs (Tables 37 and 38) were estimated with the linear regression presented in Table 35. The costs of medication for patients who are treated with medication only were included in this background cost. An example is presented below for a patient from the known CAD population who is obese and defined TP in the ICA-only strategy.
TABLE 37
Monthly background costs EUROPA: suspected CAD population (£)
TABLE 38
Monthly background costs EUROPA: known CAD population (£)
The average age of an obese patient with known CAD and a TP test outcome is 63 years; 34% have diabetes mellitus and 25% are symptomatic. Creatinine clearance below 80 ml/minute is on average 6.9 ml/minute, nitrates usage at baseline 44%, the presence of existing vascular disease is 10.1%, calcium channel blocker usage at baseline 32% and lipid-lowering therapy at baseline 55.9%. So, in total, £298.05 is assigned per cycle of 3 months as a background cost (Table 36).
TABLE 36
Example background cost calculation
Non-fatal event costs
For the year in which a NFE occurs, £11,805 was added to the background cost. For subsequent years, the additional cost was estimated as £986. In the year that a fatal CV event occurs, the additional cost was estimated as £3641. When a fatal non-CV event occurred, an additional cost of £12,421 was added.
Utilities for patients with coronary artery disease
Health-related quality-of-life estimates were assigned to the states in the Markov model based on age, gender, baseline CCS classification and whether or not the patient had undergone treatment. Patients modelled through the disease progression model are assumed to have a CCS class (Campeau102) of 2. The HRQoL estimates were based on three sources: population norm for the EQ-5D,100 EQ-5D scores per CCS class103 and treatment effect on quality of life (QoL) based on the Randomized Intervention Treatment of Angina (RITA-2) trial.64
Baseline EQ-5D – untreated patients with CAD
Combining the population norm values with the EQ-5D scores per CCS class (0–4) (Tables 39 and 40) generates relative HRQoL by CCS class and gender. Longworth's scores103 were based on a median age of 61 years and these were divided by population norms for the age group 55–64 years. To obtain HRQoL by CCS class and age, the HRQoL by CCS class was multiplied by the age-specific HRQoL scores from Kind et al.,100 assuming that the relative HRQoL by CCS class compared with the general population would hold across all ages. This multiplication was taken for the patients with CAD at baseline (without treatment).
TABLE 39
Baseline HRQoL: male
TABLE 40
Baseline HRQoL: female
Treatment EQ-5D – patients with coronary artery disease, treated
The RITA-2 trial provided data on the initial CCS class and the CCS class following revascularisation to estimate the HRQoL for a patient who is treated. The long-term effects of PCI and medical treatment in patients with CAD are compared in the RITA-2 trial. The baseline EQ5D score was combined with the RITA 2 trial to generate HRQoL scores by baseline CCS (i.e. CCS before treatment), age and gender following revascularisation (Tables 41 and 42). Improvement in HRQoL (a better CCS class) was estimated by combining the changes in CCS after treatment with association seen between baseline CCS and baseline HRQoL. A new HRQoL was calculated from the shifts to the other CCS classes. For example, 20% of the patients will have a better CCS class after treatment, 10% will have a worse CCS class after treatment and 70% will stay in the same CCS class. The product of the proportion and the HRQoL in each specific CCS class after treatment provided an updated HRQoL for a patient by baseline CCS class. The assumption was made that the effect of revascularisation on HRQoL continues. The same HRQoL values were used for patients treated with medication only.
TABLE 41
Health-related quality of life following treatment: male
TABLE 42
Health-related quality of life following treatment: female
A 3-monthly disutility of 0.010225104 was assigned to the non-fatal event states because an event has occurred. We assumed that the disutility owing to a MI is the same as for a cardiac arrest. Of the NFEs only 2.5% will be a cardiac arrest; thus, the impact of changes in the disutility of a cardiac arrest will be minimal.
Population with known coronary artery disease
For the suspected CAD population, the baseline HRQoL applies for the patients with CAD, but not treated with a revascularisation or medication (FNs). In the EUROPA model, after a while a FN patient with CAD could be identified and would be treated; for this identified patient the HRQoL following treatment applies. The TPs from the suspected CAD population have CAD and will be treated with a revascularisation or medication and therefore the HRQoL following treatment applies (Table 43).
TABLE 43
Health-related quality of life per population and test outcome
Population with known coronary artery disease
Patients from the known CAD population all have CAD irrespective of their test outcome. Therefore, they are already identified and the TPs who are treated will have the HRQoL following treatment. The TNs do not need a revascularisation; therefore they have a HRQoL of being treated because we assume that these patients are in such a good state that a revascularisation is not necessary and therefore they have the highest HRQoL, namely that of treated patients. The FPs are treated with a revascularisation although this was not necessary. Therefore, we assumed that patients being FP and who are treated have the highest HRQoL, namely that of patients who are treated. The FNs need a revascularisation so the HRQoL of patients who are not treated applies for these patients (see Table 43).
Transition probabilities
Tables 44 and 45 present the 3-monthly transition probabilities for the suspected and known CAD populations for each subgroup. These transition probabilities were based on the risk equations which are explained in Model structure and methodology, EUROPA.
TABLE 44
Transition probabilities: CAD suspected population
TABLE 45
Transition probabilities: known CAD population
Stroke model
The costs and effects of the patients who experience a stroke due to the initial ICA or revascularisation are modelled with a relatively simple alive–dead model based on estimates by Sandercock et al.66 for thrombolytic therapy of stroke.
Survival
Mortality rates were based on UK life tables65 and a relative risk of 2.5 to reflect the increased risk of mortality following a stroke.105 Survival for each subgroup modelled in this study was therefore not simply dependent on stroke but also on the average age in that subgroup.
Costs
Sandercock et al.66 estimated a cost of approximately £6260 in the first year after a stroke. As Sandercock et al.66 presented both 12-month and lifetime costs, we estimated the average annual costs of treating stroke patients after the first year to be approximately £3400. These costs were then inflated to reflect costs for 2009–10 and then discounted at a rate of 3.5%.
Quality-adjusted life-year
Calibration of the model to fit with the results by Sandercock et al.66 resulted in an average health utility of 0.37. This value was combined with survival and the resulting QALYs were discounted using at a rate of 3.5%.
York Radiation Model
The following tables show the key parameters for the base-case scenario for the YRM when modelling the effect of radiation on CAD patients. Table 46 shows the mean parameter values (costs and QALY loss due to cancer) for the cancer module of the YRM. If the age at first exposure to radiation is < 40 years, the average age of incidence for breast cancer is assumed to be 40 years; for higher ages the average is assumed to be 60 years. In the CAD patient population all patients are aged > 40 years. This can be seen clearly in Table 51, with demographic characteristics of the patient population. The lifetime risk of cancer incidence by age and sex for a one-time exposure to 10 mSv, based on the HPA model, is shown in Table 47. Table 49 shows the age-specific utilities used to calculate the QALYs for non-cancer patients. Table 50 shows the life expectancy for the general population, i.e. patients who do not get cancer, based on the 2007 England and Wales life table. Note that in various tables values are presented for younger age groups, as those values are required for the analysis presented below (see Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease).
TABLE 51
Demographic characteristics of the CAD patient population
TABLE 50
Overview of age-specific remaining life expectancy
Table 52 presents the radiation doses for each of the analysed scanning strategies for patients with CAD. Te value for NGCCT is based on an expert survey (response: n = 2) for this particular patient group, whereas the average radiation doses for ICA and PCI are taken from literature.61
For all of the scanning strategies, the uncertainty in the costs (Table 48) and remaining QALYs of the cancer module in the YRM are modelled via a PSA. Te values for the input are shown in Table 46.
TABLE 48
Cost per scan for CT64 and NGGCT (base case)
Proportions of patients in difficult-to-image subgroups
Difficult-to-image patient group-specific costs and QALYs were calculated. Te aim was to calculate an overall ICER for the three strategies and for the two populations (suspected and known CAD). Expert opinion was used to gather information on the relative proportions of patients in the different difficult-to-image groups in a known or suspected CAD population. Primary data collection from patient records was considered, but due to time constraints a questionnaire distributed to experts in the field was used to derive a reasonable estimate of the relative proportions. Multiplying the relative proportions with the subgroup-specific costs and effects produced an overall ICER for the suspected CAD population and an overall ICER for the known CAD population.
The questionnaire was distributed to six experts, four of whom completed and returned it. Means are calculated from the proportions that the experts filled in. See Appendix 7 for details on the experts. Table 53 shows the relative proportions for each population. According to the experts it is impossible to have a revascularisation before the test is performed in a population with suspected CAD.
TABLE 53
Mean proportion difficult-to-image subgroups, per expert and overall
Results
Initially the costs of using the NGCCT instead of an ICA are lower but what is the influence of the lower sensitivity and specificity on the effectiveness side and the costs side? The cost-effectiveness of the three strategies is described below. First intermediate results are given for the three strategies for each subgroup.
Intermediate outcomes
In addition to the cost-effectiveness of the NGCCT, intermediate outcomes in terms of mortality, morbidity and the percentages of correct diagnostic classification (TP, FP, TN and FN) are also important. Tables 55 and 56 show, for both CAD populations and for each difficult-to-image group, these three intermediate outcomes.
TABLE 55
Intermediate outcomes: suspected CAD population
TABLE 56
Intermediate outcomes: known CAD population
Population with suspected coronary artery disease
As expected, the ICA had 100% correct diagnostic classification due to the assumption of 100% sensitivity and 100% specificity. Unfortunately, this comes with higher mortality and morbidity rates due to the complications of the test itself. The strategy where each patient will undergo an ICA had the highest test-induced mortality and morbidity rate, and the strategy that uses only the NGCCT to diagnose patients has test-induced mortality and morbidity rates of zero. Conversely, revascularisation-induced mortality and morbidity rates were highest in the NGCCT-only strategy due to the FPs who undergo unnecessary revascularisations with the associated complications. The NGCCT–ICA strategy had the lowest revascularisation-induced mortality and morbidity rates because only TPs are treated and the FNs who are not correctly diagnosed will not receive a revascularisation where they should have. The NGCCT-only strategy has the lowest overall mortality rate in the suspected population. The NGCCT-only strategy, as expected, had the lowest correct classification proportion.
Population with known coronary artery disease
The same results apply for the known CAD population; the ICA classifies 100% of patients correctly, the ICA strategy has the highest test mortality and morbidity rates and the NGCCT-only strategy has the highest revascularisation mortality and morbidity. However, in the known population the overall mortality and morbidity is lowest in the NGCCT–ICA strategy. ICA only has the highest overall mortality and morbidity rate.
Costs per model
Table 57 shows the costs assigned to the patients in the diagnostic model, the EUROPA model, the YRM and costs from the stroke model per subgroup. The presented costs are after including the probabilities; adding the cost per model gives the total costs.
TABLE 57
Costs per model (£)
Population with suspected coronary artery disease
Most of the costs in the EUROPA model do not differ significantly between the three strategies. The difference in costs between the strategies is mainly due to the difference in the costs in the diagnostic model. The ICA-only strategy has the highest costs in the diagnostic model because the test itself is much more expensive than NGCCT. The impact of treating FPs unnecessary with a revascularisation in the NGCCT-only strategy is marginal because the proportion that receives a revascularisation is just 18%. The incremental cost induced due to radiation is lowest in the NGCCT-only strategy because the radiation dose is lowest in the NGCCT-only strategy. Also, not surprisingly, the costs in the stroke model are the highest for the ICA-only strategy due to the largest proportion having non-fatal complications of the initial ICA and revascularisations.
Population with known coronary artery disease
In the known population, the costs in the diagnostic model are still the highest for the ICA-only strategy. However, the NGCCT–ICA strategy instead of the NGCCT-only strategy has the lowest cost in the diagnostic model. This is different from the suspected CAD population because the treatment decision differs between the two models. The known FPs of the NGCCT-only strategy are always treated with a revascularisation with accompanying extra costs. In the suspected CAD population, only 18% of FPs receive a revascularisation and, as medication costs are modelled in the EUROPA model, it will lead to fewer costs for the FPs.
The same applies for the stroke model because the non-fatal complication rate of the NGGCT-only strategy in the known group is higher than that of the NGCCT–ICA strategy and in the suspected population the NGGCT-ICA has a higher non-fatal complication rate. The proportion of the suspected CAD population that receives a revascularisation after a positive test is 18%, and corresponding proportion in the known population is 100%, therefore the proportion that experience a stroke due to the revascularisation is higher in the known population.
Quality-adjusted life-years per model
Table 58 shows an overall QALY estimate and a separate QALY estimate per model for every strategy, subgroup and population. The presented QALYs are after including the probabilities; adding up the QALYs of the different models leads to the total QALYs per strategy. The YRM provides disutilities, as it induces QALY loss due to radiation.
TABLE 58
Quality-adjusted life-years per model
Population with suspected coronary artery disease
In the EUROPA model the ICA-only strategy obtains, in every difficult-to-image patient group, the highest number of QALYs. This is because of the lower HRQoL FNs experienced in the NGCCT-only strategy and in the NGCCT–ICA strategy owing to lower sensitivity and specificity of the NGCCT. FNs do not occur in the ICA-only strategy; they will all be classified as TP with a higher HRQoL. The QALYs in the healthy population model are the lowest in the ICA-only population because the proportion of TNs is the lowest for this strategy. The NGCCT–ICA and NGCCT-only strategies have larger proportion in the TNs because less ICA-related mortality occurs. Table 19 shows the four test outcomes; the proportion that is modelled with the healthy population model is the sum of the proportions classified as TN and FP. The QALYs from the stroke model are highest in the ICA-only strategy because in this strategy the largest proportion of patients is modelled with this model due to the highest morbidity induced by the initial treatment and initial ICA.
Population with known coronary artery disease
In the known population there is little difference between the three strategies, as all test outcomes are modelled with the EUROPA model. In the known population, every patient has CAD and therefore the healthy population model is not used for this population. In all cases, the ICA only has the lowest QALYs in the EUROPA model. This could be because ICA only has the largest overall mortality rate and therefore fewer people are modelled with the EUROPA model. The morbidity rate was the highest for the ICA-only strategy and therefore it accumulates the highest number of QALYs in the stroke model. The NGCCT–ICA strategy has the lowest morbidity rate and therefore it obtains fewer QALYs than the other strategies in the stroke model. More QALYs obtained in the stroke model can lead to less QALY gain in the EUROPA model; as the HRQoL in the stroke model is lower than in the EUROPA model, the higher complication rate of ICA is not favourable for the ICA-only strategy. The disutilities associated with the YRM are the largest (Table 58 shows no difference between the first two strategies but this is due to rounding) for the ICA-only strategy owing to the higher radiation dose of the ICA compared with the NGCCT.
Cost-effectiveness
The aim of this assessment was to estimate the cost-effectiveness of the NGCCT in difficult-to-image patients for a suspected and for a known CAD population. ICERs are presented below for the suspected CAD population (see Table 59) and for the known CAD population (see Table 60). The cost-effectiveness is based on probabilistic modelling as the models are non-linear. After running the subgroup-specific probabilistic sensitivity analyses we combined them into one population by using each subgroup-specific costs and effects (mean and SE), the correlations between the costs and effects, and the relative frequencies of the subgroups. The uncertainty regarding these relative frequencies was included in the probabilistic analyses. The relative proportions were based on expert opinion, as described above (see Proportions of patients in difficult-to-image subgroups and Table 53).
TABLE 59
Cost-effectiveness: suspected CAD population (sorted by QALYs)
TABLE 60
Cost-effectiveness: known CAD population (sorted by QALYs)
Population with suspected coronary artery disease
Table 59 presents very small differences in QALYs; however, the ICA-only strategy is in general more effective than the other two strategies. In most subgroups, the NGCCT–ICA strategy achieves fewer QALYs than the other strategies. The ICA-only strategy is the most expensive strategy; the NGCCT-only strategy is cost saving compared with the other strategies. The negative incremental costs of the NGCCT-only strategy are due to the lower costs in the diagnostic model. The lower costs in the diagnostic model are the result of the large difference between the cost prices of the NGCCT and the ICA. After combining the results of the subgroups, we see that the NGCCT-only strategy might be considered the most attractive. The ICER of NGCCT–ICA compared with NGCCT only is so high (£71,000) that, given conventional willingness-to-pay threshold of £20,000–30,000, it is unlikely that commissioners of health care would consider this a cost-effective use of NHS resources.
Population with known coronary artery disease
In the known CAD population the cost-effectiveness differed by subgroup (Table 60). The NGCCT–ICA and the NGCCT-only strategies are, in all subgroups, more effective than the ICA-only strategy. In the subgroups obese, HCS, HHR, and beta-blocker intolerance, the NGCCT–ICA strategy dominated the other strategies, being more effective and of lower cost than the other two strategies. In all subgroups, the NGCCT–ICA strategy was less expensive than the other strategies. When results of the subgroups are combined, the most attractive strategy would be to perform a NGCCT with ICA; this scenario yields the highest cost saving, and dominates ICA only. The ICER of NGCCT only compared with NGCCT–ICA is so high (£726,230) that it is unlikely to be considered cost-effective, given conventional willingness-to-pay threshold of £20,000 to £30,000.
Sensitivity analyses
Probabilistic sensitivity analyses were performed to explore the robustness of the outcomes. The NGCCT accuracy parameters, the prior likelihood of CAD for both populations, treatment decisions, complication and mortality rates, cost of events, cost of radiation, disutilities due to radiation, the QoL and transition rates in the disease progression model are varied in the sensitivity analysis. The test accuracy parameters of the ICA were not varied in the sensitivity analysis. Cost-effectiveness acceptability curves are presented in this section per population after combining the difficult-to-image subgroups into one population group. Table 61 presents the distributions of the parameters. Subgroup-specific parameters such as sensitivity, specificity, etc., are presented for only the obese subgroup of the suspected CAD population.
The acceptability curves in Figures 22 and 23 are in line with the base-case results presented in Tables 59 and 60. In the suspected population, in the range of thresholds of < £30,000, the NGCCT-only strategy has the highest probability of being cost-effective. Once thresholds are > £70,000, the three different strategies are equivalent. For the known CAD patients, the NGCCT–ICA strategy has the highest probability of being cost-effective, over the whole range of thresholds, while the ICA-only strategy has always the smallest probability of being cost-effective.

FIGURE 22
Suspected CAD population: CEAC.

FIGURE 23
Known CAD population: CEAC.
Scenario analyses
Scenario analyses based on a probabilistic analysis were performed to estimate the influence of the cost price of the NGCCT, the prior likelihood of the CAD suspected population, and the influence of the complication rates on the cost-effectiveness. In the first two scenarios, the cost price of the NGCCT is fixed at £150 and at £207, respectively. All other parameters are varied as in the PSA. Tables 62 and 63 show the results for the lower cost price of the NGCCT in both CAD populations for each subgroup. Tables 64 and 65 present the results of the higher cost price.
TABLE 62
Scenario analysis: NGCCT £150, CAD-suspected population
TABLE 63
Scenario analysis: NGCCT £150, known CAD population
TABLE 64
Scenario analysis: new-generation cardiac computed tomography £207, CAD-suspected population
TABLE 65
Scenario analysis: NGCCT £207, known CAD population
The prior likelihood of the suspected population was increased to 0.3. Table 66 presents the results of this scenario analysis.
Worst-case and best-case scenario analyses were performed to show the influence of the revascularisation and test complications on the cost-effectiveness. The influence of the rates on the cost-effectiveness in the suspected CAD population is shown below; see Tables 67 and 68.
TABLE 67
Best-case scenario analysis: upper limit complication rates in suspected CAD population
TABLE 68
Worst-case scenario analysis: lower limit complication rates in suspected CAD
Scenario analysis: new-generation cardiac computed tomography £150, coronary artery disease
A lower cost price means that the NGCCT–ICA and the NGCCT-only strategies become less expensive. The overall results do not change.
Scenario analysis: new-generation cardiac computed tomography £207, coronary artery disease
This scenario shows the impact of a higher NGCCT cost price on the cost-effectiveness. There is little change in the incremental costs, even when the cost of the NGCCT increases. In the suspected population the ICA-only strategy is still the most expensive strategy and NGCCT only the least expensive strategy. The higher price of the NGCCT led to a change in cost rank in the known CAD population. In the base case ICA only was the most expensive strategy but when the price is increased the NGCCT-only strategy is the most expensive strategy. Based on the ICER, for the suspected population NGCCT only remains the most favourable strategy, whereas for the known population the most favourable strategy remains NGCCT–ICA.
Scenario: prior likelihood, suspected population 0.3
‘ICA only’ is still the most expensive strategy and it gains the most QALYs. However, a higher prior likelihood leads to an increase in costs and a decrease in QALYs for all strategies. A higher prior likelihood means that more patients will have CAD and therefore more patients must be treated, which leads to higher costs. Furthermore, fewer patients will be modelled with the healthy population model resulting in a decrease in QALYs and more costs in the EUROPA model. With regards to the ICER, the NGCCT-only strategy remains the most favourable.
Scenario analysis complication rates
In the best-case scenario (Table 67) for the NGCCT, the complication rates are set at the upper limit of the 95% CI. ICA only is still the most effective strategy. However, the incremental QALYs gained by the ICA-only strategy have become smaller in comparison with the base-case analysis. As the ICA induces more complications than the NGCCT, this scenario analysis can be seen as the best-case scenario for the NGCCT strategies.
In the worst-case scenario (Table 68) for the NGCCT, the complication rates are set at the lower limit of the 95% CI, the ICA-only strategy is the most effective strategy. The incremental QALYs gained by ICA only increased compared with the base-case analysis. When assessing the balance between costs and effects, in both scenarios NGCCT only remains the most favourable strategy.
Scenario analysis covariates used in risk equation for obese subgroup
A study by Oreopoulos et al.106 examines the association between obesity and HRQoL in patients with CAD. It gives a good representation of an obese population with CAD (BMI of 25–30 kg/m2, n = 2310; BMI of 30–35 kg/m2, n = 1331; BMI of 35–40 kg/m2, n = 446; BMI of > 40 kg/m2, n = 178). The baseline characteristics that were found in the Oreopoulos et al. study106 are similar to the baseline characteristics used in our model. The baseline characteristics in the model are based on the systematic review and on the EUROPA trial. Not all covariates for the risk equations are presented in the Oreopoulos et al. study106 but gender, diabetes, existing vascular disease and previous MI are presented. We have performed scenario analyses within the obese group to study the effect of changing these covariates. The baseline values used in the obese known subgroup are existing vascular disease (stroke, TIA and peripheral vascular disease) 9.8%, female 34.1%, previous MI 64.7% and diabetes milletus proportion 34.1%. These values were changed to the following: existing vascular disease 7.5% and 13.5%; female 30%; previous MI 50% and diabetes milletus proportion 60%. These analyses (results not shown) show that these changes have no impact on our conclusions.
Cost-effectiveness of new-generation cardiac computed tomography in congenital heart disease
Model structure
The main model structure of the YRM for patients with congenital heart disease is identical to the structure discussed in detail above (see York Radiation Model). For the patients with congenital heart disease a number of scenario analyses were conducted, for example varying the age of cancer incidence. These are variations only in key parameters, not in the model structure. Further details are provided below. Regarding the potentially repetitive nature of the imaging in patients with congenital heart disease, experts emphasised that, owing to radiation exposure considerations, these patients are mostly imaged with echocardiography and MRI. We therefore assumed that the NGCCT would be used in a single instance for treatment planning, rather than for ongoing monitoring.
Model parameters
Base case
In the base case for patients with congenital heart disease, the key parameters of the YRM (i.e. utility, costs per scan, probability of cancer incidence given radiation, and cancers models) remain the same as for patients with CAD. The only difference is in the radiation doses for patients with congenital heart disease. These were based on an expert opinion, accounting for the particular diagnostic circumstances of patients with congenital heart disease (Table 69). We used these results to define five different age groups: 1-year-olds (infants), 5- to 10-year-olds (young children) and 25- to 35-year-olds (adults).
Patients with congenital heart disease can suffer from a range of cyanotic or non-cyanotic heart diseases. The timing of diagnosis and treatment and, hence, the use of a CT, depends on the particular lesion in question, but in most cases occurs in the first years of life. Depending on the lesion, further investigations and treatment might be necessary later in life. For aortic arch abnormalities (double aortic arch, vascular ring), for example a CT is undertaken at the time of diagnosis, usually in the first year of life. Similarly, for pulmonary atresia with MAPCAs either echocardiography, followed by cardiac catheterisation with invasive angiography or cross-sectional imaging (MRI or CT), is carried out in the first year of life and then again as required but often at the age of 2 or 3 years; for total anomalous pulmonary venous drainage/scimitar, echocardiography followed by cross-sectional imaging (MRI or CT) is undertaken at time of diagnosis and often again immediately before surgery (age 2–3 years). For lesions with both a vascular and airway component a CT may be carried out at diagnosis, which is usually soon after birth. In some cases, where a lesion has been previously treated using stents or pacemakers, MRI is unsuitable and patients require the use of CT when clinically indicated.
No clear evidence exists on to what extent NGCCT reduces the radiation dose at each scan. The general, NGCCT favourable assumption, based on information from one expert (see Appendix 7) was to assume a reduction of 50% compared with standard 64-slice CT.
Scenario analysis
In the scenario analyses a number of key parameters for patients with congenital heart disease were varied. These were (a) using the minimum radiation dose, (b) the maximum radiation dose, (c) an earlier age at cancer diagnosis, and (d) using the Biological Effects of Ionizing Radiation (BEIR) model for the effects of radiation on cancer incidence. Lastly, we ran (e) a scenario combining the least favourable assumption for the comparator, i.e. an NGCCT-friendly scenario that uses maximum radiation dose for a 64-slice CT scan, early onset of cancer, and the BEIR cancer radiation model.
The values for the (a) minimum and (b) maximum scenarios were based on the data shown in Table 64. The values for (c), the earlier age at cancer incidence scenario, were taken from the cancer model in the YRM.61 The earlier age with the corresponding disease costs and remaining QALYs is shown in Table 70. Note that for the age group of patients with congenital heart disease (age at exposure < 40 years), the YRM takes the incidence age of 40 years for breast cancer by default. The values for the BEIR model (Table 71) were published by the National Research Council for a 1999 US population.107 The BEIR study developed a more conservative risk model to estimate the relationship between exposure to ionising radiation and harmful health effects, primarily based on the cancer incidence data from the Life Span Study for the period 1958–98 and based on Dosimetry System 2002 (DSO2) dosimetry data.61
TABLE 70
Mean total costs and mean QALYs lost due to cancer, discounted at 3.5% per annum to age at cancer diagnosis assuming an early age at cancer incidence
TABLE 71
Probability for lifetime incidence of cancer for an exposure to 10 mSv according to the BEIR model for age groups indicated for NGCCT
For all of the scenarios, the uncertainty in the costs and remaining QALYs of the cancer module are modelled via a PSA. The values for this are shown in Table 46. For prostate cancer no data for the uncertainty exists. In addition, we varied for all scenarios (including the base case) the price of a 64-slice CT scan; the alternative value is shown in Table 72.
TABLE 72
Cost per scan for 64-slice CT in scenario analysis
Base-case results
Table 73 shows the intermediate result of the probability of lifetime cancer incidence for a given patient, group for the average radiation dose and the ranges as given by expert survey (HPA radiation/cancer model, assuming 50% male patients). The probability depends on overall radiation dose and age at exposure. Table 74 shows the absolute QALYs for each age group by scanner type. NGCCT leads to higher overall QALYs because of the lower probability of cancer. The number of patients needed to be scanned in each age group to gain 1 QALY (in absolute terms) is shown in Table 75.
TABLE 73
Probability of lifetime cancer for different ages in the base-case scenario for patients with congenital heart disease
TABLE 74
Absolute QALYs for both strategies in the base-case scenario for congenital heart disease (SD in parenthesis)
TABLE 75
Number of patients needed to scan (NGGCT) to gain 1 QALY, compared with 64-slice CT, in the base-case scenario
The costs caused by radiation-attributable cancer are shown in Table 76. Table 77 shows the maximum admissible cost that makes an NGCCT cost-effective, only accounting for the costs of radiation-induced cancer, for two different threshold values, i.e. a willingness to pay per gained QALY of £20,000 or £30,000, respectively. Table 78 shows the ICERS for the base-case scenario using two different costs for a 64-slice CT scan (£132.66 and £105.55, respectively); the price for the NGCCT is identical in both cases.
TABLE 76
Mean absolute radiation-induced cancer costs (£) of base case for patients with congenital heart disease (SD in parentheses)
TABLE 77
Threshold analysis showing the maximal additional price per patient that is admissible to make a NGCCT scan cost-effective
TABLE 78
Incremental cost-effectiveness ratio for base-case scenario (cost per NGCCT scan: £169.26)
Sensitivity analysis and scenario analysis results
In this section the results for the sensitivity analysis and different scenario analysis are presented. In the sensitivity analysis the inputs for the age at cancer incidence, expected disease costs and the expected remaining QALYs are varied (for details see Table 46). The key parameters for the scenario analysis are outlined above.
Table 79 shows the intermediate results of the probability of lifetime cancer incidence given radiation dose and age at exposure for the five patient groups using the BEIR model, and assuming 50% male patients.
TABLE 79
Probability of lifetime cancer for different ages (BEIR radiation-cancer model)
Sensitivity analysis
In Figure 24 the cost-effectiveness plane for the five different age groups of the base-case scenario is shown. The sensitivity analysis accounts for the uncertainty of the mean age of incidence, disease cost of cancer, and remaining QALYs in the YRM cancer module. In Table 80, selected summary statistics of the outcome distribution of the PSA are shown.

FIGURE 24
Cost-effectiveness plane for PSA of base-case scenario for five different age groups (note: origin not included).
TABLE 80
Summary statistic of the distribution of the incremental effects, the incremental costs, and the ICER of the PSA in the base-case scenarios
Scenario analysis
In this section the results of the five different scenario analyses are shown. These were (a) using the minimum radiation dose, (b) the maximum radiation dose, (c) an earlier age at cancer diagnosis and (d) using the BEIR model for the effects of radiation on cancer incidence. Lastly, we ran (e) a scenario combining the least favourable assumption for the comparator, i.e. an NGCCT-friendly scenario that uses maximum radiation dose for a 64-slice CT scan, early onset of cancer, and the BEIR cancer-radiation model.
Tables 81 and 82 show the disease in the costs of radiation-induced cancer and the expected absolute QALYs for each age group in the five different scenario analyses. The corresponding differences are reported in Tables 83 and 84.
TABLE 81
Absolute radiation-induced cancer costs for scenario analysis in GBP
TABLE 82
Absolute QALYs for the five different age groups in the scenario analysis
TABLE 83
Differences in absolute radiation-induced cancer costs for scenario analysis between CT64 and NGCCT
TABLE 84
Differences in absolute QALYs between CT64 and NGCCT
Tables 85 and 86 show the maximum admissible cost that makes an NGCCT cost-effective for two different threshold values, i.e. a willingness to pay per gained QALY £20,000 or £30,000, respectively. Tables 87 and 88 report the ICERs for the scenario analyses in each age group, for a 64-slice CT price of £132.62 and £105.55, respectively.
TABLE 85
Threshold analysis showing the maximal additional price per patient that is admissible to make a NGCCT scan cost-effective at a willingness to pay of £20,000 for scenario analysis
TABLE 86
Threshold analysis showing the maximal additional price per patient that is admissible to make a NGCCT scan cost-effective at a willingness to pay of £30,000 for scenario analysis
TABLE 87
Incremental cost-effectiveness ratio (£ per QALY gained) for scenario analysis with cost per NGCCT scan: £169.26 and cost per CT64 scan £132.62
TABLE 88
Incremental cost-effectiveness ratio for scenario analysis with cost per NGCCT scan: £169.26 and cost per CT64 scan £105.55
Only in the NGCCT-friendly scenario do the ICERs decrease significantly, ranging from £28,000 per QALY gained for the youngest patients to £4300 per QALY gained for the adult patients. Looking at Tables 83 and 84, it is clear that of all key parameters, setting the radiation dose to the maximum of the range given by the expert has the highest impact on the cancer-related costs to be saved and QALYs to be gained. However, this upper value of the range of 25 mSv should be regarded with caution. It is very likely that the expert has implied a range of values ever used in his/her patient population, and it is very unlikely that it was implied that the average dosage could range from 4 to 25 mSv. The fact that for all other scenarios the ICER remains > £30,000 indicates that, even with the uncertainty about the various assumptions in mind, it can reasonably be concluded that the use of NGCCT instead of 64-slice CT in order to reduce radiation exposure is not cost-effective in this patient group.
Summary
In this chapter, we assessed the cost-effectiveness of NGCCT in two different populations (Table 89). The first is the comparison of NGCCT compared with ICA in difficult-to-image CAD patients and the second is the comparison of NGCCT compared with 64-slice CT in patients with congenital heart disease.
TABLE 89
Summary baseline cost-effectiveness
The CAD population was divided into two subpopulations: the suspected CAD population and the known CAD population. Patients suspected of CAD are patients who have chest pain or other symptoms suggestive of CAD. Patients with known CAD are patients who have previously been diagnosed with CAD and whose symptoms are no longer controlled by drug treatment and/or being considered for revascularisation. The use of NGCCT has different purposes in the two CAD populations: for the suspected CAD population the purpose is to diagnose patients with CAD and for the known CAD population the purpose is to aid decision-making regarding a revascularisation.
For the CAD population, five different models were combined to estimate the cost-effectiveness of the NGCCT:
- a decision tree that models the diagnostic pathway
- an alive–dead Markov model for ‘healthy’ patients without CAD65
- a stroke model to estimate the impact of test and treatment-related stroke
- a model for the prognosis of patients with CAD (the EUROPA model)69
- a model to assess the impact of imaging due to radiation on cancer morbidity and mortality.61
The last of these five models, the YRM, was also used to assess the cost-effectiveness of the use of NGCCT to lower radiation exposure in patients with congenital heart disease.
The health economic analysis of the use of NGCCT in difficult-to-image patients with CAD showed that the use of NGCCT instead of invasive CA may be considered cost-effective. In patients with suspected CAD, the NGCCT-only strategy might be considered the most attractive. The ICER of NGCCT–ICA compared with NGCCT only is so high (£71,000) that it is unlikely to be considered cost-effective, given a conventional willingness-to-pay threshold of £20,000 to £30,000. In patients with known CAD, the most attractive strategy would be to perform a NGCCT with ICA; this scenario yields the highest cost saving and dominates ICA only. The ICER of NGCCT only compared with NGCCT–ICA is so high (£726,230) that it is unlikely to be considered cost-effective.
When taking uncertainty into account, these findings are confirmed. In the suspected population, in the range of thresholds of < £70,000, the NGCCT-only strategy has the highest probability of being cost-effective. For thresholds above £70,000, the three different strategies are more or less equivalent. For the patients with known CAD, the NGCCT–ICA strategy has the highest probability of being cost-effective, over the whole range of thresholds, whereas the ICA-only strategy has always the smallest probability of being cost-effective.
The key drivers behind these results are the percentage of patients being misclassified (as a results of test accuracy data and prevalence of disease) and the complication rate for ICA and revascularisation (see Table 55). In the ICA-only strategy, all patients are at risk for ICA-induced morbidity and mortality, whereas the TPs are also at risk for the revascularisation-induced morbidity and mortality. In the NGCCT-only strategy, misclassification leads to FPs who undergo unnecessary revascularisations with the associated complications, whereas ICA complications cannot occur. Overall, in the population of suspected CAD, the NGCCT-only strategy has the lowest overall mortality rate – less than half of that of ICA only. To some extent, the same results apply for the known CAD population; here the overall mortality and morbidity is lowest in the NGCCT–ICA strategy. ICA only has the highest overall mortality and morbidity rate, regardless of the population.
As noted previously, it is important to realise that the percentage of patients being misclassified is a function of both diagnostic accuracy and the prior likelihood. If the prior likelihood increases, the percentage of FNs also increases while the percentage of FPs decreases. This explains to some extent why the results for the suspected CAD population are slightly different than for the known CAD population, even though for both populations the same accuracy was assumed.
Currently, there is uncertainty about the estimate of the cost price of a NGCCT scan, as we had to make various assumptions. Therefore, we performed a scenario analysis changing this cost price to £207 per scan, and this did not alter our conclusions.
The disaggregated results in Tables 57 and 58 show that the inclusion of the reduced radiation effects has only very minimal impact on the outcomes.
The cost-effectiveness analysis of the use of NGCCT in congenital heart disease showed that, when only considering the radiation exposure, the use of NGCCT instead of 64-slice CT is not cost-effective in this group. The ICER ranged from £521,000 per QALY gained for the youngest patients to £90,000 per QALY gained for the adult patients. The reduction in radiation by replacing a single 64-slice CT scan by a NGCCT scan is small and leads to only a minor decrease in radiation-related cancer incidence, therefore it cannot justify the additional costs of the NGCCT scan.
Various scenarios were explored to assess the impact of the main assumptions. Only in the most unlikely scenario, i.e. an average radiation dose of 25 mSV for a 64-slice CT, do the ICERs decrease significantly. The fact that for all other scenarios the ICER remains > £30,000 indicates that, even with the uncertainty about the various assumptions in mind, it can reasonably be concluded that the use of NGCCT instead of 64-slice CT in order to reduce radiation exposure is not cost-effective in this patient group.
- Assessment of cost-effectiveness - A systematic review and economic evaluation o...Assessment of cost-effectiveness - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
- Meta-analysis of diagnostic tests for acute kidney injury - The future for diagn...Meta-analysis of diagnostic tests for acute kidney injury - The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation
- Assessment of clinical effectiveness - A systematic review and economic evaluati...Assessment of clinical effectiveness - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
- Health economic results - Saline in Acute Bronchiolitis RCT and Economic evaluat...Health economic results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review
- Methods - Preoperative intravenous iron for anaemia in elective major open abdom...Methods - Preoperative intravenous iron for anaemia in elective major open abdominal surgery: the PREVENTT RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...