Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Olson J, Sharp P, Goatman K, et al. Improving the economic value of photographic screening for optical coherence tomography-detectable macular oedema: a prospective, multicentre, UK study. Southampton (UK): NIHR Journals Library; 2013 Nov. (Health Technology Assessment, No. 17.51.)

Improving the economic value of photographic screening for optical coherence tomography-detectable macular oedema: a prospective, multicentre, UK study.
Show detailsVersion 2.1 (4th June 2009).
1. Study summary
Full title
Improving the value of screening for diabetic macular oedema using surrogate photographic markers.
Short title
Screening for diabetic MO using surrogate photographic markers.
Official website
Funding
£432,174 from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. Grant reference 06/402/49.
Ethical approval
Main REC: North of Scotland Research Ethics Committee.
REC ref: 07/S0801/107.
Date of approval: 17/12/07.
Important dates
Start date: 1st May 2008.
50% target recruitment point: 1st May 2009.
100% target recruitment point: 31st October 2009.
End date: 30th April 2010.
Draft final report due: 14th May 2010.
Collaborators
Aberdeen University School of Medicine, NHS Grampian, NHS Tayside, NHS Lothian and Borders, Royal Liverpool and Broadgreen University Hospital NHS Trust, Queen Margaret Hospital, Dunfermline, Oxford Radcliffe Hospitals NHS Trust, Heart of England NHS Trust, Norfolk and Norwich University Hospitals NHS Trust.
Study objectives
The purpose of this study is to determine the best method for detecting sight-threatening macular oedema using photographic surrogate markers for people with diabetes in the context of national screening programmes.
Background
Macular oedema is associated with several conditions which cause irreversible vision loss, including diabetic retinopathy. It may be classified according to the fluid distribution: diffuse oedema is a general thickening of the central retina caused by either extensive capillary dilation or capillary closure, while focal oedema is centred on specific vascular abnormalities, such as microaneurysms. Accumulated fluid defocuses the image on the retina, reducing visual acuity. If oedema persists, the increased pressure may lead to irreparable photoreceptor damage or retinal detachment.
Since retinal thickening is not visible directly on the retinal photographs used by screening programmes, people are referred to ophthalmology clinics on the basis of a range of surrogate photographic markers, such as exudates within a certain distance of the foveal centre. Evidence from the Early Treatment of Diabetic Retinopathy Study suggests that exudates may be a sensitive marker of macular oedema (Bresnick 2000). However, as the ETDRS excluded patients with mild retinopathy in the absence of exudate, the results are not applicable to screening programmes in the United Kingdom, where 60% of patients have no visible signs of retinopathy and over 30% have only mild retinopathy. Evidence from the Grampian Retinal Screening Programme suggests that only 12% of patients with surrogate markers referred to an ophthalmologist have indications of macular oedema when examined by slit-lamp biomicroscopy. Similarly, a retrospective analysis from Liverpool, including 257 patients referred from the screening programme to the ophthalmology clinic between December 2001 and June 2002, found that only 14% had evidence of macular oedema (unpublished data).
Macular oedema has traditionally been assessed clinically using a combination of slit-lamp biomicroscopy, stereo photography and stereo fluorescein angiography. However, these techniques have a number of limitations. Foremost is that they are only qualitative assessments, which are relatively insensitive to thickness changes. Furthermore, slit-lamp examination does not provide a pictorial record and, together with stereo photography, is known to be biased by the presence or absence of exudates. Although the angiogram is a sensitive test for leakage, the assessment of thickening is very subjective. Best corrected visual acuity has also been used as a surrogate indication of thickening, but is neither sensitive nor specific, being affected by several factors besides macular thickness.
Study design
A total of 4000 patients with photographic signs of maculopathy (exudates within two disc diameter radius, blots or dot haemorrhages within one disc diameter radius) shall be recruited from the seven study centres. Each subject will have photography and optical coherence tomography on both eyes where possible. 10% of patients are expected to have ungradeable images and will be unsuitable for the study.
All relevant lesions visible in the colour photographs will be annotated by the research nurse in Aberdeen using computer-assisted software. The optical coherence tomography images will be analysed both quantitatively and qualitatively by the research nurse. Software will be developed to analyse the distribution of retinal lesions in order to find the significance of the photographic marker patterns on the likelihood of clinically significant macular oedema.
Proposed outcome measures
A sample of patients attending diabetic retinopathy-screening programmes will receive an optical coherence tomography examination in addition to the standard digital photograph. An expert grader will assess all digital images independently for the presence of different surrogate photographic markers. The sensitivity and specificity of standard referral criteria (based on the presence of surrogate markers) will be assessed using optical coherence tomography as the reference standard.
Although manual grading will always have a role in the assessment of retinal images, the scale of the current diabetes epidemic means that automation will have to play a key role if the Liverpool Declaration's target of offering annual systematic screening to at least 80% of the estimated 35 million people with diabetes in Europe is to be achieved. Furthermore, given the low specificity of current referral criteria, it is important to investigate whether specificity can be improved using more complex patterns of surrogate markers, using either computer-assisted annotation or fully automated computer analysis, so that scarce ophthalmology resources are used most effectively.
Derived sensitivity/specificity estimates will be used to assess the costs and consequences (i.e. the number of appropriate/inappropriate ophthalmology referrals) of using alternative surrogate markers, and patterns of surrogate markers, for the detection of macular oedema, using manual, computer-assisted annotation and fully automated detection systems.
It is important to look at the implications for health care delivery of introducing this technology into systematic screening programmes for diabetic retinopathy. We will therefore model long-term costs and outcomes (visual loss and quality adjusted life years) of the alternative screening strategies using epidemiological literature and available cost estimates.
Sample size calculation
If 33333 patients are screened across five centres, 4000 patients would be expected to have surrogate markers (12%) of whom 400 would be expected to have macular oedema. If there are 4000 patients with any markers, the power for detecting a change in the referral specificity of at least 20% will be much higher than 80%.
However, a new diagnostic test (using a more specific combination of features) which has increased specificity may also have decreased the sensitivity. Therefore, since there are fewer true cases with macula oedema than true controls, the crucial issue for the power of this study is whether or not a small change in sensitivity can be detected.
With 400 cases of macula oedema there will be 80% power to detect a difference in sensitivity of 3% (99% vs. 96%) between two diagnostic tests (any markers vs. a specific combination of markers) when the percentage of true cases where the diagnostic tests disagree is expected to be 5%. A McNemar's test of equality of paired proportions has been used with a 0.05 significance level.
Research governance
Research activities at each of the participating centres will be carried out in accordance with the Department of Health's Research Governance Framework for Health and Social Care. The project will be registered with the Institute of Applied Health Sciences (University of Aberdeen) and each appropriate NHS trust. Copies of the protocol and records of all important documents will be cross referenced and filed so that they are available at any given time. Records relating to all procedures carried out will be kept so that the research process is clearly understandable and repeatable. NHS Grampian has agreed to act as the sponsors for the study.
A Trial Steering Committee has been set up. The chair, Dr Caroline Styles, is a consultant ophthalmologist in Fife with experience of retinal screening (she was later replaced by Dr Rod Harvey). Professor Alex Elliot, a medical physicist from Glasgow, brings medical image processing experience. Mr Steve Graham is the committee patient representative. Ms Alison Farrow is a retinal photographer in Aberdeen (she was later replaced by Dr A Manivannan). Finally Dr John Olson and Professor Peter Sharp are also members.
2. Centre contact details
| Centre | Principal investigator(s) | Other contacts |
|---|---|---|
| University of Aberdeen and NHS Grampian | Dr John Olson | Dr Keith Goatman |
| Heart of England NHS Trust | Professor Paul Dodson | Ms Jane Pitt |
| NHS Tayside | Professor Graham Leese Dr John Ellis | |
| NHS Lothian | Dr Ken Swa | Dr Shyamanga Borooah |
| Royal Liverpool & Broadgreen University Hospitals Trust | Professor Simon Harding Professor Deborah Broadbent | Dr Yalin Zheng |
| Oxford Radcliffe Hospitals NHS Trust | Professor Victor Chong | Ms Sue Beatty |
| Queen Margaret Hospital, Dunfermline | Dr Caroline Styles |
Dr John Olson is the project Chief Investigator (ten.shn@noslo.nhoj). For general enquiries about the study contact Dr Keith Goatman (ku.ca.ndba@namtaog.a.k).
Dr Caroline Styles (ten.shn@selyts.enilorac) is the chair of the Trial Steering Committee (later replaced by Dr Rod Harvey).
3. Patient selection and recruitment
Each centre shall collect data from 800 patients fulfilling the inclusion criteria below.
Inclusion Criteria
Age 18 or older.
A trained grader confirms that the fundus photograph of at least one eye shows diabetic eye disease including any of:
Microaneurysms/dot haemorrhages within one disc diameter radius of the macula.
Blot haemorrhages within one disc diameter radius of the macula.
Exudates within two disc diameter radius of the macula.
Able and willing to provide signed informed consent.
Exclusion Criteria
The patient has had macular or pan-retinal laser treatment [clarification to protocol added version 2.0].
The patient has had an intraocular injection [added in protocol version 2.0].
The patient is pregnant (since oedema may be present independent of gestational diabetes) [Added in protocol version 2.0].
Intraocular surgery within one year of enrolment.
The screening fundus photograph is a technical failure (i.e. the macula vessels are not clearly visible and/or the field of view does not include a region of diameter two disc diameter radius s about the centre of the macula).
Contraindications to pupillary dilation/intolerance or hypersensitivity to mydriatics in either eye if dilation is necessitated.
How patients are recruited will depend on the local set-up at each centre. In Aberdeen patients with exudates or blots within 1 disc diameter radius (grade code M2) are routinely referred from the screening service to see an ophthalmologist. These patients are now routinely offered optical coherence tomography scans and therefore only need to be consented to allow their data to be used in the study. Patients with exudates within 1 disc diameter radius and 2 disc diameter radius (grade code M1) will need to be invited for an optical coherence tomography scan they would not normally receive, so will need to be consented both for the imaging and use of data. Finally, patients with microaneurysms/dot haemorrhages within 1 disc diameter radius do not trigger a specific grade in the Scottish grading system and will have to be manually chosen during screening grading. As for the M1 category, these patients will have to be invited for an optical coherence tomography they would not normally receive, so will need to be consented both for imaging and use of their data.
4. Retinal photography, visual acuity and Glitazone.
Visual acuity
Log-MAR (or Snellen if Log-MAR not available) pin-hole or best-corrected visual acuity measurement for both eyes. [Best-corrected added version 2.1]
Record if the patient has a long-standing, non-diabetic reason for a low visual acuity measurement, for instance due to amblyopia. This must be recorded on the web upload form. [Added version 2.1]
Photography
Single views of both eyes:
Approximately 45 degree field of view.
Approximately macula centred.
Colour digital photograph, at least 3 megapixels (ideally less than 7 megapixels and, if using JPEG compression, set for highest quality).
Adequate field of view (should show 2 disc diameter radius around centre of macula).
Adequate clarity (i.e. adequate to see macular microaneurysms, if present). If there are problems obtaining a retinal photograph it is very likely to be difficult obtaining an adequate optical coherence tomography scan.
Mydriasis only necessary if pupil size too small for imaging. Camera small pupil facility acceptable providing 2 disc diameter radius visible around macula centre.
Note that the maximum allowed time between photography and optical coherence tomography is four weeks to reduce errors due to lesion changes between photograph and optical coherence tomography.
Example images showing adequate and inadequate clarity and field of view are available from the website. (http://www.abdn.ac.uk/ismo/).
Glitazone [Added version 2.1]
The glitazone class of drugs appears to be associated with diabetic macular oedema [1]. If the patient has used any of the following drugs within the past six months this must be recorded on the web upload form:
Rosiglitazone: Avandia, Avandamet.
Pioglitazone: Actos Competact.
[1] Fong DS and Contreras R. Glitazone use associated with diabetic macular edema. American Journal of Ophthalmology 2009;147:583–586.
5. Optical Coherence Tomography
Optical coherence tomography scanner choice
When the project was first proposed every centre had use of the Zeiss Stratus optical coherence tomography scanner (often known as OCT3). Since the funding was granted a number of new instruments based on a spectral domain method have become available. Initial tests on the Zeiss Cirrus, Topcon OCT1000 and Heidelberg Spectralis show these new scanners have some advantages for this study. Hence centres will not be required to use the Stratus but instead encouraged to use the best available equipment.
In order to reduce variation due to different manufacturers, models and operators:
Only accredited operators shall acquire scans.
It is recommended that, where possible, the same scanner is used for the duration of the study. If more than one scanner is used the scanner used must be indicated. If a scanner must be replaced it may be replaced by any model.
Dates of services and faults should be recorded in the study folder.
Optical Coherence Tomography protocol
Two types of data are required from the optical coherence tomography scanner:
A thickness map divided into nine Early Treatment Diabetic Retinopathy Study regions centred on the centre of the macula and with a diameter of 6 mm.
Orthogonal B-mode 6-mm cross-sections centred on the centre of the macula.
Specific instructions for different optical coherence tomography models are given in appendix A to the protocol.
Accreditation process
As in other multicentre imaging studies, to avoid inter-centre imaging variation all optical coherence tomography operators are required to be accredited before submitting data for the study. Operators should submit a simple portfolio of images (described below) collected using the optical coherence tomography scanner intended for the study. Images will be checked for macula position, adequate image quality and differences between thicknesses in the nine Early Treatment Diabetic Retinopathy Study regions of the repeat scans. The accreditation images are as follows:
(a) Normal eyes
Repeat macula maps of the same normal eye as per scanner model protocol above.
(b) Macular oedema
Repeat macula maps of the same eye showing obvious macular oedema (i.e. central thickness at least 300 microns).
For scanners (such as the Stratus) which do not automatically generate high-resolution orthogonal cross-sections (“cross hair scans”) also perform the cross-hair scan.
Accreditation Upload procedure
Go to the study website: http://www.abdn.ac.uk/ismo/
Login using your supplied username and password. The admin functions will appear once you are logged in. Select “Accreditation” from the menu and follow the instructions for uploading images.
6. Data transfer
Photographs and optical coherence tomography scans are transferred to Aberdeen using the web-based upload service at:
Log in using your supplied username and password and the admin functions will appear. Select “upload data” and follow the on-screen instructions.
Note: the server automatically generates an anonymous subject ID. If you enter the subject name and hospital ID number these will print a front sheet for your study folder, but will not be transmitted to Aberdeen.
Data protection and personal information
The personal patient information you enter is not transferred to the webserver, it is just used to produce the print out.
However, patient information may appear on the photographs or optical coherence tomography images which are transferred to Aberdeen. Although the University of Aberdeen webserver has been registered to allow the storage of personal information, personal information is not required for this study we do not wish to keep it, and have not sought ethical permission to do so. Therefore all personal information will be automatically removed from images that are uploaded to Aberdeen. It is therefore vital that if centres wish to be able to cross reference data from their own clinical records that they keep a local copy of the anonymous study ID and real patient ID.
Image grading
(a) Colour photographs
All fundus photographs will be assessed in Aberdeen by a trained reference grader. An experienced grader will train the reference grader in the identification of the features of diabetic retinopathy and in the use of the software. The trainer will quality assure the annotations of 100 images as part of the training phase. The following features will be noted:
Presence of microaneurysms/dot haemorrhages and blots within 1 disc diameter radius.
Presence of exudates within 1 disc diameter radius and 2 disc diameter radius.
All the above lesions will be annotated on images, using computer-assisted annotation software, to enable pattern analysis of the lesion distributions.
(b) Optical Coherence Tomography data
All the optical coherence tomography images will be graded quantitatively and qualitatively for area, amount and site of retinal thickening based on the generated thickness maps. Optical coherence tomography studies suggest that oedema may be reliably detected by slit-lamp biomicroscopy if it has a retinal thickness of at least 300 µm (Hee 1998). This agrees with the Early Treatment Diabetic Retinopathy Study, which used a reference thickness of 250 µm for “clinically significant” macular oedema.
In this study, macular oedema will be defined as an optical coherence tomography retinal thickness of 300 µm or greater (relative to Zeiss Stratus measurements) in any map region within one disc diameter radius of the centre of the fovea (i.e. in any of the five central Early Treatment Diabetic Retinopathy Study map regions).
Appendix A: Specific Optical Coherence Tomography scanner instructions
(a) Zeiss Stratus Optical Coherence Tomography
Data acquisition
For each eye, use the fast macular map protocol to obtain six intersecting radial lines with a length of 6 mm. Total acquisition time is approximately two seconds.
Scans will be considered acceptable if: (1) the standard deviation of the central foveal measurements from the six cross-sections is less than 10% of the mean central measurement, and (2) the recorded signal strength is at least 4, and (3) there are no warnings about low analysis confidence, missing data or high variance, and (4) there are no visible boundary tracking errors.
Data export
Two pieces of evidence are required to confirm the presence of macular oedema: (1) the thickness map giving the Early Treatment Diabetic Retinopathy Study region thicknesses in microns and (2) a cross-sectional image showing the thickening is due to dark, fluid-filled spaces.
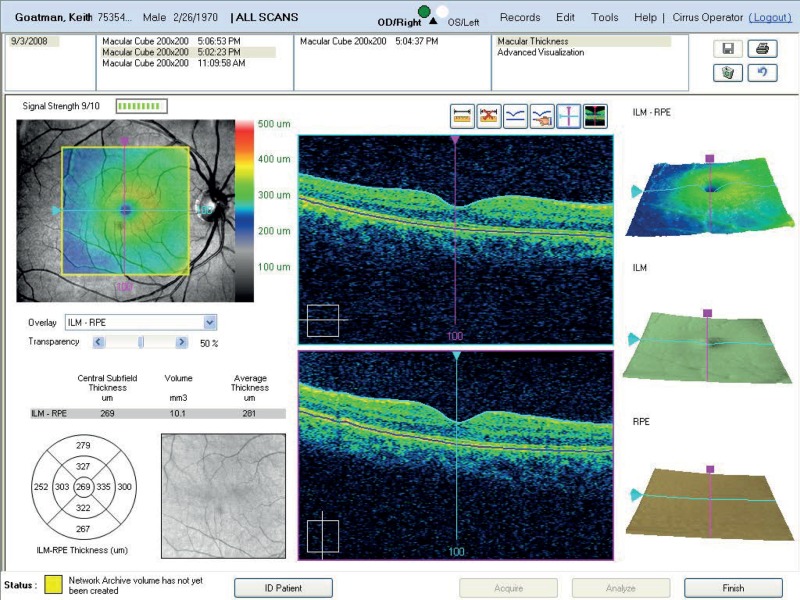
Thickness maps: Generate thickness maps by selecting the best scans for the left and right eyes and clicking on the “analysis” tab and selecting “Retinal Thickness/Volume (OU)”. Export the result as a PDF document using the “Print to PDF file” option. An example is shown in figure Stratus 1.
FIGURE
Stratus 1 – example thickness map (PDF file).
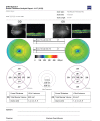
Does the horizontal cross-section image included on the thickness map (a) pass through the region of greatest thickening and (b) clearly show the thickening? If not, export an additional cross-sectional image for one or both eyes as below:
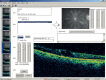
Cross-sectional image(s): The PDF report includes a low-resolution/low-quality image of the horizontal cross-section for each eye. If one or both images either does not pass through the region of thickening, or is insufficient quality to show the oedema, a higher quality image of the cross-section which best shows the thickening should be exported. Select one eye at a time. Click on the analysis tab and click on the “Scan Selection” button at the bottom of the analysis section. The six cross-sections are shown on the left of the screen (see figure Stratus 2). Select the one which best shows the thickening and click on the “Export JPEG” button to save the cross-section as a JPEG image (see figure Stratus 3). Repeat for the other eye if necessary.
FIGURE
Stratus 2 – Cross-section selection for export.

FIGURE
Stratus 3 – Exported cross-section (JPEG file).
(b) Zeiss Cirrus Optical Coherence Tomography
Data acquisition
The Cirrus has two macular mapping protocols. The first (512 × 128) acquires 128 horizontal cross-sections, each containing 512 A-scans. The second (200 × 200) acquires 200 horizontal cross-sections, each containing 200 A-scans. We recommend use of the 200 × 200 protocol as it produces equally high-quality maps in a slightly shorter time; less time for patient movement, increasing the probability of obtaining a successful scan. High-resolution orthogonal cross-sections are also acquired automatically during the scan.
Scans will be considered acceptable if: (1) the signal strength is at least 5/10, and (2) there are no obvious signs of movement, and (3) there are no visible boundary tracing errors, and (4) the fixation point is within 250 microns of the centre of the foveal pit (where visible).
Data export
Two pieces of evidence are required to confirm the presence of macular oedema: (1) the thickness map values and (2) dark fluid-filled spaces on the cross-sectional image(s). If the orthogonal cross-sections do not intersect the region of thickening then a second image is required with the cross hairs moved to pass through the thickened region.
To export the data select print and choose the option to save the printout as a TIFF image (note the quality of the PDF image is too poor for the study).
Upload the optical coherence tomography images and colour photographs using the web-upload system at: http://www.abdn.ac.uk/ismo/
(c) Topcon OCT-1000 Mark I
The Topcon OCT-1000 is essentially a non-mydriatic fundus camera with an optical coherence tomography spectrometer attached. As well as the spectral domain optical coherence tomography data it also acquires 3MP colour photographs which are ideal for use in this study.
Important note about volume scaling
The OCT-1000 has an option to change the relative horizontal and vertical scale of the B-mode slice display. Topcon call this the “volume scale”, and it has options of 1 : 1, 1 : 2 or 1 : 3. You do not need to change this setting for the study but you should inform Aberdeen of your preferred local setting. The value may be viewed/changed by selecting Tools from the main menu bar, Options => Volume scale.
Data capture
Use the “3D scan” acquisition mode.
Select the 6 × 6 mm rectangular scan area and 512 × 128 lines (128 lines of 512 A-scans per line).
Use the camera macular-centred (M) fixation point.
Once an acceptable scan has been acquired save the data.
Colour photograph export
The colour photograph is exported by selecting “Export” from the main menu bar and selecting “Fullsize fundus”. Select PNG filetype (the jpeg quality is too poor for the study) and choose a filename, ideally including the anonymous patient ID (e.g. for Aberdeen A0123_photo_left.png).
Optical Coherence Tomography data export
Three pieces of information are required for the study:
The nine region Early Treatment Diabetic Retinopathy Study thickness map.
The central horizontal B-mode section (slice 64).
The horizontal B-mode section which best includes the severest thickening within the Early Treatment Diabetic Retinopathy Study region, if different from (b) above.
These are obtained from the captured data as follows:
Analyse data: Make sure the raw study data has been analysed (this estimates the region boundaries on the 128 B-mode sections).
The default view shows B-mode slice 64 on the left hand side and the colour photograph on the right.
Display Early Treatment Diabetic Retinopathy Study grid: Click on the Early Treatment Diabetic Retinopathy Study icon by the colour photograph to overlay thickness values on the colour photograph (see figure Topcon example 1).

FIGURE
Topcon example 1 showing B-mode section 60 on the left hand side and the colour photograph with Early Treatment Diabetic Retinopathy Study region thicknesses on the right.
Optional reposition: In images where the foveal pit is obvious, place a cursor on the deepest part of the pit and check that this corresponds to the centre of the Early Treatment Diabetic Retinopathy Study grid. If the difference is more than approximately 200 microns (for reference the central Early Treatment Diabetic Retinopathy Study region is 1000 microns diameter) reposition the Early Treatment Diabetic Retinopathy Study grid using the “reposition” option in the grid menu to the left of the colour photograph. This is done by dragging the grid while holding down the left mouse button. Note that moving the grid will mean part of one or more of the outer regions will be outside the captured data (indicated by the green box). If the fixation error is too large and the grid is moved too far then N/A will appear in place of a thickness measurement. This will be counted as a technical failure and should be repeated. However, the N/A designation appears too conservative. For example, figure Topcon example 1 shows a small corrective shift of approximately 130 microns. Figure Topcon example 2 shows a much larger correction shift of approximately 1000 microns, where 55% of the area of the outer inferior region is outside the collected optical coherence tomography image. This is not acceptable and consequently images requiring a corrective shift of more than 500 microns will be considered a technical failure and should be repeated.
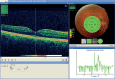
FIGURE
Topcon example 2 showing an excessive reposition of the Early Treatment Diabetic Retinopathy Study grid.
Central section screen-shot: take a screenshot showing the central horizontal section (slice 64) and the colour photograph with the Early Treatment Diabetic Retinopathy Study grid overlaid. Select Export from the main menu bar followed by “Screenshot”. Save the screenshot as a PNG format image, ideally using a filename convention which includes the patient anonymous ID. Figure Topcon 1 shows an example screenshot.
Maximum thickness screen-shot: if the central slice does not include the area of thickening, change the displayed section by scrolling the mouse wheel until the section with the thickening is displayed and repeat the screenshot as above. If the central section adequately shows the thickening then this image is not required.
For each eye there will be one colour photograph and one or two optical coherence tomography images to upload using the web-based system at http://www.abdn.ac.uk/ismo/
(d) Heidelberg Spectralis
The Heidelberg Spectralis is currently the fastest optical coherence tomography scanner on the market, acquiring 40,000 A-scan lines per second. It has two features not found on any other optical coherence tomography scanner: eye tracking and multiple lines acquisition to reduce noise (ART). It also has more user adjustable parameters than any other scanner.
The default macular thickness protocol acquires 19 horizontal sections with horizontal and vertical field of views of 20 and 15 degrees respectively. By default each line is acquired multiple times (with ART enabled). Note: This default protocol is not suitable for the study, as it does not cover the required 6 mm diameter region about the centre of the macula.
Data acquisition
The field of view should be centred on the macula.
Change the horizontal field of view (using the right cursor key) from 20 degrees to 30 degrees. The current field of view and number of sections is displayed at the bottom of the screen in acquisition mode.
Change the vertical field of view (using the UP cursor key) from 15 degrees to 25 degrees (which also increases the number of slides from 19 to 31).
The number of ART repeat scans can be set between 2 and 100. A setting of 3 is adequate for this study.
When the correct field of view is obtained lock the position using the large black circular button on the touch pad.
Signal strength should be greater than 15/40 (image quality bar blue rather than red).
Data analysis and export
Three pieces of information are required for the study:
The nine region Early Treatment Diabetic Retinopathy Study thickness map.
The horizontal B-mode section through the centre of the macula.
The horizontal B-mode section which includes the severest thickening within the Early Treatment Diabetic Retinopathy Study region. If this is the same slice as (b) above another image is not required.
(a) Nine region thickness map
For the nine region thickness map select concentric circle diameters of “1, 3, 6 mm Early Treatment Diabetic Retinopathy Study” (see figure Spectralis 2).

FIGURE Spectralis 2
Example screen print showing macular thickness map.
If the foveal pit is visible and there is an obvious fixation error the Early Treatment Diabetic Retinopathy Study grid may be moved using the mouse so as to be centred on the foveal pit. If the require shift is too large one or more of the outer regions will go outside the field of view and the thickness value will be blank. These are considered a technical failure and should be repeated.
Export the screenshot image in PNG format (Please do not use JPEG. Even using the highest quality setting the images are saved with the colour information at half the resolution of the brightness information, which blurs coloured features with sharp edges, such as the text.)
(b) and (c) Horizontal cross-section
Produce a screenshot of the horizontal slice centred on the macula (see figure Spectralis 1) and save in PNG format.

FIGURE Spectralis 1
Example screen print of horizontal section.
Optionally, if the region of greatest thickening is not visible on the central slice, select the slice which shows the greatest thickening and produce a screenshot as above.
Upload the data using the web-based system at: http://www.abdn.ac.uk/ismo/
For each eye there will be one colour photograph and two or three (where the thickening is not visible on the central section) optical coherence tomography images to upload.
- Protocol - Improving the economic value of photographic screening for optical co...Protocol - Improving the economic value of photographic screening for optical coherence tomography-detectable macular oedema: a prospective, multicentre, UK study
- Acknowledgements - Adalimumab, etanercept and ustekinumab for treating plaque ps...Acknowledgements - Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation
- List of supplementary material - Strategies for older people living in care home...List of supplementary material - Strategies for older people living in care homes to prevent urinary tract infection: the StOP UTI realist synthesis
- List of supplementary material - Validation and development of models using clin...List of supplementary material - Validation and development of models using clinical, biochemical and ultrasound markers for predicting pre-eclampsia: an individual participant data meta-analysis
- List of abbreviations - A pragmatic randomised controlled trial of the effective...List of abbreviations - A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of ‘PhysioDirect’ telephone assessment and advice services for physiotherapy
Your browsing activity is empty.
Activity recording is turned off.
See more...