Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Allen SJ, Wareham K, Wang D, et al. A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE). Southampton (UK): NIHR Journals Library; 2013 Dec. (Health Technology Assessment, No. 17.57.)

A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE).
Show detailsA total of 17,420 in-patients aged ≥ 65 years and who had been exposed to one or more antibiotics were assessed for eligibility (Figure 1). Exclusion criteria were present in 3202 (18.4%) patients, 9068 (52.1%) declined to participate, 2130 (12.2%) were too unwell to join the study and 39 (0.2%) were nil by mouth. We recruited 2981 (17.1%) patients, at randomisation 1493 (50.1%) were allocated to the probiotic and 1488 (49.9%) to the placebo arm.
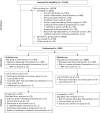
FIGURE 1
Trial profile.
In total, 2941 (98.7%) were included in the analysis according to treatment allocated; 23 in the probiotic arm and 17 in the placebo arm were excluded. The identity of the IMP was unknown in seven participants (six allocated to the probiotic and one to the placebo arm) due to an error in IMP labelling at one hospital site. No outcome data were available in 23 patients who were lost to follow-up. In each arm of the study, these included six patients who declined further participation shortly after randomisation without giving a reason and contact was lost with four patients from each arm. Exclusion criteria were present at recruitment in three patients and the details of antibiotic treatment could not be determined in one patient in the probiotic arm. Six participants were recruited to the study for a second time and all were allocated to the placebo arm. Possible carry-over effects from their first involvement in the study could not be excluded and; therefore, data from their second involvement were excluded.
Participant characteristics according to intervention arm
Consent to participate in the trial was provided directly by 1398 patients (95.1%) in the probiotic arm and 1392 patients (94.6%) in the placebo arm. For patients unable to give consent themselves, assent was usually provided by a family member: daughter [in 24 cases (1.6%) in the probiotic arm and 34 cases (2.3%) in the placebo arm], wife [in 19 cases (1.3%) in the probiotic arm and 13 cases (0.9%) in the placebo arm], or son [in 15 cases (1.0%) in the probiotic arm and 18 cases (1.2%) in the placebo arm].
Baseline demographic and patient characteristics were similar in the two intervention arms except for a greater proportion of males than females in the probiotic arm and vice versa in the placebo arm (Table 2). The frailty of the study population is reflected in the median age of 77.1 years and common occurrence of comorbid illnesses: 54.6% of participants suffered from hypertension, 24.1% from chronic obstructive pulmonary disease (COPD) and 22.9% from diabetes. Participant age ranged from 65.0 to 107.5 years in the probiotic arm and from 65.0 to 104.4 years in the placebo arm. More participants were recruited during the winter than in the summer months. The majority of patients were admitted to hospital from home and approximately one-third had been admitted to hospital within the previous 8 weeks. Very few people of non-white ethnic origin were recruited. Cigarette smoking was uncommon, but approximately one in three participants drank alcohol. Recent consumption of foods containing live bacteria was uncommon among all participants and occurred with a similar frequency in both study arms.
TABLE 2
Demographic variables and participant characteristics according to intervention arm
Participant characteristics according to centre
Overall, 1873 (63.7%) inpatients were recruited in hospitals in ABMUHB (Singleton, Morriston and Princess of Wales) and 1068 (36.3%) in CDDFT (Durham and Darlington). In ABMUHB, recruitment began with a pilot study of 50 patients in Morriston Hospital on 1 December 2008 to evaluate the recruitment methods and data collection forms. Methods were found to be reliable and these patients were included in the final analysis. Recruitment continued until 28 February 2012 and a total of 1479 patients (50.3% of total) were recruited (see Table 2). Recruitment in Singleton Hospital began on 9 June 2009 but was terminated on 9 February 2011, after 203 (6.9%) patients had been recruited, because of falling numbers of eligible patients due to service reconfiguration. To maintain patient numbers, recruitment was undertaken at Princess of Wales Hospital from 5 May 2011 to 10 January 2012 and 191 (6.5%) patients were recruited. The start of recruitment was delayed in CDDFT for operational reasons. Darlington Memorial Hospital recruited 521 (17.7%) patients from 12 October 2009 to 27 February 2012 and University Hospital of North Durham recruited 547 (18.6%) patients from 17 November 2011 to 28 February 2012 (see Table 2).
Baseline participant characteristics were generally similar across the hospital sites (Table 3) with some exceptions. The greater proportion of males in the probiotic arm and females in the placebo arm occurred in all centres except for Singleton Hospital, where there were more females than males in the probiotic arm (data not shown). Participants recruited at Singleton Hospital were more likely to be female and tended to be older than participants from other hospitals. The period during which recruitment in each centre occurred was reflected in the lower proportion of patient recruitment during the winter months in Princess of Wales than in other hospitals. The frequency of COPD and hospital admission in the previous 8 weeks were both more common in hospitals in CDDFT than in ABMUHB.
TABLE 3
Demographic variables and participants characteristics according to centre
Indications for initial antibiotic treatment
Indications for antibiotic treatment classified according to the MedDRA SOC43 were similar in the two study arms (Table 4). The most common indication was ‘respiratory, thoracic and mediastinal disorders’. Antibiotic treatment for suspected sepsis where the site was unclear was given to a small proportion of patients. About one in four patients in each arm of the study received antibiotics for prophylaxis rather than the treatment of infection and nearly all of these were for surgical and medical procedures.
TABLE 4
Indications for initial antibiotic treatment according to MedDRA SOC and intervention arm
In keeping with differences in the frequency of COPD according to centre, a greater proportion of the patients in hospitals in CDDFT than ABMUHB were treated for ‘respiratory, thoracic and mediastinal disorders’ (Table 5).
TABLE 5
Indications for initial antibiotic treatment according to MedDRA SOC and centre
Antibiotic exposure
All of the participants were receiving one or more antibiotics when they started the IMPs. The date the participant began taking the antibiotics before recruitment was known in 1448 participants in the probiotic and 1443 in the placebo arm. The median (IQR) period of exposure to antibiotics before starting the IMP was 3.0 days (2.0–6.0 days) in both study arms (p = 0.38).
During the period 7 days before, and 8 weeks following, recruitment, the most commonly used antibiotic class was the penicillins, with over half of all participants receiving a broad-spectrum penicillin. About one in four participants were exposed to a cephalosporin. Antibiotic exposure was similar in the two study arms (Table 6).
TABLE 6
Antibiotic exposure according to intervention arm. The number and percentage of participants who received therapy with the antibiotic during the period 7 days before recruitment to the end of follow-up at 8 weeks
Antibiotic exposure varied according to centre (see Appendix 9, Table 31). In hospitals in CDDFT, exposure to broad-spectrum penicillins was greater than in hospitals in ABMUHB (67.8–70.6% vs. 47.3–57.1%, respectively), but exposure to cephalosporins was lower (1.9–3.3% vs. 13.6–29.1%, respectively), as was exposure to quinolones (6.9–7.5% vs. 8.4–21.2%, respectively).
Fewer than 1 in 10 participants received only a single dose of an antibiotic and most received antibiotics for ≥ 7, with one-third treated for at least 14 days (Tables 7 and 8). The majority of participants were exposed to antibiotics from two or more classes. Exposure to combination therapy and duration of antibiotic therapy was similar in the two study arms.
TABLE 7
Combination of antibiotic therapies according to intervention arm
TABLE 8
Duration of antibiotic therapy according to intervention arm
Non-antibiotic drug treatment
Use of drugs other than antibiotics was common, with many participants receiving antihypertensive therapy, aspirin, PPIs or angiotensin-converting enzyme (ACE) inhibitor therapy. Non-antibiotic drug treatment was similar in the two study arms (Table 9).
TABLE 9
Non-antibiotic drug treatment according to intervention arm
Primary outcomes
Antibiotic-associated diarrhoea (including CDD) occurred with a similar frequency in the probiotic arm (159 participants, 10.8%) and placebo arm (153 participants, 10.4%; RR 1.04; 95% CI 0.84 to 1.28; p = 0.71; Table 10). This included 12 participants with frequent stools that they described as looser than normal but who were unable to describe stool consistency using the Bristol Stool Form Scale.38
TABLE 10
Primary outcomes according to intervention arm
Clostridium difficile diarrhoea was uncommon and occurred in 12 (0.8%) participants in the probiotic arm and 17 (1.2%) participants in the placebo arm (RR 0.71; 95% CI 0.34 to 1.47; p = 0.35; see Table 10). Based on this effect size and the low prevalence of CDD, the number needed to treat to prevent one case is 295. This would be reduced to 95 for an effect size at the lower limit of the 95% CI (a threefold reduction in CDD in the probiotic arm). The corresponding number needed to harm (the upper 95% CI) is 267.
Secondary outcomes
Clostridium difficile was isolated from stools in two participants with mild loose stools (not meeting the study criteria for diarrhoea) in the probiotic arm. One participant in each arm had an episode of CDD after an initial episode of AAD that was not associated with CDI; the participant allocated to the placebo arm required surgery for CDD and the participant allocated to the probiotic arm had gallstones and died during the episode of CDD. One patient with known carcinoma of the head of the pancreas with a biliary stent in situ died during an episode of CDD that occurred after withdrawal from the trial.
The adjusted treatment effect on occurrence of AAD from covariate analysis was similar to the unadjusted effect after controlling for nine prespecified covariates. Covariate analysis identified that the occurrence of AAD could be predicted by the duration of antibiotic treatment, antacid therapy and duration of hospital stay (Table 11).
TABLE 11
Adjusted treatment effect and potential risk factors for AAD: covariate analysis
The frequency of AAD was similar in each centre: Morriston 162/1479 (11.0%), Singleton 20/203 (9.9%), Princess of Wales 21/191 (11.0%), Durham 56/547 (10.2%) and Darlington 53/521 (10.2%; p = 0.97). Subgroup analyses showed that the distribution of cases of AAD according to prespecified potential risk factors for AAD, including those identified as risk factors in covariate analysis, was similar in the two intervention arms and there was no evidence of a statistically significant interaction between prespecified potential risk factors for AAD and intervention arm (Table 12).
TABLE 12
Subgroup analyses of AAD by prespecified risk factors
Most episodes of AAD (73.7%) occurred within 4 weeks of recruitment. On average, episodes of AAD lasted for 2 days with four stools in 24 hours and of consistency seven on the Bristol Stool Form Scale (Table 13). The most commonly associated symptoms were urgency, abdominal pain and nocturnal diarrhoea. The latter tended to occur more frequently in the placebo than the probiotic group (p = 0.051) and other characteristics of the diarrhoea episodes were similar in the two study arms (see Table 13). Most episodes of AAD were managed in hospital and stool samples were collected and tested for diarrhoeal pathogens in 58.6% of all cases. For many episodes of AAD, the short duration and occurrence after discharge from hospital complicated the collection of a stool specimen for testing for pathogens.
TABLE 13
Severity of AAD and frequency of associated symptoms according to intervention arm
As with AAD, the adjusted treatment effect for CDD was similar to the unadjusted estimate (Table 14). Covariate analysis showed that duration of antibiotic treatment was associated with CDD.
TABLE 14
Adjusted treatment effect and potential risk factors for CDD: covariate analysis
The frequency of CDD was similar in each centre: Morriston 21/1479 (1.4%), Singleton 2/203 (1.0%), Princess of Wales 0/191 (0.0%), Durham 3/547 (0.5%) and Darlington 3/521 (0.6%; p = 0.15). In subgroup analysis, there was a statistically significant interaction between intervention arm and age (p = 0.0015; Table 15). In patients aged > 77 years, the frequency of CDD was significantly lower in the probiotic arm than in the placebo arm. In contrast, the frequency of CDD was similar in the two intervention arms for patients aged ≤ 77 years. In addition, the interaction between treatment group and duration of antibiotic treatment was of borderline statistical significance (p = 0.054). There was no evidence of a significant interaction between the intervention arm and other prespecified potential risk factors for CDD (Table 15).
TABLE 15
Subgroup analyses of CDD by prespecified risk factors
The timing of onset of CDD was similar to that of AAD, with 75.8% cases occurring within 4 weeks of recruitment. On average, the duration of CDD was 6.5 days and duration was similar in the two study arms (Table 16). Bloating was less common in the placebo arm than in the probiotic arm (risk difference 40.7%; 95% CI 7.4% to 74.0%) and median stool frequency tended to be lower in the placebo arm than in the probiotic arm (see Table 16). Otherwise, gastrointestinal symptoms, clinical findings and investigations and classification of severity were similar in the two study arms. During follow-up, no patient was identified as having peritonitis, ileus, toxic megacolon or life-threatening CDD or as having died from CDD. The majority of patients in both study arms were managed in hospital.
TABLE 16
Severity of CDD, frequency of associated symptoms and investigations according to intervention arm
Seven (0.5%) participants in the probiotic arm and 10 (0.7%) participants in the placebo arm had diarrhoea due to other causes (RR 0.70; 95% CI 0.27 to 1.84). In the probiotic arm, six had norovirus diarrhoea and one was diagnosed with non-specific colitis. In the placebo arm, six had norovirus diarrhoea, one had diarrhoea after taking laxatives, two patients attributed diarrhoea to drinking a large volume of fruit juice and one had melaena associated with abnormal clotting.
Overall, 2927/2940 (99.6%) participants took at least one dose of the IMP, with a similar proportion in the probiotic (1462/1469, 99.5%) and placebo arms (1465/1471, 99.6%; p = 0.78; compliance unknown for one participant in the probiotic arm). The median number of days that participants were observed or reported taking the IMP in the first 3 weeks was similar in the probiotic [n = 1469, 21 days (IQR 14–21 days)] and placebo arms [n = 1471, 21 days (IQR 14–21 days); p = 0.55; Figure 2]. The full 21-day course was completed by 52.5% of participants. Overall, 1076/2934 (36.7%) participants reported that they disliked taking the IMP, and this proportion was similar in the probiotic (529/1466, 36.1%) and placebo arms (547/1468, 37.3%; p = 0.51). Taking account of compliance in covariate analysis did not materially alter the risk of AAD (OR 1.02; 95% CI 0.80 to 1.30) or CDD (OR 0.66; 95% CI 0.30 to 1.47).

FIGURE 2
Total number of days participants took the IMPs according to intervention arm.
Unused IMPs were collected opportunistically at three time points during the study, from participants who had withdrawn or died, for assessing correct identity according to active versus placebo and number of viable organisms in the probiotic preparation. Thirty-four probiotic capsules were tested and all contained ≥ 1.62 × 1010 viable bacteria. All of the 33 placebo capsules tested were sterile.
During the first 3 weeks while participants were taking the IMPs, the duration of hospital stay was similar in the probiotic [n = 1469, median 6 days (IQR 2–13 days)] and placebo arms [n = 1470, median 6 days (IQR 2–13 days); p = 0.65]. The most commonly reported gastrointestinal symptoms were nausea (14.9%), abdominal pain (13.4%) and diarrhoea (any loose stools reported by the participants; 12.3%; Table 17). The frequency of gastrointestinal symptoms was similar in the two study arms with the exception of flatus, which was marginally less common in the placebo than the probiotic arm (risk difference 2.3%; 95% CI 0.0% to 4.6%). Futhermore, although very few participants had a NGT in situ, this was significantly more common in the probiotic than placebo arm. With these two exceptions, the duration that symptoms were present was also similar in the two study arms (p > 0.17 for all comparisons).
TABLE 17
Frequency of gastrointestinal symptoms and other morbidity in the first 3 weeks according to intervention arm
There were no statistically significant differences in either the frequency or duration of gastrointestinal symptoms or other morbidity according to study arm during weeks 4–8 of the study (data not shown). Overall, average duration of hospital stay was known in 2899 participants and was similar in the probiotic [n = 1452, median 4 days (IQR 1–11 days)] and placebo arms [n = 1447, median 4 days (IQR 1–11 days); p = 0.87; Figure 3].
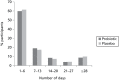
FIGURE 3
Duration of hospital stay according to intervention arm.
Eighteen participants were excluded from PP analysis. Seven patients in the probiotic and six in the placebo arm declined to take any of the IMPs. Investigation of the IMP labelling error that occurred at one centre resulted in the IMPs being withdrawn before completion of the 21-day course in one participant in the probiotic arm and four in the placebo arm. Among these participants excluded from PP analysis, none developed CDD, but one allocated to the probiotic arm developed AAD. Analysis of primary outcomes in the PP population did not materially alter the assessment of the efficacy of the intervention (data not shown). In addition, the risk of developing AAD or CDD was as similar among those participants who took all 21 IMP doses, 14 or more doses or seven or more doses as it was in all participants (data not shown).
Serious adverse events
Serious adverse events were common in the study population with 578 (19.7%) participants experiencing one or more SAE (Table 18). The most common MedDRA SOC classifications for SAEs were respiratory, thoracic and mediastinal disorders, gastrointestinal disorders, and cardiac disorders.
TABLE 18
Serious adverse events classified according to MedDRA SOC, and intervention arm
Serious adverse events classified as gastrointestinal disorders occurred in 79 (2.7%) participants with a similar frequency in both study arms (see Table 18). With SAEs classified according to MedDRA PTs43 (see Appendix 9, Table 32), gastrointestinal haemorrhage occurred in 15 participants in the probiotic arm (specified as upper gastrointestinal haemorrhage in four participants and lower in five participants) and 11 participants in the placebo arm (specified as upper gastrointestinal haemorrhage in one participant and lower in seven participants). Peptic ulcer occurred in four participants in the probiotic arm (specified as duodenal ulcer in one participant and perforated peptic ulcer in two participants) and one participant in the placebo arm experienced a perforated duodenal ulcer. Abdominal pain occurred in four participants in the probiotic arm and three in the placebo arm. Gastroenteritis occurred in three participants in the probiotic arm and two in the placebo arm. Constipation occurred in one participant in the probiotic arm and two in the placebo arm. Peritonitis, volvulus and dysentery each occurred in one participant in the probiotic arm and appendix abscess, colostomy performed, diarrhoea, liver abscess and pancreatitis each occurred in one participant in the placebo arm. There was no occurrence of intestinal ischaemia.
The frequency of SAEs that were, or may have been, due to bacterial infection occurred with similar frequency in each arm (see Appendix 9, Table 32). Pneumonia occurred in 52 participants in the probiotic arm (specified as caused by Pseudomonas sp. in two of these participants) and 53 in the placebo arm (specified as caused by Pseudomonas sp. in three of these participants). Abscesses occurred in one participant in the probiotic arm (specifically, a groin abscess) and four participants in the placebo arm (specifically groin, mediastinal, liver and psoas abscesses). Urinary tract infection occurred in 15 participants in the probiotic arm and 12 in the placebo arm, wound infection or cellulitis occurred in 16 participants in the probiotic arm and nine in the placebo arm, infected implant site occurred in two participants in the probiotic arm and one in the placebo arm and infected haematoma occurred in one participant in the probiotic arm. Sepsis occurred in 10 participants in the probiotic and 12 in the placebo arm and organ failure in one participant in each study arm.
The most frequent SAEs classified according to MedDRA PTs43 were pneumonia (3.3%), obstructive pulmonary disorder (1.6%) and falls (1.1%). Overall, 143 (4.9%) participants experienced a SAE that resulted in death, 10 (0.3%) SAEs that were considered to be life-threatening, 447 (15.2%) SAEs that prolonged hospitalisation, four (0.1%) SAEs that resulted in persistent or significant disability or incapacity and 11 (0.4%) experienced other SAEs that were considered to be significant medical events (see Appendix 9, Table 32). The proportion of patients in each SAE severity category, the frequency of individual SAEs within each category and the proportion of participants experiencing one or more SAE were similar in the two study arms. (see Appendix 9, Table 32).
Following the occurrence of a SAE, the patient’s clinical team withdrew the IMP from 14 (0.5%) participants and discontinued them temporarily in 90 (3.1%) participants, with a similar proportion in each study arm (see Appendix 9, Table 33). A common reason for discontinuing the IMPs was to reduce the number of medications for the patient rather than any concern regarding the safety of the bacterial organisms.
Quality of life analysis
European Quality of Life-5 Dimensions
There was a tendency for the EQ-5D visual analogue scale (VAS) and index values to increase over time from baseline to 4 weeks and then 8 weeks, indicating an improvement in health status over time within each of the study arms. Median scores were similar in the two study arms (Table 19).
TABLE 19
Summary statistics of EQ-5D VAS and index values by intervention arm and visit
The change from baseline EQ-5D VAS to that at 4 weeks was similar in both study arms but at 8 weeks there was a statistically significant difference between the two arms. However, this was a change of less than 2 points on the 100-point scale and, therefore, is unlikely to represent a clinically important change in health status (Table 20).
TABLE 20
Mixed model analysis of EQ-5D change from baseline in VAS health status scores and index score
Generic Short Form questionnaire-12 items version 2
As with the EQ-5D, there was a tendency for SF-12 v2, MCS, PCS and subdomain scores, with the exception of vitality, to increase over time, also indicating that the level of health increased in both study arms (Table 21).
TABLE 21
Generic Short Form questionnaire-12 items version 2, MCS, PCS and subdomain scores by intervention arm and visit
Analysis of changes from baseline in SF-12 v2 summary and subdomain scores at 4 weeks and 8 weeks by treatment allocated showed no statistically significant differences between the two study arms (Table 22).
TABLE 22
Mixed model analysis of SF-12 v2 change from baseline in MCS, PCS and subdomain scores
Economic analysis
Resource use and costs
Health-care contacts
Average duration of initial hospital stay was 0.03 days longer in the probiotic arm than in the placebo arm (Table 23). Overall, during the 8-week follow-up period, 18.3% patients were readmitted to hospital with a similar frequency in each study arm; however, patients in the probiotic arm remained in hospital for 0.62 days less than those in the placebo arm during each readmission. In the probiotic arm, 38.4% of patients reported other health-care contacts for a new problem compared with 40.9% in the placebo arm (costed as GP visits) and spent an additional 0.11 days, on average, in care facilities. None of these differences was statistically significant.
TABLE 23
Health-care contacts per patient by intervention arm
The mean cost of health-care contacts per patient was similar in the two trial arms (Table 24).
TABLE 24
Mean cost of health-care contacts per patient by intervention arm: base case
Antibiotics
The mean cost of antibiotics was £105.38 in the probiotic arm and £90.94 in the placebo arm. Staff costs for administration of antibiotics was £759.71 in the probiotic and £738.34 in the placebo arm. Overall antibiotics cost per patient was £35.80 less in the placebo arm than in the probiotic arm, but the difference was not statistically significant (Table 25).
TABLE 25
Mean cost of antibiotics, probiotics and episodes of diarrhoea per patient by intervention arm: base case
Intervention implementation
The mean nursing time required to administer the probiotic course was 39 minutes at a cost of £63.02. Including the retail cost of the formulation and accounting for duration of hospital stay, the mean implementation cost of the probiotic was £73.02 (range £10.00–179.68; Table 25). No adverse events requiring additional health-care contacts were observed.
Episodes of diarrhoea
A summary of costs associated with gastroenteritis while patients are in hospital and collected outside the trial can be found in Table 26. When all causes of diarrhoea were included but the costs of antibiotics, other health-care contacts and increased duration of hospital stay were excluded, an episode of diarrhoea cost £402.63 more in the placebo arm than in the probiotic arm. When only AAD was considered, the differential cost was £478.23 more in the placebo arm than in the probiotic arm (see Table 25). However, these differences were not statistically significant.
TABLE 26
Cost components of diarrhoea episodes
Independent of study arm, the mean duration of hospital stay was 22.31 days for patients with AAD versus 16.73 days for non-diarrhoea patients. This difference of 5.58 days (95% CI 2.78 to 8.39 days) accrued, on average, £4531.36 (95% CI £3439.80 to £5622.92) more health-care costs (p = 0.01). In addition to increased length of hospital stay, the main cost drivers for this difference were additional costs of £1976.66 (95% CI £1677.24 to £2276.09) attributed to assessment and management of diarrhoea episodes including microbiology, staff time, diagnostics, cleaning, laundry and infection control measures.
Total health-care cost
Total health-care cost was £8.74 greater in the probiotic arm than in the placebo arm (see Table 25). According to our analysis, the main cost drivers that make up a high proportion of the total health-care costs were the duration of the initial hospital stay and readmissions, staff time for antibiotic and probiotic administration and diarrhoea-associated costs (including microbiology, clinical review and assessment, diagnostic and therapeutic procedures, disposables, cleaning, laundry and infection control procedures).
Utility and quality-adjusted life-years
Mean EQ-5D index values at the baseline were 0.51 for both the probiotic and placebo arms, and this value increased over time. At 4 weeks, scores for both trial arms were 0.60, and at 8 weeks this had further increased to 0.64 in the probiotic and 0.63 in the placebo arm. The slightly better 8-week follow-up outcome for the patients who were administered the probiotic (average utility difference of 0.01) was not statistically significant. Extrapolated to 1 year, the total QALY gain in the probiotic group was 0.0004 as no further QALY gain was to be expected after 8 weeks and any further changes in QoL would probably be due to general recovery (Table 27).
TABLE 27
Cost–utility of probiotic in comparison to placebo: base case
Cost-effectiveness and uncertainty
Base-case analysis showed only a small total health-care cost difference between the probiotic and placebo arms (see Table 25). This was mainly due to the relatively small implementation cost of the probiotic and the marginal cost savings for diarrhoea episodes in the probiotic arm. The cost difference resulted in an ICER of £22,701 per QALY at 1 year with a probability of the intervention being cost-effective at a £20,000 willingness-to-pay threshold of 48%. The CEAC depicts the probability of the intervention being cost-effective at different willingness-to-pay thresholds. (Figure 4).
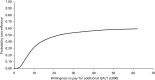
FIGURE 4
Cost-effectiveness acceptability curve, base case analysis: total health-care cost.
If the implementation costs of the probiotics only are taken into account, without consideration of any downstream effects, the ICER increases to £189,662 per QALY at 1 year with a probability of cost-effectiveness at £30,000 of 2% (Figure 5). Thus, based on a £30,000 willingness-to-pay threshold and implementation costs, probiotics are not cost-effective.

FIGURE 5
Cost-effectiveness acceptability curve, base case analysis: probiotics implementation cost.
Results of the cost–consequences analysis are reported in Table 28. As overall differences in costs and clinical outcomes between the two arms were small, the clinical effectiveness and cost-effectiveness of probiotics in the prevention of AAD in this study can be considered limited. Even though probiotics appeared cost-effective in the cost–utility analysis based on total health-care costs, no significant budgetary impact can be anticipated. This is due to the small differences in total cost between the probiotic and placebo arms and the lack of statistical significance in the primary outcomes. Subgroup analysis was not undertaken, as the covariate analysis did not identify any specific population that clearly benefited from receiving the probiotic. Cost per case of diarrhoea averted was not analysed as the study did not demonstrate a difference in diarrhoea frequency between the two groups.
TABLE 28
Clinical effectiveness and cost-effectiveness outcomes: cost–consequences analysis
Sensitivity analysis
Changes in the parameters included in the microcosting of a diarrhoea episode and changes in the cost of an inpatient day (the average cost per day amounted by a patient while in hospital) did not result in significant changes to the difference in overall cost between the probiotic and the placebo arms (Table 29). Furthermore, a decrease or increase in staff time for probiotic administration did not significantly change the cost-effectiveness results (Table 30). Considering probiotic implementation costs only, a reduction in staff time by 50% resulted in an ICER of £107,818 per QALY and a probability of cost-effectiveness at £30,000 of 16%, whereas doubling of staff time increased the ICER to £353,402 per QALY and was associated with a probability of cost-effectiveness at £30,000 of < 1%. The CEACs for these results can be found in Figures 6 and 7.
TABLE 29
Sensitivity analysis: changes in mean health-care cost per patient following parameter change within defined ranges
TABLE 30
Sensitivity analysis: changes of ICER based on probiotic implementation cost following parameter change within defined ranges

FIGURE 6
Cost-effectiveness acceptability curve, sensitivity analysis: probiotic implementation cost when staff cost halved.
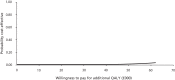
FIGURE 7
Cost-effectiveness acceptability curve, sensitivity analysis: probiotic implementation cost when staff cost doubled.
Summary of cost-effectiveness results
- Cost and duration of hospital stays, cost of diarrhoea, cost of antibiotics and total health-care cost per patient were very similar between the probiotic and the placebo arms. No statistically significant cost differences were found between the two study arms.
- Incremental total health-care cost of participants who suffered from AAD was £4531.36 (95% CI £3439.80 to £5622.92), which was significantly higher than for non-diarrhoea patients, independent of study arm. This was mainly due to increased length of hospital stay and additional diarrhoea-associated costs.
- Duration of hospital stay (initial stay and readmissions), staff time for antibiotic administration and diarrhoea-associated costs were identified as main components of the total health-care costs across both study arms.
- Between baseline and 8 weeks, mean QoL increase was 0.01 QALY higher in the probiotic than in the placebo arm; however, this difference was not statistically significant.
- The ICER associated with probiotic use at 1 year was estimated at £22,701 per QALY gained when total health-care costs were considered and £189,662 per QALY gained considering probiotic implementation costs only. However, the similarity in total cost, number of diarrhoea cases and patient QoL between the two trial arms limits the relevance of the ICERs.
- One-way sensitivity analyses did not show any significant effect on difference in total health-care costs between the trial arms and the overall conclusion of the cost-effectiveness assessment.
- Participant characteristics according to intervention arm
- Participant characteristics according to centre
- Indications for initial antibiotic treatment
- Antibiotic exposure
- Non-antibiotic drug treatment
- Primary outcomes
- Secondary outcomes
- Serious adverse events
- Economic analysis
- Summary of cost-effectiveness results
- Results - A high-dose preparation of lactobacilli and bifidobacteria in the prev...Results - A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE)
- Discussion - OPTIMA prelim: a randomised feasibility study of personalised care ...Discussion - OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer
- The psychological and physiological aspects of placebo for surgical trials - Pla...The psychological and physiological aspects of placebo for surgical trials - Placebo comparator group selection and use in surgical trials: the ASPIRE project including expert workshop
- What is ‘placebo’ in the context of surgical trials? - Placebo comparator group ...What is ‘placebo’ in the context of surgical trials? - Placebo comparator group selection and use in surgical trials: the ASPIRE project including expert workshop
- Introduction - A high-dose preparation of lactobacilli and bifidobacteria in the...Introduction - A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE)
Your browsing activity is empty.
Activity recording is turned off.
See more...