Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Taylor AH, Thompson TP, Greaves CJ, et al. A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers. Southampton (UK): NIHR Journals Library; 2014 Jan. (Health Technology Assessment, No. 18.4.)

A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers.
Show detailsExercise Assisted Reduction then Stop work sampling form
Exercise Assisted Reduction then Stop work sampling form (PDF download) (PDF, 1.8M)
Estimating the resource use and cost for the Exercise Assisted Reduction then Stop Intervention
Training and set-up costs
Experience in the pilot trial indicates that training for HTs in EARS intervention delivery requires 30 hours of training time (5 × 6-hour sessions), with an expectation that one lead trainer undertakes training for three trainees. Table 1 sets out the cost estimates for training activity based on these assumptions.
Health trainer contact time with participants (electronic data)
Analysis of within-trial data (electronic data reportage by HTs) provided the following summary statistics:
- EARS participants n = 49
- Missing (no contact time) n = 0
- Using n = 49:
- mean number of intervention sessions attended = 4.10 (SD 2.69)
- mean number of DNA events = 0.65 (SD 1.13) (conservative)
- mean number of contacts with EARS (intervention + continued support for quitters) = 4.26.
TABLE 2
Breakdown of time spent delivering the intervention by health trainers. Mean time (minutes) and standard deviation (where calculable) per participant (n = 49)
Health trainer non-contact time
Electronic records
The data/information we have from HT electronic record suggest only a small proportion of non-face-to-face activities delivering EARS (37% of HT time), the majority of which was captured in identification and recruitment of smokers for the EARS service. It is apparent that electronic methods of data reportage failed to fully capture time spent in non-contact activities.
Work sampling records
Work sampling of two HTs was carried out over a 2-week period. Data collection in the pilot indicated that some developments were needed to the data collection form and also, given that data were collected from only two HTs, it is difficult to draw firm conclusions from the pilot study. However, the method was tested out, it was acceptable to HTs, and the pilot study indicates that it is an important source of future data (within cost estimates). In theory this approach is able to provide data that will allow an approximation to be made over the ratio of contact/non-contact HT time in EARS delivery. In the pilot data, we found the ratio difficult to determine as feedback from HTs on work sampling indicated that HTs used research-related data collection questions to launch into intervention delivery. Adjusting for this bias by excluding all data collection activities suggests a considerable and substantial proportion of non-contact activities for the two HTs sampled (57% of total HT time).
Identification of ‘hard-to-reach’ smokers and recruitment
In the pilot trial, a number of different modes of identification of participants for EARS were explored. In addition, some identification work (screening and searching for ‘hard-to-reach’ participants) was taken by the principal investigator and trial manager, whereas in practice this work is likely to be done by a HT. Within the trial, the most efficient models of recruitment were from GPs and previous treatment failures from SSS (in the region of 1–1.5 hours of recruitment activity per participant randomised). We therefore assumed that a mean of 1.25 hours of recruitment-based activity might be required. This may be conservative as anecdotal evidence suggests that recruitment into a trial is much more difficult than recruitment into a service.
Supervision time
Current costs assume 1 hour’s supervision per HT per week and intervention turnover of cases every 2 months. The assumption is based on experiences in the trial with 49 EARS participants.
Exercise aids
Data estimated within trial (electronic reporting) showed the per-participant costs to be in the region of £19. The data/information we have from the trial suggested that only a low proportion of participants had financial subsidies. Further details are provided in Table 3. Accelerometers were used in the trial to measure exercise outcomes, but unit cost of intervention was assumed to be for a cheaper pedometer.
TABLE 3
Estimated mean cost of exercise aids per participant
Sensitivity analysis to explore inclusion of indirect costs associated with intervention
Effect of implementation on Stop Smoking services
It is possible that the EARS intervention could increase the number of participants wishing to make a quit attempt via SSS over and above control. The sample size of the pilot trial made it difficult to make inference on this, but a full RCT may be more informative. In the pilot trial, 2/49 participants in the EARS active arm were referred to SSS compared with 0/50 in control. The analysis of intervention cost of EARS at present makes no projection about how services could be integrated. Assuming a scenario where 50% (rounded up) of the 11 making a quit attempt in EARS seek help through SSS, using a cost of SSS per participant at £350 – as reported by Carr et al.1 – we explored including this data (along with pharmacotherapies) with the components of the total service cost for EARS in a sensitivity analysis presented in Table 4. The figures overestimate the cost of SSS within the trial period.
TABLE 1
Training calculation
We restrict our base-case analysis of costs to those incurred as a direct result of the intervention on the basis that it is questionable as to whether or not there would be additional indirect intervention costs over time with EARS relative to comparator. Smoking is a chronic condition, and many smokers typically receive multiple interventions before achieving long-term quit.
Effect of implementation on pharmacotherapy usage
As with smoking services, it is plausible that the EARS intervention could result in additional usage of pharmacotherapy compared with alternatives (NRT, buproprion and/or varenicline) to support a quit attempt. It is also possible that an EARS-type intervention could result in lower usage of pharmacotherapy if exercise alone proves effective at minimising cravings.
In a sensitivity analysis, we assumed that an additional 50% of the 11 out of 12 making a quit attempt made pharmacotherapy-assisted quit attempts on prescription. One course of pharmacotherapy equates to £62 per participant; this assumption is based on cost of GP advice plus prescription by Wang et al.,2 inflated from 2006 prices using the PSSRU Hospital and Community Pay and Prices index.3
Results
Including additional pharmacotherapy and SSS resource cost increases the total per-participant cost of the EARS service by £50.45, that is to say a total of £242.62.
TABLE 4
Additional indirect costs associated with EARS-type intervention
Effect of implementation on other services
For pragmatic reasons this is assumed to be zero cost.
Economic evaluation search strategy used to identify cost-effectiveness analysis studies in smoking cessation
The following electronic databases were searched from their inception up to August 2012: MEDLINE, EMBASE (both via Ovid) and Cochrane Reviews and Dissemination (CRD). The search terms used included smoking, tobacco and terms relating to types of economic evaluation.
Search strategy (MEDLINE)
cost benefit analysis/
cost effectiveness analysis/
cost minimization analysis/
cost utility analysis/
(technology assessment).tw.
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
or/1-6
(nicotine$ or smok$ or tobacco).ti.
and/ 8-9
Search strategy (EMBASE)
cost benefit analysis/
cost effectiveness analysis/
cost minimization analysis/
cost utility analysis/
(technology assessment).tw.
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
or/1-6
(nicotine$ or smok$ or tobacco).ti.
and/ 8-9
Search strategy (CRD)
We searched for economic models and economic evaluations performed by a search of all publications using the terms smoking, tobacco and nicotine.
Evidence review summary table
TABLE 5
Detailed/summary detail table for modelling studies discussed in evidence review
| Wang et al.2 (UK) | |
|---|---|
| (a) Model structure | Aim: to assess the cost-effectiveness of making NRT available in the context of a CDTQ programme for a suitable population of smokers Model: decision-analytic model (structure reported – decision tree), parameters estimated from published literature Time horizon: lifetime Viewpoint: UK NHS/Personal Social Services Viewpoint justification: none reported Alternatives: No NRT. In the full model, for attempts made with NRT the attempts were categorised as either ‘abrupt’ or CDTQ. Within each of these possibilities, the form by which smokers CDTQ or quit modelled are OTC NRT, prescription NRT or a smokers’ clinic. The authors also show a schematic for a mixed analysis. The reporting does not lend itself to a clear distinction between the approaches Study population: current smokers (previous quit attempts or exposure to other interventions in the past not dealt with. Future quit attempts – assumption that risk of relapse is independent of current quit attempt) Evaluation method: cost-effectiveness analysis |
| (b) Data inputs – effectiveness data | Effectiveness data: model refers to meta-analyses conducted elsewhere for probability of successful quit attempt (at 6 months, 12 months and lifetime) Method used to estimate effectiveness: sustained abstinence measures from meta-analysis of individual patient data at 6 months. Evidence synthesis (meta-analysis or other method, NR, for abrupt quitters) of relapse rates from 12 studies for the period between 6 and 12 months using: 12 months’ sustained abstinence = 6 months’ sustained abstinence × risk of relapse Lifetime abstinence estimated by assumption that 30% relapse after 12 months, i.e. 70% of 12-month quitters succeed Valuation of health benefits: CQG (authors estimate the QALY gain using data from Doll et al., they do not estimate aggregate/total QALYs as these data apparently unavailable) Indirect benefits: NR |
| (b) Data inputs – resource use | Resource use: therapy and clinicians’ time, OTC treatments resulted in zero cost to NHS as defrayed to patient Unit cost data: reported in appendix Resources reported separately from cost: yes Currency (exchange rate): £ Price year (adjustment for inflation): 2008 |
| (c) Consideration of uncertainty/consistency | Discount rate: 3.5% costs and QALYs Uncertainty: simple deterministic analysis, main focus is on the CDTQ quit rate vs. the abrupt quit rate. Authors refer presentation of ICERs by subgroup sensitivity analysis The authors also perform a sort of threshold analysis to examine the trade-off (to examine possible effect of smokers who would otherwise have made an abrupt quit now cutting down vs. those making no attempt) Authors’ conclusion: CDTQ delivers ICERs within margins considered cost-effective. CDTQ is less effective and more costly than abrupt quitting but may address a different population |
| (d) Presentation of CEA findings | Outcome of model: (as identified by authors) expected lifetime QALYs. In reality, the model only estimates the CQG associated with a quit attempt. The summary outcome measure is the ICER and is therefore an incremental analysis (CQG) Probability of lifetime quit rate: values reported for different options within the range of 0.0137–0.1119 (abrupt quit) Total cost: (£0–153.79) ICER: range of ICERs presented for different service strategies |
| NICE guidance PH14 | |
| (a) Model structure | Aim: cost-effectiveness of brief intervention and referral (multiple scenarios) GP and nurse delivered and referral to specialist SSS for smoking cessation Model: decision tree, projection of final outcomes Time horizon: lifetime (analysis). Decision tree corresponds to 12 months Viewpoint: NHS and Personal and Social Services Viewpoint justification: NICE guidance Alternatives: various. Brief opportunistic advice from GP alone (with adjunct NRT, telephone helpline, self-help material). Nurse-led brief interventions (30 minutes) in primary or hospital setting Study population: UK smokers Evaluation method: CUA |
| (b) Data inputs – effectiveness data | Probability data: 12-month quit rate Method used to estimate comparative effectiveness: ORs applied to 12-month quit rates for different types of brief intervention Primary outcome measure: 12 month quit rate (in some instances calculated from ORs) obtained from evidence synthesis of trial data. Other outcome measures: adjustment for risk of longer-term risk of relapse from Yudkin et al.5 Detailed methods not provided Valuation of health benefits: secondary EQ-5D data from an unpublished survey of 15,000 current and ex-smokers Indirect benefits: not considered |
| (b) Data inputs – resource use | Resource use: GP/nurse time, nurse training in brief advice, cost of self-help materials, provision of telephone helpline service Unit cost data: staff costs from Curtis and Netten,6 NRT costs from BNF 2005, other overheads (telephone calls, literature) estimated, source not reported Cost savings in terms of smoking-related diseases per long-term quitter estimated from published source Godfrey7 Resources reported separately from cost: no; however, itemised resource use presented alongside total costs Currency (exchange rate): £ Price year (adjustment for inflation): 2005 |
| (c) Consideration of uncertainty/consistency | Key parameters varied: effectiveness and background quit rates, length of intervention. Discussion of other factors: socio-economic deprivation, tobacco dependence, extending model to include previous smokers not responding, alternative treatment settings Discount rate: 3.5% Uncertainty: deterministic – series of univariate scenario analyses. Threshold analysis to explore minimum effect size required to remain cost-effectiveness |
| (d) Presentation of CEA findings | Outcome: Cost per QALY (£) Probability of lifetime quit rate: assumes maximal period of follow-up by Yudkin5 at 8 years reflects lifetime rate Total cost: not presented ICER: Dependent on age and sex the ICERs relating to a brief (5-minute) intervention range from £636 to £1677 Authors’ conclusion: cost-effective results can be generated in all settings and age-group for all forms of brief advice. ICERs tend to increase with cohort age |
| Bauld et al.8 | |
| (a) Model structure | Aim: cost-effective analysis of smoking-cessation programs Model: decision tree (52-week model) with a Markov model (lifetime model) Time horizon: lifetime Viewpoint: NHS health service perspective (NHS Scotland) Viewpoint justification: not explicitly discussed Alternatives: both a pharmacy and a group-based SSS were compared against self-quit attempts. The authors suggest that direct comparison of pharmacy and group-based services would be inappropriate as they attract different ‘types’ of smoker Study population: smokers using NHS smoking cessation services in Glasgow Evaluation method: CEA |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: verified 52-week abstinence rates to determine the numbers of current and ex-smokers in each cohort. Relapse rates applied for 8 years post quit. Risk of smoking related mortality applied 12 years post quit. Primary outcome measure: 52-week abstinence as verified by CO monitor Other effectiveness data considered: relapse rates and risk of smoking related death in ex-smokers. The methods for calculating smoking attributable mortality appear to be based on methods based on Thun et al.,9 and are outlined in a report commissioned by NHS Health Scotland Valuation of health benefits: secondary sources. Health state utility values from Tengs and Wallace10 – 0.8 smoker, 0.87 ex-smoker Indirect benefits: not considered |
| (b) Data inputs – Resource use | Resource use: all relevant health service costs associated with NRT, professional time, overheads and materials Unit cost data: BNF, PSSRU and within study Resources reported separately from cost: in summary form Currency (exchange rate): £ Price year (adjustment for inflation): 2007 |
| (c) Consideration of uncertainty/consistency | Key parameters varied in sensitivity analysis: scenario analyses based on (i) incorporating the future cost of smoking-related disease, (ii) not discounting QALYS, (iii) including self-reported quit, (iv) including a small cost (to the NHS) based on self-quit attempts Probability data: 12-month quit rates from within-trial analysis Discount rate: 3.5% QALYs only Uncertainty: scenario analyses, one of which includes future health costs of smokers |
| (d) Presentation of CEA findings | Outcomes: cost per client, cost per 52-week quitter, cost per QALY Probability of lifetime quit rate: applied by calculating year-on-year transitions (time dependent probabilities) Total cost: pharmacy cost per client £79.00. Group cost £368.00 ICER: (vs. self-quit) pharmacy service cost per 52-week quitter = £7800, group-based service cost per 52-week quitter = £9200 Authors’ conclusion: both pharmacy and group based services represent cost-effective interventions to help smokers to quit. Observed quit rates were lower than in some other studies; while this is commonly true in observational study designs, the lower effectiveness rates may also reflect a greater resistance to smoking cessation in Glasgow |
| Howard,11 BENESCO model | |
| (a) Model structure | Aim: to assess the cost-effectiveness of varenicline vs. alternative smoking-cessation strategies Model: Markov Time horizon: 20-year and lifetime Viewpoint: US health care Viewpoint justification: US budget holders Time horizon: 20-year and lifetime Alternatives: Buproprion, NRT, unaided quitting Study population: overall US population (of smokers) Evaluation method: CEA, CUA, budget impact |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: continuous abstinence rates, hazard ratios (published literature Thun et al.9) (used to calculate number of smoking related diseases and deaths) Valuation of health benefits: QALYs from published literature (Fiscella and Franks12) Indirect benefits: not included |
| (b) Data inputs – resource use | Resource use: cost of treatments Unit cost data: drugs, dispensing fees, physician fees and treatment costs Resources reported separately from cost: yes Currency (exchange rate): $ Price year (adjustment for inflation): 2005 (inflation index detail not provided) |
| (c) Consideration of uncertainty/consistency | Key parameters: costs, efficacy rates, relapse rates, disease incidence, prevalence and mortality rates Time horizon: 2, 5,10, 20 years and lifetime Discount rate: 3% per annum Uncertainty: scenario analysis, probabilistic sensitivity analysis (details of distributions provided) |
| (d) Presentation of CEA findings | Outcomes: incremental cost and incremental QALYs, presented in disaggregated form for the entire modelled cohort (just under 12 million smokers). No summary statistic presented cost per QALY due to dominance of modelled intervention Probability of lifetime quit rate: Relapse rates assumed to be higher in first 5 years Total cost: for the US health system and a cohort of 11.9 million smokers, the total costs for varenicline were $328,541M over a lifetime compared with $330,958 for bupropion, $332,662 for NRT and $333,283 for unaided cessation ICER: varenicline dominated all alternatives in model base case Authors’ conclusion: varenicline is very likely cost-effective (based on probabilistic output presented in cost-effectiveness acceptability curves) Comments: population-based US model with a focus on pharmacological aids to smoking cessation |
| Hurley,13 Quit Benefits Model | |
| (a) Model structure | Aim: to create a tool to evaluate tobacco control programs when the number of quitters is known Model: Markov – model included four smoking associated diseases: MI, stroke, lung cancer, COPD Time horizon: 10 years (after quitting smoking) Viewpoint: health care system (primary care) Viewpoint justification: Australian policy-makers and health program funders Alternatives: N/A Study population: simulated quitters (Australians aged between 15 and 74) Evaluation method: CEA, CUA |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: relative risks from observational studies (Australian) Valuation of health benefits: reduction in smoking related disease and death, life-years saved, QALYs Utilities of less than one for smoking related sequelae only. Utilities from CEA registry Indirect costs/benefits: not included |
| (b) Data inputs – resource use | Resource use: cost of treating smoking-related diseases Unit cost data: no, total cost of smoking-related diseases reported for direct health care costs only. Some information about different cost categories Resources reported separately from cost: no Currency (exchange rate): $AUS Price year (adjustment for inflation): 2001 (no inflation adjustments) |
| (c) Consideration of uncertainty/consistency | Key parameters: relative risks Probability data: some modelling of source data to derive transition probabilities (i.e. using exponential models in the case of smoking-related diseases. Model and assumptions well documented Time horizon: 10 years (after quitting smoking) Discount rate: 0, 3% and 5% Uncertainty: univariate and multivariate sensitivity analysis of all model inputs (+/–) 10% |
| (d) Presentation of CEA findings | Outcomes: life-years saved, QALYs, costs Probability of lifetime quit rate: N/A Total cost: average saving AUS $373,000 per 1000 quitters (men and women combined) ICER: not presented in a summary outcome measure as quitters had lower costs and better outcomes than non-quitters Authors’ conclusion: QBM highlights the clinical and economic benefits of a tobacco control programme showing the impact on the individual Comments: this model was implicitly compared with no intervention, i.e. illustrates the burden of disease and how it might be reduced by an effective tobacco control programme |
| Woolacott et al.14 | |
| (a) Model structure | Aim: to estimate the cost-effectiveness of NRT and/or bupropion sustained release for smoking cessation Model: decision-analytic (appears to be decision tree) Time horizon: unclear; not lifetime Viewpoint: UK NHS Viewpoint justification: not provided (HTA report) Alternatives: brief advice alone and in combination with either buproprion-sustained release or NRT. Counselling (individual or group) Study population: primary care Evaluation method: CEA, CUA |
| (b) Data inputs – effectiveness data | Effectiveness data: from published studies Method used to estimate effectiveness: continuous quit rate at 12 months (control/treated) Valuation of health benefits: life-years saved (from published review). QALYS (from Fiscella and Franks12) Indirect costs/benefits: not included |
| (b) Data inputs – resource use | Resource use: resource use relating to intervention only Unit cost data: yes, but not all intervention costs have sources which are referenced Resources reported separately from cost: yes Currency (exchange rate): £ Price year (adjustment for inflation): not reported – 2002? |
| (c) Consideration of uncertainty/consistency | Key parameters: continuous quit rate p.a. (advice) = 3% Continuous quit rate (counselling) p.a. = 9% Spontaneous cessation p.a. = 1% Probability data: for buproprion/NRT derived from meta-analysed or indirect comparisons of ORs to calculate annual probabilities for decision tree nodes Time horizon: unclear; not lifetime Discount rate: not applied Uncertainty: univariate and multivariate sensitivity analysis of all model inputs (+/–) 10% |
| (d) Presentation of CEA findings | Outcomes: ICER/life-years saved Probability of lifetime quit rate: 40% risk of relapse (in what period?) Total cost: £67–202M (NHS England and Wales) ICER: £1459–1777 when compared with advice alone Authors’ conclusion: NRT/buproprion-sustained release are cost-effective Comments: this model demonstrates the budget impact on a hypothetical NHS population for England and Wales |
| Godfrey et al.7 | |
| (a) Model structure | Aim: to assess the cost-effectiveness of English smoking treatment services Model: not presented formally as a model, but analysis is conformant with decision-analytic modelling. Epidemiological data was combined with observational/survey data to form the basis of CEA. Regression-based techniques used to account for within-service variations Time horizon: lifetime (not explicitly stated) Viewpoint: UK NHS (perspective of the unit of the smoking service, not the individual smoker) Viewpoint justification: to establish if actual practice is cost-effective Alternatives: as this was an evaluation of treatment services, this reflected variation in actual practices (e.g. different service configurations, staffing, interventions) Study population: English NHS smoking treatment services Evaluation method: CEA and regression based analysis of determinants of service-level total and incremental costs (per life-years) |
| (b) Data inputs – effectiveness data | Effectiveness data: from published studies Method used to estimate effectiveness: epidemiological model Valuation of health benefits: Doll et al.15 as basis for estimating life-years saved Indirect benefits: not included |
| (b) Data inputs – Resource use | Unit cost data: yes, relying on published sources (Curtis3) Resources reported separately from cost: yes Currency (exchange rate): £ Price year (adjustment for inflation): 2000–1 Costs of smoking-related diseases: included in sensitivity analysis |
| (c) Consideration of uncertainty/consistency | Key parameters: background quit rate, relapse rate Discount rate: varied at 0%, 3.5% (base case), 6% Uncertainty: series of univariate analyses (varying background cessation, relapse after 4 weeks, life-years gained from smoking cessation. A worst- and best-case scenario analysis was additionally performed |
| (d) Presentation of CEA findings | Outcomes: cost per life-year saved, cost per person setting a quit data Probability of lifetime quit rate: 65% of those quitting at 4 weeks relapse at 12 months. 54% relapse between 1 and 7 years (from Yudkin et al.5). Total cost per person setting a quit date: mean cost of £123.40 Summary measure of cost-effectiveness: average cost per life-year gained per smoker is £684 (£483 if future savings in smoker’s health care costs are considered) Authors’ conclusion: Comments: this model demonstrates the cost-effectiveness of English smoking services. As such, it presents evidence of cost-effective actual practice of English smoking services. As such, it presents evidence of cost-effective actual practice. The use of observational data from 10 years ago might render some of the studies estimates slightly out of date, not least because it was conducted when SSS had only recently been implemented. The model compares results with other studies |
| Coleman16 | |
| (a) Model structure | Aim: to derive cost-effectiveness of interventions (NRT, buproprion, varenicline) for preventing relapse in recently abstinent smokers Model: cohort simulation Time horizon: lifetime Viewpoint: UK NHS Viewpoint justification: HTA standard Alternatives: no intervention Study population: England and Wales (hypothetical) smokers Evaluation method: CUA |
| (b) Data inputs – effectiveness data | Effectiveness data: from published studies Method used to estimate effectiveness: Indirect comparisons of interventions Valuation of health benefits: Doll17 – mortality rates used to determine odds ratios and smoking attributable deaths. Tengs and Wallace for the majority of comorbid states.10 Tilman and Silcock18 for former and current smokers Indirect benefits: not included |
| (b) Data inputs – resource use | Unit cost data: partial, use of published sources Resources reported separately from cost: partial (intervention cost) Currency (exchange rate): £ Price year (adjustment for inflation): Not stated, but unit costs for therapies sourced from 2008 formulary prices Costs of smoking related diseases: included |
| (c) Consideration of uncertainty/consistency | Key parameters: background quit rate = 2%, relapse rate Discount rate: 3.5% (benefits, as costs are incurred immediately) Uncertainty: deterministic – changes in intervention cost, effectiveness and background quit rates |
| (d) Presentation of CEA findings | Outcomes: cost per QALY Probability of lifetime quit rate: N/A Total cost per person setting a quit date: N/A Summary measure of cost-effectiveness: no synthesis of dominant interventions. Very cost-effective at commonly applied UK NHS threshold Authors conclusion: interventions appear cost-effective and similar in magnitude to other studies Comments: paper discusses limitations: lack of data on comorbidities and smoking status; only single interventions considered in projected ICERs; efficacy from RCTs may be better than routine clinical care; paper makes distinction that 1 QALY today (after application of a 3.5% discount rate) is worth 0.25 QALYs in 40 years’ time – i.e. health gains are reduced fourfold |
| Feenstra19 | |
| (a) Model structure | Aim: estimate CEA of five face-to-face smoking-cessation interventions Model: dynamic simulation model Time horizon: 75 years Viewpoint: Dutch (third-party payer) health care Viewpoint justification: to support Dutch smoking-cessation guidelines Alternatives: face-to-face smoking-cessation interventions in community and primary care Study population: Dutch population (smokers, non-smokers, former smokers) Evaluation method: CEA, CUA |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: detailed and complex demographic and epidemiologic data. In terms of smoking cessation rates, 12-month continuous abstinence rates (dynamic) converted to risk ratios for current and former smokers Valuation of health benefits: Dutch quality of life weights from published sources for smoking-related diseases Indirect benefits: not considered |
| (b) Data inputs – resource use | Unit cost data: Dutch sources Resources reported separately from cost: yes, intervention Currency (exchange rate): € Price year (adjustment for inflation): 2000 |
| (c) Consideration of uncertainty/consistency | Key parameters varied in sensitivity analysis: national population statistics and disease incidence Discount rate: 4% Uncertainty: univariate analyses including varying the: discount rate, time horizon, resource usage and cessation rate |
| (d) Presentation of CEA findings | Outcomes: Probability of lifetime quit rate: NR as this was a dynamic model Total cost: costs presented are for the entire population over time, so difficult to interpret. Costs per life-year gained are presented and in the region of 1400–6800 dependent on intervention and period of implementation ICER: €1100–4900 for telephone and intensive counselling Authors’ conclusion: all interventions considered were cost-effective compared with current practice and minimal GP counselling was cost saving |
| Fiscella and Franks12 (US) | |
| (a) Model structure | Aim: to assess the cost-effectiveness of nicotine patch Model: decision-analytic model (calculated analytically) Time horizon: lifetime Viewpoint: US health care payer Viewpoint justification: to inform payer decision re reimbursement Alternatives: physician counselling alone Study population: primary care population of smokers Evaluation method: cost-effectiveness analysis |
| (b) Data inputs – effectiveness data | Effectiveness data: quit rate from brief counselling. Quit odds rate from within trial for nicotine patch Method used to estimate effectiveness: quit odds ratio for the relative success of NRT active/placebo (from within trial). Acceptance rate of the patch was considered Lifetime abstinence estimated by assumption (35%) Valuation of health benefits: years of healthy life measure (YOLS) Indirect benefits: discussed, not included in analysis |
| (b) Data inputs – resource use | Resource use: therapy and clinicians’ time, OTC treatments resulted in zero cost to NHS as defrayed to patient Unit cost data: yes Resources reported separately from cost: yes Currency (exchange rate): US$ Price year (adjustment for inflation): 1995 US$ |
| (c) Consideration of uncertainty/consistency | Discount rate: 3.5% costs and QALYs Uncertainty: series of one-way sensitivity analyses. PSA using the normal distribution as candidate for 10,000 trials |
| (d) Presentation of CEA findings | Outcome of model: cost per quitter/cost per QALYS Probability of lifetime quit rate: 35% Total cost: $7332 per additional quitter (base case) ICER: cost-effective at $2422–14,112 per QALY from (PSA) |
| HECOS/Orme et al.20 | |
| (a) Model structure | Aim: to estimate the health and economic outcomes associated with smoking and the benefits of smoking cessation Model: cohort-level Markov (complex) developed analytically via application of difference equations Time horizon: lifetime Viewpoint: UK NHS Viewpoint justification: authors were tasked with creating a tool for use by health care payers, government policy makers and health-care organisations Alternatives: pharmacological, GP advice, group therapy Study population: hypothetical UK population Evaluation method: cost-effectiveness analysis |
| (b) Data inputs – effectiveness data | Effectiveness data: populated with UK data. Details restricted to online appendices Method used to estimate effectiveness: construction of an epidemiological model. Reporting not transparent due to complexity of model and aim of paper Lifetime abstinence estimated by assumption that a fixed percentage relapse per year Valuation of health benefits: via consideration of change in health status to morbid states and death. Life-years not adjusted for quality Indirect benefits: included |
| (b) Data inputs – Resource use | Unit cost data: yes Resource use: Not itemised, total cost only presented by cessation strategy Resources reported separately from cost: no – relied on secondary source Currency (exchange rate): £ Price year (adjustment for inflation): 1999 |
| (c) Consideration of uncertainty/consistency | Discount rate (varied): 6% (0–10%) Uncertainty: extensive deterministic sensitivity analysis around base-case assumptions of the model |
| (d) Presentation of CEA findings | Outcome of model: cost per life-year saved/cost per death averted Probability of lifetime quit rate: 30% Total cost: intervention costs reported by strategy per participant (secondary source) ICER: £1200 per life-year saved. £22,000 per death averted |
CUA, cost–utility analysis; N/A, not applicable; NR, not reported; OTC, over the counter; CQG, cost per QALY gained.
Data used in probabilistic analysis
TABLE 6
Parameter table
| Parameters | Point estimates (base case) | SEa | Candidate distribution |
|---|---|---|---|
| Quit rate control (proportion starting as ex-smoker) | 0.04 | 0.20 | Beta |
| Quit rate comparator (proportion starting as ex-smoker) | 0.14 | 0.05 | Beta |
| Intervention cost for EARS (total) | £192 | 19.61 | Gamma |
| Smoking-related disease (cost estimate) | £27,120 | £2767.40 | Gamma |
| Relapse rate in year 1– (post intervention) | 0.28 | 0.03 | Beta |
| Hazard rate (annual) based on Yudkin et al. | 0.0874 | 0.0089 | Beta |
| Health state values/utilities (by age and sex cohort) | See table 10 in chapter 4 | Beta | |
| Mortality rates (by age and sex cohort) | See table 2 below | Beta | |
SE, standard error.
- a
Correct to two decimal places.
TABLE 7
Adjusted age-specific annual mortality rates (as Table 42, Health state values by smoking status, age, sex, smoking intensity) from Vogl et al.21 and estimated standard errors in a general population with no long-standing illness
| Adjusted mortality rates (SE) | ||||
|---|---|---|---|---|
| Age (years) | Current | Former smoker by age stopped | ||
| < 10 years | < 20 years | ≥ 20 years | ||
| Males | ||||
| 35–44 | 0.001302 (0.0434) | 0.000964 (0.0001) | – | – |
| 45–54 | 0.002479 (0.0001) | 0.001575 (0.0002) | – | – |
| 55–64 | 0.006684 (0.0003) | 0.005123 (0.0005) | 0.002811 (0.0003) | – |
| 65–74 | 0.018818 (0.0007) | 0.013510 (0.0015) | 0.011766 (0.0013) | 0.008425 (0.0010) |
| 75–84 | 0.045752 (0.0056) | 0.032571 (0.0040) | 0.032202 (0.0039) | 0.028202 (0.0034) |
| Females | ||||
| 35–44 | 0.000987 (0.0434) | 0.000731 (0.0047) | – | – |
| 45–54 | 0.002460 (0.0001) | 0.001563 (0.0001) | – | – |
| 55–64 | 0.006878 (0.0003) | 0.005271 (0.0002) | 0.002892 (0.0003) | – |
| 65–74 | 0.021015 (0.0007) | 0.015087 (0.005) | 0.013139 (0.0012) | 0.009409 (0.0009) |
| 75–84 | 0.054728 (0.0019) | 0.038961 (0.0014) | 0.038520 (0.0033) | 0.033735 (0.0029) |
Other results/cost-effectiveness analyses/analyses

FIGURE 1
Cost-effectiveness plane for 60-year-old male, base-case CEA. Scatterplot of costs and effects.

FIGURE 2
Cost-effectiveness acceptability curve for 60-year-old male, base-case CEA.

FIGURE 3
Cost-effectiveness acceptability curve for 40-year-old female, base-case CEA.

FIGURE 4
Cost-effectiveness acceptability curve for 50-year-old female, base-case CEA.
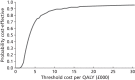
FIGURE 5
Cost-effectiveness acceptability curve for 60-year-old female, base-case CEA.
References
- 1.
- Carr SM, Lhussier M, Forster N, Geddes L, Deane K, Pennington M, et al. An evidence synthesis of qualitative and quantitative research on component intervention techniques, effectiveness, cost-effectiveness, equity and acceptability of different versions of health-related lifestyle advisor role in improving health. Health Technol Assess 2011;15(9). [PMC free article: PMC4781312] [PubMed: 21329611]
- 2.
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12(2). [PubMed: 18093448]
- 3.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- 4.
- NICE. Brief Interventions and Referral for Smoking Cessation: Economic Modelling Report. 2008.
- 5.
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 2003;327:28–9. [PMC free article: PMC164239] [PubMed: 12842953]
- 6.
- Curtis L, Netten A. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2005.
- 7.
- Godfrey C, Parrott S, Coleman T, Pound E. The cost-effectiveness of the English smoking treatment services: evidence from practice. Addiction 2005;100(Suppl. 2):70–83. [PubMed: 15844290]
- 8.
- Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, et al. One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services. Nicotine Tob Res 2011;13:135–45. [PubMed: 21196451]
- 9.
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA 2000;284:706–12. [PubMed: 10927778]
- 10.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583–637. [PubMed: 10843310]
- 11.
- Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics 2008;26:497–511. [PubMed: 18489200]
- 12.
- Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA 1996;275:1247–51. [PubMed: 8601956]
- 13.
- Hurley SF, Matthews JP. The Quit Benefits Model: a Markov model for assessing the health benefits and health care cost savings of quitting smoking. Cost Eff Resour Alloc 2007;5:2. [PMC free article: PMC1796848] [PubMed: 17241477]
- 14.
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a sytmeatic review and economic evaluation. Health Technol Assess 2002;6(16). [PubMed: 12269277]
- 15.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. [PMC free article: PMC437139] [PubMed: 15213107]
- 16.
- Coleman T, Agboola S, Leonardi-Bee J, Taylor M, McEwen A, McNeill A. Relapse prevention in UK Stop Smoking Services: current practice, systematic reviews of effectiveness and cost-effectiveness analysis. Health Technol Assess 2010;14(49). [PubMed: 21040645]
- 17.
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994;309:901–11. [PMC free article: PMC2541142] [PubMed: 7755693]
- 18.
- Tillmann M, Silcock J. A comparison of smokers’ and ex-smokers’ health-related quality of life. J Public Health Med 1997;19:268–73. [PubMed: 9347449]
- 19.
- Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, Rutten-van Molken MP. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health 2005;8:178–90. [PubMed: 15877590]
- 20.
- Orme ME, Hogue SL, Kennedy LM, Paine AC, Godfrey C. Development of the health and economic consequences of smoking interactive model. Tob Control 2001;10:55–61. [PMC free article: PMC1763982] [PubMed: 11226362]
- 21.
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12:203. [PMC free article: PMC3352300] [PubMed: 22429454]
- Exercise Assisted Reduction then Stop work sampling form
- Estimating the resource use and cost for the Exercise Assisted Reduction then Stop Intervention
- Economic evaluation search strategy used to identify cost-effectiveness analysis studies in smoking cessation
- Evidence review summary table
- Data used in probabilistic analysis
- Other results/cost-effectiveness analyses/analyses
- References
- Cost-effectiveness data collection materials - A pilot randomised trial to asses...Cost-effectiveness data collection materials - A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers
- Post-discharge letters to patients and general practitioners, follow-up forms an...Post-discharge letters to patients and general practitioners, follow-up forms and case report form - A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial
- References - Home environmental assessments and modification delivered by occupa...References - Home environmental assessments and modification delivered by occupational therapists to reduce falls in people aged 65 years and over: the OTIS RCT
- Type of clinical complications reported in the included studies - Contrast-enhan...Type of clinical complications reported in the included studies - Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: a systematic review and economic evaluation
- Trial Steering Committee: terms of reference and membership - A clinical and eco...Trial Steering Committee: terms of reference and membership - A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial
Your browsing activity is empty.
Activity recording is turned off.
See more...