Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Farmer AJ, Stevens R, Hirst J, et al. Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness. Southampton (UK): NIHR Journals Library; 2014 Feb. (Health Technology Assessment, No. 18.14.)

Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness.
Show detailsBackground
In this chapter, we have applied the methods described in Chapter 3 to data from the Collaborative Atorvastatin Diabetes Study (CARDS) to examine the impact of screening interval on detection of kidney disease in a UK population with type 2 diabetes. As in Chapter 3, we use statistical estimation to quantify between-patient variation in both renal function and rate of change of renal function, as well as the natural variability of biological measures that leads to error in diagnosis. We use simulation models to estimate whether screening annually, or at 2- or 3-yearly intervals, is likely to produce more false- than true-positive diagnoses of microalbuminuria: that is, more likely to detect ‘measurement error’ than ‘true change’ in albuminuria status.
Methods
Data
The randomised trial CARDS was a trial of atorvastatin versus placebo in people with type 2 diabetes. The protocol and the characteristics of the study cohort have been described in full elsewhere.151–153 Briefly, 132 clinical centres in the UK and Ireland recruited 2832 people with type 2 diabetes aged 40–75 years with low-density lipoprotein cholesterol ≤ 4.14 mmol/l, fasting triglycerides ≤ 6.78 mmol/l and at least one of the following conditions: hypertension, retinopathy, micro- or macroalbuminuria and current smoking status. Exclusion criteria included previous coronary heart disease or stroke, previous surgery for peripheral vascular disease, plasma creatinine ≥ 150 µmol/l, and HbA1c ≥ 12%. Patients were recruited between 1997 and 2001. Safety parameters, including urine albumin and urine creatinine, were measured 1, 2, 3 and 6 months after randomisation, and at 6-monthly intervals thereafter. Albumin and creatinine were measured using a Hitachi 747 autoanalyser (Boehringer Mannheim Corp, Indianapolis, IN) on urine samples that were confirmed free of infection by dipstick testing. At the recommendation of the data monitoring board, the study was stopped in June 2003 following an interim analysis of the primary cardiovascular end point, which showed a highly significant (p < 0.001) benefit of atorvastatin.
Statistical analysis
As described in Chapter 3, we fitted longitudinal models for trends and variation in log-ACR. Log-ACR at diagnosis of diabetes was modelled as a patient-level, normally distributed random effect. Change over time in log-ACR was modelled as a patient-level, normally distributed random effect, correlated with actual value of ACR. Within-measurement variability in log-ACR was modelled using a t-distribution with three degrees of freedom, uncorrelated with actual level of ACR. The model parameters were estimated using WinBUGS (MRC Biostatistics Unit, Cambridge, UK).139 We also fitted models for trends and variation in eGFR using the same methods as described above, except for the modelling of the within-measurement variability, for which we used a normal distribution. Residual plots indicated an approximate normal distribution for eGFR and, therefore, a log transformation was not required. We allowed mean eGFR and mean rate of change to vary between men and women, but held other parameters the same for men and women. Models were stratified by baseline urine albumin status. We report means and standard deviations of log- and eGFR, geometric means of ACR and the average signal-to-noise ratio as defined in Chapter 3.
Although we did not have data with which to validate individual tests for microalbuminuria in the CARDS database, the model estimates of within-person variability allowed us to estimate the proportion of positive tests that were false positive – that is, attributable to measurement variability rather than to true change in underlying renal function. With the same methodology as described in Chapter 3, we used simulation modelling to calculate the proportion of those identified with true- and false-positive tests in a population similar to that of CARDS, under the assumption of annual screening according to NICE guidelines. Our primary analysis models an annual screening programme as described above, and compares this to 2-yearly screening (biennial) and 3-yearly screening (triennial), while maintaining the NICE protocol for use of confirmatory samples when the first sample measures above threshold. In sensitivity analyses, we assess the degree of change in model parameters that would be required to reach different clinical conclusions. We also used simulation to estimate rates and accuracy of diagnoses of stage 3b kidney disease, defined by eGFR < 45 ml/minute in normoalbuminuric patients.
Results
The characteristics of the participants in the CARDS included in this analysis are shown in Table 13. Data from 2562 participants were eligible for inclusion in this analysis.
TABLE 13
Characteristics at baseline of included participants
Albumin-to-creatinine ratio
Table 14 reports the parameters used in defining the model that estimates trends and variation in log-ACR. The mean log-ACR of 0.41 (95% CI 0.28 to 0.53) in men and 0.29 (95% CI 0.11 to 0.48) in women are equivalent to geometric mean ACR at diagnosis of diabetes of 1.51 (95% CI 1.32 to 1.70) mg/mol and 1.34 (95% CI 1.12 to 1.62) mg/mol, respectively. The average change in log-ACR per year of 0.018 corresponds to a 2% per year increase in ACR in both sexes (Figure 12). The standard deviation around this average, 0.022 on the log-ACR scale, corresponds to variation in the rate of change: the estimated 25th and 75th centiles of the annual increase in ACR are a 0.3% and 3.3% increase per year, respectively. The estimated standard deviation of within-measurement variability in log-ACR is 0.85 (95% CI 0.74 to 1.00), which corresponds to a within-measurement coefficient of variation in ACR > 100%. The average signal-to-noise ratios, defined as average change divided by within-measurement standard deviation, are therefore 0.021, 0.042 and 0.063 for annual, biennial and triennial screening, respectively.
TABLE 14
Parameter estimates (95% CIs) in a random-effects model of trend and variation in log-ACR based on the CARDS cohort
Tables 15 and 16 report the estimated rates of identification of microalbuminuria modelled from these estimates. Results for men and women are shown separately (see Tables 15 and 16). The tables show the cumulative rate of positive test results, the percentage of those that are false positive and the percentage of those repeatedly testing negative that are false negative. Each of these outcomes is shown under assumptions of annual, biennial and triennial screening.
Over the first 6 years after diagnosis of diabetes, annual, biennial and triennial screening would, respectively, identify 451, 394 and 365 cases of microalbuminuria per 1000 men with type 2 diabetes. Over 18 years, annual, biennial and triennial screening would identify 582, 534 and 505 cases, respectively.
In an initial screening test, an estimated 78% of those identified with microalbuminuria would be true positive, and 22% would be false positive. By year 6, with assumptions of annual, biennial and triennial screening, respectively, 36%, 30% and 27% of diagnoses of microalbuminuria in men would be false positive, and, by year 18, 35%, 29% and 26%. By year 18, estimated false-negative rates for men were 0.1%, 0.8% and 1.7% for annual, biennial and triennial screening, respectively.
Table 16 shows the corresponding estimates for women with type 2 diabetes. Over 18 years, we estimate that annual, biennial and triennial screening, respectively, would identify 431, 382 and 354 cases of microalbuminuria per 1000 women with type 2 diabetes, 50%, 44% and 40% of which would be false positive. Of those testing negative for microalbuminuria, 0.1%, 0.3% and 0.7% would be false negative.
In summary, in men and women with type 2 diabetes, 36% (95% CI 32% to 42%) and 48% (95% CI 41% to 55%), respectively, of patients tested annually for microalbuminuria over 6 years would be inaccurately identified as having microalbuminuria. If screening intervals were extended to 3-yearly, the corresponding figures would be 27% (95% CI 23% to 31%) and 37% (95% CI 30% to 44%).
Figure 13 shows the degree to which the model reproduces the CARDS data by comparing model predictions to observed data. Figure 14 examines the extent to which the resulting model can be generalised by comparing model-predicted rates of microalbuminuria identification to observed prevalence of microalbuminuria in other cohort studies, with the limitation that different studies have used different criteria (e.g. number of confirmatory tests) for diagnosing microalbuminuria.
To illustrate the sensitivity of our conclusions to the exact parameter values, Figure 15 shows how the estimated false-positive rate would vary under different assumptions about the average rate of progression of log-ACR. This illustrates the change in the number of false-positive tests when the average rate of change in ACR per year used within the model is increased above that observed in our cohort.
Estimated glomerular filtration rate
Data on 2012 CARDS participants with normoalbuminuria at baseline and 550 CARDS participants with microalbuminuria at baseline were used in obtaining model estimates. Table 17 reports the parameters used in defining the model that estimates trends and variation in eGFR in people with type 2 diabetes and normoalbuminuria. The estimated mean eGFR at 10 years since diagnosis of diabetes is 69 (95% CI 69 to 70) ml/minute in men and 62 (95% CI 61 to 64) ml/minute in women, and the between-person standard deviation is 8.7 (95% CI 8.1 to 9.3) ml/minute. The estimated average change in eGFR per year is 0.26 (95% CI 0.13 to 0.40) ml/minute in men, and 0.24 (95% CI 0.061 to 0.42) ml/minute in women. The estimated between-person standard deviation of the reduction per year is 0.50 (95% CI 0.28 to 0.69) ml/minute. The estimated standard deviation of within-measurement variation in eGFR is 4.9 (95% CI 4.8 to 5.1) ml/minute. The signal-to-noise ratio is therefore 0.053 for annual monitoring, 0.11 for biennial monitoring and 0.16 for triennial monitoring. Table 17 also shows the corresponding parameter estimates for participants with normoalbuminuria and microalbuminuria at baseline. Figure 16 illustrates the observed mean eGFR by duration of diabetes compared with the modelled estimates of progression and shows how the modelled rate of change matches the original cohort data.
TABLE 17
Parameter estimates (ml/minute) in a random effects model of trend and variation in eGFR in people with type 2 diabetes
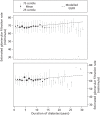
FIGURE 16
Observed mean (dot) and 25th and 75th centiles (vertical lines) of eGFR in men (top) and women (bottom) with type 2 diabetes and normoalbuminuria, and model estimate of trends (dashed line).
Table 18 shows the modelled estimates of the proportion of normoalbuminuric men classified as having stage 3b kidney disease (eGFR < 45 ml/minute) over time for annual, biennial or triennial eGFR screening. In annual screening, for example, there would be 20 men identified with stage 3b kidney disease (95% CI 16 to 26) per 1000 after 18 years, and 67% (95% CI 53% to 83%) of these would be false positive. Similar estimates were obtained for women (data not shown).
Discussion
As for type 1 diabetes (Chapter 3), we found that the short-term variability of ACR measurement is so great that the variability itself accounts for a high proportion of microalbuminuria diagnoses, even though these diagnoses are based on repeated testing of an individual. Replacing current practice with biennial or triennial screening would reduce the total number of false-positive diagnoses. Under all schemes the false-negative rate – people with underlying microalbuminuria incorrectly classified as normoalbuminuria – is small compared with the false-positive rate. We also found that, in the majority of patients, eGFR does not decline until after the onset of microalbuminuria, which implies that urine albumin testing has a more important role to play than serum creatinine in the monitoring of renal function in type 2 diabetes.
Our model is subject to limitations such as the degree to which the CARDS cohort is typical of the general UK type 2 diabetic population. For example, the extent to which ACEi and A2RB treatment rates within a randomised trial of HME-CoA reductase inhibitors (statins) are representative of the general diabetic population is not known. However, Figure 14 suggests that microalbuminuria rates predicted by our model are not atypical of type 2 diabetes. The microalbuminuria results in Figure 14 also show good model fit over a wide timescale despite the relatively short follow-up time in CARDS. Although our exact numerical results are subject to limitations, the general principle that high within-measurement variability brings about repeated opportunity for false-positive results is not in doubt (Box 1), and Figure 15 illustrates that our broad conclusions can be quite robust even to large changes in a key model parameter.
The clinical implication of the high number of false-positive diagnoses of microalbuminuria is overtreatment, with ACEi and A2RB, of patients whose diagnosis of microalbuminuria is in fact a result of measurement error rather than underlying renal dysfunction. The systematic review in Chapter 2 found that, in type 2 diabetes, even patients with normal renal function can benefit from ACEi and A2RB. This contrasts with the situation in type 1 diabetes, in which there is little evidence of benefit from treatment in normoalbuminuric people. It follows that the cost-effectiveness of monitoring people with type 2 diabetes for onset of microalbuminuria is difficult to predict. This, and the relative value of different monitoring intervals, is therefore the subject of a full cost-effectiveness modelling exercise in Chapter 6.
- Modelling progression of kidney disease in type 2 diabetes - Optimal strategies ...Modelling progression of kidney disease in type 2 diabetes - Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness
- Qualitative process evaluation - Patient-reported outcome measures for monitorin...Qualitative process evaluation - Patient-reported outcome measures for monitoring primary care patients with depression: the PROMDEP cluster RCT and economic evaluation
- List of abbreviations - Feasibility of a RCT of techniques for managing an impac...List of abbreviations - Feasibility of a RCT of techniques for managing an impacted fetal head during emergency caesarean section: the MIDAS scoping study
- Discussion and conclusions - Optimal strategies for identifying kidney disease i...Discussion and conclusions - Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness
- Methods - A high-dose preparation of lactobacilli and bifidobacteria in the prev...Methods - A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE)
Your browsing activity is empty.
Activity recording is turned off.
See more...