Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Farmer AJ, Stevens R, Hirst J, et al. Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness. Southampton (UK): NIHR Journals Library; 2014 Feb. (Health Technology Assessment, No. 18.14.)

Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness.
Show detailsThis section presents the methods and results of a series of cost-effectiveness analyses that evaluate alternative screening programmes for renal function in people with diabetes. The models described in Chapter 5 were used to estimate both costs and effects. The simulation models outlined in Chapters 3 and 4 for the progression and screening of ACR levels in type 1 and type 2 diabetes patients were incorporated into the models presented in Chapter 5. The results for screening people with type 1 and type 2 diabetes are presented first, followed by interpretation and discussion of the results to facilitate decisions regarding the implementation of renal function monitoring programmes by the NHS.
Methods
Economic evaluation
Incremental cost-effectiveness analyses were carried out for each of the various screening strategies. The incremental net cost and net cost-effectiveness were calculated in relation to the comparator, and expressed as a ratio for each strategy. As the economic evaluation perspective was that of the health-care purchaser, only direct health service costs were included. Both costs and effects were discounted at 3.5% per year for the first 30 years and 3% subsequently, in line with current guidelines.186
All outcomes are expressed as QALYs, as this measure captures both increases in life expectancy and improved quality of life resulting from prevention of complications, providing a composite outcomes measure of fatal and non-fatal events that permits comparisons among a wide range of interventions.
In the main analyses, we vary the screening interval between 1 and 10 years, initially using 5-year and then 1-year increments. Where possible, results are reported as mean values and standard deviations, or mean differences with CIs, and as ‘cost-to-clinical-effectiveness’ ratios. The effect of uncertainty surrounding costs and the utility values used in the study were examined using sensitivity analyses.
Model simulations
Using the models outlined in Chapter 5, simulations were undertaken at a patient level. The simulations include (as treatment effects) estimates of the proportion of true-positive/true-negative/false-positive/false-negative tests and the consequences of these outcomes in terms of additional benefit and cost of treatment. These models were used to examine the impact of various monitoring policies on the incidence of important health states (e.g. CVD). The results presented are the average of 1000 simulations, which stabilise the expectation of different individual Monte Carlo simulations.
Resource data and costs (type 1 and the United Kingdom Prospective Diabetes Study outcomes model)
The economic evaluation was carried out from the perspective of the health-care provider and, therefore, the following costs were included in the analysis: cost of monitoring; cost of further investigations; cost of treating diagnosed kidney disease in people with diabetes; and the cost of subsequent complications.
All costs included were obtained from published UK sources (individually referenced below) and the values and sources of cost data are described in the following sections. Costs are expressed in 2011 pounds sterling and data from previous years have been adjusted to 2011 value using the Hospital and Community Services pay and price index.186
Table 21 summarises the main sources of information on therapy unit costs, implementation unit costs and immediate (and subsequent) costs of CVD complications. As described in previous chapters, a maximum of three urinary ACR tests are taken during each year of monitoring. To determine whether a patient with normoalbuminuria has progressed to microalbuminuria or macroalbuminuria, two positive tests are required before patients are confirmed to have progression of kidney disease requiring intensification of treatment. The cost of the ACR test is estimated at £2.16 according to the Chronic Kidney Disease Costing Report, published by the National Institute for Health and Care Excellence in 2008.187 ACR tests are administered by the practice nurse, and we approximate each test to take 10 minutes of the nurse’s time. According to the Unit Costs for Health and Social Care 2010,188 a 10-minute clinic visit costs £13.50. Once a patient is confirmed to have progressed from normoalbuminuria to microalbuminuria, a GP is consulted to discuss what action to take and the administering of an ACEi, which we estimate to take around 5 minutes of a GP’s time (£15.41).188
TABLE 21
Summary of all costs used in the type 1 simulation model
If patients are confirmed to require treatment intensification through use of the ACR test, they are recommended to start an ACEi, or A2RB if they cannot tolerate ACEi. Pursuant to Prescription Cost Analysis England 2010,189 ACEi and A2RB cost £27.74 and £191.12 per year, respectively.
Furthermore, UKPDS 65 provides estimates of inpatient and outpatient costs for complications related to type 2 diabetes in the UK for fatal/non-fatal myocardial infarction, fatal/non-fatal stroke, no complications and a history of cardiovascular events.190 These were included in the model to estimate potential cost offsets from lower rates of CVD.
End-stage kidney disease costs were divided into two parts. The National Costing Report estimated a mean annual cost of £29,800 per patient for haemodialysis, which is the main mode of renal replacement therapy in the UK.192 A previous study, using UK guidelines, estimated the additional costs associated with diagnosis to be £11,479,191 which takes into account the cost of initial investigations, total referral cost and follow-up. For patients who move to end-stage kidney disease, the model includes a cost of around £40,000 in the first year (i.e. combining both diagnosis and dialysis costs), and £29,800 for every subsequent year they survive in that state.
Utility values
The economic evaluation for screening people with either type 1 or type 2 diabetes uses QALYs to adjust length of life for quality of life. A QALY value of 1 is equivalent to being in full health and 0 is equivalent to death. The values used are based on a recent meta-analysis.170 Patients without complications were assumed to have a QALY of 0.81, reflecting that people with diabetes are on average in less than full health. In the type 1 simulation model, which uses a composite cardiovascular event, we assumed two-thirds had a myocardial infarction (reducing their utility to 0.75) and one-third of patients had a stroke (reducing their utility to 0.59) over their remaining lifetime. ESRD patients are assumed to have a utility of 0.48. We also incorporated these utility values into the UKPDS outcomes model, and used the original utility values for all other diabetes complications (Table 22).
TABLE 22
A summary of diabetes complications and their respective health utility values and publication sources
Model baseline parameters
For the type 1 diabetes evaluation, a cohort of 10,000 patients was progressed through the type 1 simulation model (see Chapter 5). Screening for albuminuria commenced when patients were aged ≥ 12 years, in keeping with UK guidelines. The frequency of the screening was varied from annual to 10-yearly using the model described in Chapter 3.
A similar evaluation was undertaken for screening in patients with type 2 diabetes using the adapted version of the UKPDS outcomes model described in Chapter 5. For these simulations, the mean age of patients was assumed to be 62 years and the baseline characteristics were based on the patients in the post-study monitoring phase of UKPDS. Following UK guidelines, screening occurred annually beginning from time of diagnosis for type 2 diabetes patients. Again, the incremental cost-effectiveness ratios (ICERs) are calculated for different screening intervals from annual to 10-yearly using the model described in Chapter 4.
Analysis
Results are reported as mean values and standard deviations, or mean differences with CIs, and as cost-to-clinical-effectiveness ratios. To provide a visual representation of the results, the costs and health outcomes were mapped onto the cost-effectiveness plane and reported as acceptability curves.193 The effect of uncertainty surrounding some aspects of cost and the utility values used in the study were examined using sensitivity analyses.
A series of sensitivity analyses were carried out to examine whether the results in the main analysis were robust to a series of assumptions:
- the effect of costs: higher ACR test cost
- the effect of changing progression values of ACR in the simulation models outlined in Chapters 3 and 4 to fixed values at the upper and lower end of the reported 95% CIs
- the impact of assumptions about glycaemic control: estimating ICERs if all patients were simulated over a lifetime in two scenarios using each of the conventional and intensive treatment groups of the DCCT.
Results
Screening people with type 1 diabetes: results of the type 1 model
The characteristics of the patients in the type 1 cohort are summarised in Table 23. For the base-case scenario over a lifetime, around 40% of patients are estimated to have a cardiovascular event, and 25% progress to end-stage kidney disease. Life expectancy is estimated at 63.1 years, which is similar to the figure obtained in Soedamah-Muthu and colleagues’ study on type 1 diabetes patients in the UK. Females have a higher risk of mortality, and this is reflected in the literature.175,177–181
TABLE 23
Baseline characteristics (± standard deviation) of type 1 diabetes patients in the DCCT. Source: Nathan et al. 2005
The base-case scenario examined was annual screening of newly diagnosed type 1 diabetes patients ≥ 12 years old; the model determined total cost and QALYs gained over the duration of the simulation for the base case and for screening at greater intervals. Results of the simulation using the type 1 model are shown in Table 24. The columns of the table represent an incremental comparison of reducing the screening interval from 5 years progressively to 1 year. The costs associated with screening increase in proportion to its frequency, with the cost of annual screening being around five times greater than 5-yearly screening. The benefit in terms of incremental QALYs gained increases at a slower rate and hence the ICER increases from £3612 (£6586) to £9601 (£34,112), but all of these ratios are well within NICE’s QALY threshold of £30,000 per QALY.
TABLE 24
Mean (standard deviation) difference in cost, QALYs and incremental cost per QALY for comparisons between different screening intervals for people with type 1 diabetes
The probabilistic uncertainties surrounding these estimates are shown in Figure 22, which illustrates a cost-effectiveness plane comparing biennial with annual screening intervals, and Figure 23 shows the corresponding cost-effectiveness acceptability curve. Figure 23 shows the probability that annual screening is cost-effective based on different thresholds for a QALY. The probability the intervention is cost-saving is around 25%, and it has around an 80% chance of being below the cost-effectiveness threshold of £30,000.

FIGURE 22
Cost-effectiveness plane when comparing biennial screening intervals with annual screening intervals for patients with type 1 diabetes.
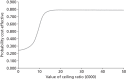
FIGURE 23
Cost-effectiveness acceptability curve for biennial screening intervals vs. annual screening intervals for patients with type 1 diabetes.
Screening people with type 2 diabetes: results of the type 2 model
Using this model, 1000 patients were simulated for 30 years; the mean age of patients newly diagnosed with type 2 diabetes was 62 years. Baseline characteristics are reported in Table 25. According to UK guidelines, screening occurred annually beginning from time of diagnosis for type 2 diabetes patients.
TABLE 25
Reported baseline characteristics (± standard deviation) for type 2 diabetes patients in the UKPDS outcomes model. Source: Holman et al. 2008
Results of the cost-effectiveness analysis are shown in Table 26. The differences in costs and QALYs between biennial and annual screening were reported as £209 (standard deviation £309) and 0.42 (standard deviation 0.12), resulting in an overall ratio of £606 (standard deviation £1782), which makes the intervention highly cost-effective. When the screening interval is increased beyond 5 years, there is a minimal difference between QALYs, suggesting that increasing the screening interval further has little impact on QALYs and that, potentially, a ‘sojourn period’ is reached.
TABLE 26
Mean difference (standard deviation) in cost, QALYs and incremental cost per QALY for comparisons between different screening intervals for people with type 2 diabetes
Similarly, the differences in costs reflect the decreasing costs of ACR tests, but the increasing number of patients being treated for hypertension and kidney disease. Figures 24 and 25 represent the uncertainty surrounding the cost-effectiveness of renal screening in patients with type 2 diabetes. The cost-effectiveness plane is illustrated in Figure 24, showing a positive incremental benefit and incremental cost for all observations. This suggests that, although annual screening provides added benefits in terms of QALYs, it comes at a higher cost. Figure 25 shows a very high probability (97%) of annual screening being below the cost-effectiveness threshold of £30,000 per QALY. Based on these results, annual screening appears to be a cost-effective option.

FIGURE 24
Cost-effectiveness plane when comparing biennial screening intervals with annual screening intervals for patients with type 2 diabetes.
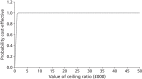
FIGURE 25
Cost-effectiveness acceptability curve for biennial screening intervals vs. annual screening intervals for patients with type 2 diabetes.
Sensitivity analysis results
The sensitivity analysis results for both type 1 and type 2 models are shown in Tables 27–29. We compared biennial screening intervals to annual screening intervals in all our analyses to help determine what factors drive our model. The results of these analyses are reported below.
TABLE 27
Sensitivity analyses conducted and differences in cost, QALYs and incremental cost per QALY for comparing yearly screening intervals with biennial screening intervals for people with type 1 diabetes
TABLE 28
Sensitivity analyses conducted and differences in cost, QALYs and incremental cost per QALY for comparing yearly screening intervals with biennial screening intervals for people with type 2 diabetes
Increasing cost of albumin-to-creatinine ratio test
For the type 1 model, the difference in cost between annual and biennial screening intervals was calculated to be £3639, with no change in the difference in QALYs of 0.26, resulting in an increased ICER of £13,997 per QALY when compared with our initial analysis. Similarly, in the UKPDS model, differences in costs increased to £811, resulting in an increased ICER of £1932 per QALY.
Albumin-to-creatinine ratio progression
Changing the values for progression of ACR in the simulation models outlined in Chapters 3 and 4 had a large impact on the results for both diabetes models. Changing the ACR progression variables to their lower 95% CI value saw a small proportion of patients progress to requiring treatment and thus ESRD, resulting in a reduction in the difference in costs and QALYs. The ICER calculated of £40,801 lies outside NICE’s recommendation, suggesting that annual screening is not cost-effective. The UKPDS model shows a similar result of £51,324 per QALY, rendering this particular scenario cost-ineffective.
When adjusting the variables to the upper 95% CI limit, annual screening was very effective in identifying patients because of their quick progression to requiring treatment, and thus ESRD. Hence, the ICERs for both models were lower when using the higher CI limit than when using the lower CI limit: £26,945 and £25,447 per QALY for type 1 diabetes and type 2 diabetes patients, respectively.
Figures 26 and 27 show the results of modelling progression of the ACR over time from diagnosis. The modelling integrates the equations that estimate the true progression of renal function in the simulation models for type 1 and type 2 diabetes patients. The simulation uses estimates of the upper and lower 95% CI limits of coefficients for the observed mean rate of ACR progression to present two alternative scenarios for progression. The figures show that, for the upper 95% CI values, progression occurs very quickly and, therefore, annual screening would identify more patients with microalbuminuria than biennial screening. However, it might be too late to treat some of these patients before they develop end-stage kidney disease.

FIGURE 26
Sensitivity analysis for rate of ACR progression based on running simulations for the 95% CI of the average rate of progression for type 1 diabetes.

FIGURE 27
Sensitivity analysis for rate of ACR progression based on running simulations for the 95% CI of the average rate of progression for type 2 diabetes.
In comparison, modelling with mean ACR progression estimates based on the lower limit of the 95% CI shows that the progression of kidney disease is very slow, and thus neither annual nor biennial screening intervals would reliably identify the small number of patients progressing to microalbuminuria. Therefore, it is likely to be less cost-effective than the upper 95% CI limit scenario, and possibly cost-ineffective.
Adjusting cardiovascular equations (type 1 model only)
If all patients in the economic evaluation were assumed to have conventional treatment as stipulated by the DCCT, the ICER is calculated at £9711.71 per QALY.45 However, if all patients were assumed to have intensive treatment, the ICER would be smaller, at £5079 per QALY. The latter scenario would see an increase in QALYs, and a decrease in cardiovascular events and thus costs.
Utility values
Adjusting utility values to the upper and lower 95% CI values for all diabetes events was shown to have little impact on the cost-effectiveness of screening, as the type 1 diabetes model and the UKPDS model found both scenarios to be very cost-effective.
Comparison of discounted and undiscounted costs
Table 29 reports the costs separately in two major categories: screening costs (i.e. tests and follow-up) and treatment and hospitalisations costs. Doubling the frequency of screening substantially increases the costs (e.g. from £2937 to £5121 in the case of type 1 diabetes). Screening and treatment costs are much higher for the type 1 diabetes patients because of the extended nature of the screening (i.e., for patients with type 1 diabetes, screening and treatment continue over their lifetime of around 50 years compared with roughly 15 years for the average person with type 2 diabetes).
Discussion and interpretation of the results
Main conclusion
Annual screening appears to be a cost-effective option for both type 1 and type 2 diabetes compared with biennial screening and other health-care interventions. For type 1 diabetes, screening produces benefit at a cost, but this cost is well below accepted thresholds used for other types of health care. For type 2 diabetes screening, the cost-effectiveness ratio is highly favourable. The data on which our analysis was based did not provide information about more frequent screening intervals than 1 year; however, the results did indicate that the benefit might be greater if the underlying rate of progression of ACR is higher than the average in the population.
Comparisons with previous literature
We have found renal screening for people with type 1 and type 2 diabetes to be cost-effective in a UK context with cost-effectiveness ratios that would compare favourably with many other funded health interventions. With regard to the benefits, it would appear that screening produces comparable or greater outcomes to that observed in a general population screening. For example, it has been previously estimated that biennial mammography for women aged 50 produces less than 1 additional month of survival and Pap smears less than 3 months.194 Here, particularly with type 2 diabetes, the gains in QALYs range from a few months to over half a year.
The findings of this study are also consistent with two studies of the cost-effectiveness of blood pressure treatment in type 2 diabetes. A study based on an analysis alongside the UKPDS indicated that tight blood pressure control policies (including use of ACE inhibitors) produces several months’ increase in QALYs at a cost of only £300 per QALY.195 Similarly, an analysis of the ADVANCE study showed treatment with a fixed combination of blood pressure therapies (again, including ACE inhibitors) produced significant increases in life expectancy and was cost-effective.196
The higher cost-effectiveness ratio in screening type 1 diabetes patients is a result of a number of factors impacting on relative outcomes and costs of screening among these groups. First, when benefits are not discounted, the gains in QALYs of screening are similar in type 1 diabetes and type 2 diabetes, but the long duration between the commencement of screening and when the majority of patient experience cardiovascular events significantly reduces discounted QALYs. Another reason for the differences across these patients is the effect of treatment on ACR. In the case of type 2 diabetes, we have assumed an average 52% reduction in ACR (see Chapter 2), whereas in type 1 diabetes the effect is 32% (see also Chapter 2). The greater incremental effect in type 2 diabetes is a result of screening older patients who are at a higher risk during the entire screening period and the treatment being more effective in slowing progression to renal failure.
The average difference in lifetime screening costs is also much higher for type 1 patients, as, currently, the guidelines for screening recommend annual screening from diagnosis with either type of diabetes. Assuming that the age at diagnosis in type 1 diabetes is around 15 years, whereas in type 2 diabetes it is 62 years, patients with type 1 diabetes will have many more tests over their lifetime.
Further, these screening costs will be occurred at a constant rate, whereas any cost offsets associated with CVD or kidney disease are likely to occur after the age of 40 years. In contrast, type 2 diabetes patients are generally already at risk of complications at diagnosis and, therefore, the cost offsets will occur much sooner.
Clinical and research implications
While these simulations provide useful insights relating, in particular, to the additional benefits of universal treatment over screening, it is difficult to draw firm policy conclusions regarding the merits of their adoption in practice because of the limited number of studies reporting continued long-term ACE inhibitor use. For example, although the ADVANCE study randomly allocated all patients with ACE inhibitors in combination with another blood pressure medication, it involved screening patients’ toleration for this treatment through an active run-in.61 Around 14% of patients withdrew during the 6-week active run-in period and the study had a mean follow-up duration of only 4.3 years. Therefore, it is necessary to collect more evidence surrounding the benefits and compliance associated with much longer periods of use before universal treatment can be considered as a practical policy option. This should be examined in future work.
A screening interval of 1 year appears optimal based on evidence of progression to renal failure of typical diabetic patients in the UK. The sensitivity analysis suggests that rates of progression would have to be substantially higher or lower than the average to have an important effect on our results. For example, within some ethnic groups (e.g. the south Asian population; see Table 5) rates of progression towards diabetic kidney disease may be substantially higher.44,197 Further empirical work could help to establish whether more frequent monitoring of ACR might be justified, or whether alternative technologies might be required to identify those at risk. Similarly, if individuals at low risk of progression could be prospectively identified, then for future implementation of protocols for personalised care it might be possible to consider screening at less frequent intervals.
Strengths and limitations
Our type 1 diabetes model is subject to limitations, such as the exclusion of other major diabetes-related complications (peripheral vascular disease, blindness and neuropathy), because of the timeframe and available secondary data sources. Nevertheless, our model appears to make predictions for type 1 diabetes patients’ life expectancy that match another large type 1 diabetes mortality study in the UK.175
The type 1 model includes important sources of major costs, including renal replacement therapy for patients who have either true-positive or false-negative test results but progress to end-stage kidney disease. Decrement of quality of life through occurrence of an ACE-related cough is not included, as the model assumes that these individuals are treated with A2RB. We have also, based on the results in Chapter 2, not added a disutility for treating patients with a false-positive test. We did not carry out a sensitivity analysis to explore the impact of the reduction in costs of A2RB with a move to generic prescribing, as this is a relatively small part of the overall costs and, in any case, would further increase the cost-effectiveness of annual screening. The impact of non-adherence to therapy is not included in this model.
The type 1 diabetes model is a synthesis of multiple data sources from a variety of different countries. Although the majority of sources are based on type 1 diabetes populations, some include type 2 diabetes patients and non-diabetes-related patients as well, which is a limitation when trying to inform NHS clinical practice for a UK-based screening programme. However, owing to the lack of published data specifically regarding type 1 diabetes populations, this was unavoidable. Further validation does, however, need to be conducted to reduce the uncertainty surrounding our estimates.
- Cost-effectiveness of monitoring renal function for type 1 and type 2 diabetes -...Cost-effectiveness of monitoring renal function for type 1 and type 2 diabetes - Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness
- List of abbreviations - Different temperature thresholds for antipyretic interve...List of abbreviations - Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT
- Intervention delivery: facilitator and referrer qualitative studies - An interve...Intervention delivery: facilitator and referrer qualitative studies - An intervention to improve the quality of life in children of parents with serious mental illness: the Young SMILES feasibility RCT
- Interpretation of placebo-controlled surgical trials and changing practice - Pla...Interpretation of placebo-controlled surgical trials and changing practice - Placebo comparator group selection and use in surgical trials: the ASPIRE project including expert workshop
- Aims and objectives of the feasibility study - An intervention to improve the qu...Aims and objectives of the feasibility study - An intervention to improve the quality of life in children of parents with serious mental illness: the Young SMILES feasibility RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...