Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Macrae D, Grieve R, Allen E, et al. A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial. Southampton (UK): NIHR Journals Library; 2014 Apr. (Health Technology Assessment, No. 18.26.)

A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial.
Show detailsRecruitment
Trial recruitment began on 4 May 2008. As indicated in Chapter 2, recruitment was slower than expected. This was mainly a result of delays in trial initiation at some sites, clinical constraints and a ‘research learning curve’ in many of the participating units which had no previous experience of recruiting critically ill children to clinical trials. These delays necessitated an application to the HTA programme for an extension to the trial. The HTA programme granted funding to allow recruitment to be extended to allow the trial to achieve sufficient power (1500 children) to identify whether or not there was a differential effect for the primary end point (VFD-30) in the two strata (cardiac and non-cardiac).
The DMEC confidentially reviewed unblinded interim analyses on two occasions. In addition, they met to discuss SAEs and recruitment rates on three further occasions.
Recruitment closed on 31 August 2011, as agreed in the HTA funding. A total of 19,924 children were screened from 13 sites. Of these, 1384 were recruited and randomised (701 to TGC and 683 to CM). The reasons for non-recruitment are shown in Table 4. Of the 1384, 15 were subsequently found to be ineligible (Table 5), leaving 1369 eligible children (694 to TGC and 675 to CM) randomised into the trial – 91% of the original target of 1500. The flow of patients is shown in Figure 3 and cumulative recruitment in Figure 4. Recruitment by site is shown in Table 6.
TABLE 4
Numbers screened with reasons for non-recruitment
TABLE 5
Ineligible patients

FIGURE 3
A flow chart showing the flow of patients. Note that the information on vital status up to 12 months was available from the Office for National Statistics, aside from for 17 non-UK nationals.

FIGURE 4
Cumulative recruitment – actual accrual vs. revised expected.
TABLE 6
Recruitment per site
Comparability at baseline
The characteristics of the children at baseline are shown in Table 7. The randomised groups were broadly comparable at trial entry. Sixty-two per cent were randomised within 1 day of admission to PICU. In terms of the prespecified stratifying factors, two-thirds were aged < 1 year, and 60% of the children were in the cardiac surgery stratum. Seven per cent of children in the cardiac surgery stratum were considered to be undergoing surgical procedures associated with a high risk of mortality (RACHS1 score 5 or 6), and 19% of children in the non-cardiac group had a PIM2 score indicative of a ≥ 15% risk of PICU mortality.
TABLE 7
Characteristics at baseline
Actual management
Table 8 describes the observed management of blood glucose after randomisation, and shows a clear difference between the two arms of the study. In the TGC arm, 461 of the children (66%) received insulin compared with 109 of 675 (16%) in the CM arm. Children in the TGC arm received more insulin, and continued on insulin for longer. Figure 5 shows the mean daily blood glucose level by arm. There was a clear separation between the two randomised arms, with children in the TGC arm having a significantly lower blood glucose profile than those in the CM arm.
TABLE 8
Actual management after randomisation

FIGURE 5
Mean glucose level.
Thirty-day clinical outcomes
Primary outcome
Results for the primary outcome are shown in Table 9. The mean number of VFD-30 from randomisation was 23 in both trial arms (mean difference 0.36; 95% CI –0.42 to 1.14).
TABLE 9
Primary outcome 30 days post randomisation
Secondary outcomes
The secondary outcomes up to 30 days are shown in Table 10, and the duration of ventilation in Figure 6 and of vasoactive drug use in Figure 7. In general, the secondary outcomes are similar between the arms over the 30-day period, although less RRT was undertaken in the TGC arm (odds ratio 0.63; 95% CI 0.45 to 0.89). Additionally, mean caloric intake (Figure 8) was similar between the two groups.
TABLE 10
Secondary outcomes 30 days post randomisation

FIGURE 6
Proportion of patients on mechanical ventilation.
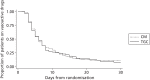
FIGURE 7
Proportion of patients receiving vasoactive drugs.

FIGURE 8
Mean caloric intake.
In terms of adverse effects, there were 135 patients whose blood glucose level was below the threshold that defined moderate hypoglycaemia; 61 of these had one or more episodes that were considered severe. Hypoglycaemia occurred in 33 (4.1%) patients not given insulin, but was more commonly observed in patients who received insulin [102 (17.9%)].
Hypoglycaemia occurred in a greater proportion of patients in the TGC arm than in the CM arm of the study (moderate, 12.5% vs. 3.1%, p < 0.001; severe, 7.3% vs. 1.5%, p < 0.001). Of the patients who experienced any hypoglycaemic episode, 11.1% died as opposed to 4.4% of those who did not experience any hypoglycaemic episode (p = 0.001).
Stratified analyses
Table 11a–e shows the primary outcome for the main prespecified stratification factors. None of the interaction tests between the intervention and prespecified subgroups was statistically significant, suggesting that there is no difference in the effect of TGC on VFD-30 in the different strata [p = 0.63 (cardiac vs. non-cardiac); p = 0.28 (age < 1 vs. ≥ 1 year); p = 0.09 (RACHS1 1–4 vs. 5–6); p = 0.88 (PIM2 < 5% vs. 5–15% vs. ≥ 15%) and p = 0.66 (run-in cases vs. non-run-in cases)]. One of the prespecified stratified analyses (TBI or not) was not included, as only 13 TBI patients were followed up at 1 year.
TABLE 11a
Ventilator-free days at 30 days post randomisation stratified by cardiac and non-cardiac patients
TABLE 11e
Ventilator-free days at 30 days post randomisation stratified by run-in (first 100 cases) and non-run-in
TABLE 11b
Ventilator-free days at 30 days post randomisation stratified by age < 1 year and age ≥ 1 year
TABLE 11c
Ventilator-free days at 30 days post randomisation stratified by operative complexity (cardiac)
TABLE 11d
Ventilator-free days at 30 days post randomisation stratified by predicted risk of mortality (non-cardiac)
In both cardiac and non-cardiac strata, hypoglycaemia occurred in a greater proportion of patients in the TGC arm than in the CM arm of the trial (moderate: cardiac 10.9% vs. 1.4%, p < 0.001; non-cardiac 15.4% vs. 5.8% p < 0.001; severe: cardiac 5.5% vs. 0.5%, p < 0.001; non-cardiac 10.3% vs. 3.1%, p = 0.001). Cardiac cases receiving insulin were not at a greater risk of hypoglycaemia than non-cardiac cases (16.4% vs. 20.3%).
Thirty-day economic outcomes
For the index hospital episode, the mean PICU bed-days, LOS on GM wards and total LOS for the index hospital episode were similar between arms (Table 12). The mean total number of hospital days up to day 30, including both the initial episode and readmissions to the initial PICU before day 30, were similar between arms (see Table 12). For the stratum admitted for cardiac surgery, the mean total LOS was again comparable between arms (Table 13). As regards the non-cardiac stratum, for the initial hospital episode, the mean numbers of PICU days, LOS on GM wards and total LOS were lower for the TGC than the CM arm (Table 14).
TABLE 12
Lengths of stay (days) within 30 days post randomisation: whole study cohort
TABLE 13
Lengths of stay (days) within 30 days post randomisation: cardiac surgery subgroup
TABLE 14
Lengths of stay (days) within 30 days post randomisation: non-cardiac surgery subgroup
Tables 15–17 report that the mean numbers of PICU bed-days, by HRG level, were similar between arms.
TABLE 15
Mean (SD) PICU bed-days by HRG level within 30 days post randomisation: whole study cohort
TABLE 17
Mean (SD) PICU bed-days by HRG level within 30 days post randomisation: non-cardiac surgery subgroup
TABLE 16
Mean (SD) PICU bed-days by HRG level within 30 days post randomisation: cardiac surgery subgroup
Overall, the mean total costs at 30 days post randomisation were similar between arms (Table 18). For the cardiac subgroup, the mean total costs per patient were £16,228 (TGC) and £17,005 (CM) (Table 19). For the non-cardiac subgroup, the TGC arm had lower mean costs than the CM group, with an incremental cost of –£2319 (95% CI –£4702 to £124) (Table 20). Including the treatment by cardiac interaction term led to a statistically significant improvement in model fit (p < 0.001).
TABLE 18
Total and incremental costs (£) within 30 days post randomisation: whole study cohort
TABLE 19
Total and incremental costs (£) within 30 days post randomisation: cardiac surgery subgroup
TABLE 20
Total and incremental costs (£) within 30 days post randomisation: non-cardiac surgery subgroup
Twelve-month results
Index hospital episode and readmissions to paediatric intensive care unit within 30 days post randomisation
Table 21 reports the mean total number of hospital days up to 12 months post randomisation, including the initial hospital episode and any readmissions to PICU within 30 days. A lower proportion of patients in the TGC than in CM arm had an index hospital admission or relevant readmission that continued beyond day 30. Between 30 days and 12 months post randomisation, the mean number of days in PICU, on GM wards and in total, was lower for the TGC than the CM arm (see Table 21).
TABLE 21
Lengths of stay (days) within 12 months post randomisation: whole study cohort
Four patients were still in hospital at the date of administrative censoring, with LOS ranging from 119 to 359 days. Each of these patients was assumed to have the mean total LOS taken across the whole sample still in hospital at the respective time point. (For example, for the patient censored at a LOS of 119 days, the assumed LOS was 228 days, according to the mean across the 46 patients still in hospital 118 days post randomisation.) One patient withdrew consent for participation in the study, after 8 days in hospital, and was assumed to have a total hospital LOS of 60 days, the mean across the whole sample of patients who were still in hospital after day 8.
For the cardiac stratum, the mean total LOS at 12 months was similar between arms (Table 22). For the non-cardiac subgroup, the TGC arm had a lower proportion of patients who had a hospital admission that continued beyond 30 days post randomisation, and on average reported fewer days on PICUs, and on GM wards (Table 23), than the CM arm. For the non-cardiac stratum, the mean total LOS for the initial episode and readmissions to PICU within 30 days was 31.0 days for the TGC arm compared with 44.5 days for the CM arm (see Table 23).
TABLE 22
Lengths of stay (days) within 12 months post randomisation: cardiac surgery subgroup
TABLE 23
Lengths of stay (days) within 12 months post randomisation: non-cardiac surgery subgroup
Figure 9 plots the proportion of patients over time who were still in hospital following the index admission. For the overall sample, and the cardiac patients, the proportion still in hospital was similar between arms at each time point. For the non-cardiac patients, a higher proportion of the CM than the TGC arm were still in hospital 60 and 90 days post randomisation.

FIGURE 9
Proportion of patients remaining in hospital (index admission) up to 180 days post randomisation. Proportion of patients remaining in hospital: (a) whole study cohort; (b) cardiac surgery subgroup; and (c) non-cardiac surgery group.
Mortality
Mortality at 12 months was similar between the randomised groups (Table 24). The CIs around each of the odds ratios all encompassed 1.
TABLE 24
Vital status within 12 months post randomisation: overall, cardiac and non-cardiac
Assessment of attention and behaviour in patients with traumatic brain injury
No differences were found between the two arms of the trial in attention and behaviour measures for those patients with TBI (Table 25).
TABLE 25
Attention and behaviour measures at 12 months (subgroup comprising children diagnosed with brain injury at trial entry)
Other hospital and community service use (after discharge from index hospital episode but excluding any readmissions to the initial paediatric intensive care unit within 30 days)
Figure 10 shows the flow of patients from randomisation to response to the service-use questionnaire. In the overall sample, a total of 397 patients (203 in the TGC arm, 194 in the CM arm) were randomised after 30 October 2010 and could not be followed up for 1 year; that is, for the purposes of collecting information on service use, these patients were administratively censored. Patients were also ineligible for the service-use questionnaire if their GP did not confirm that it was appropriate to contact them to administer the questionnaire. Of the eligible patients, the response rate to the service-use questionnaire was 63% in the TGC arm and 61% in the CM arm. For those who responded to the questionnaire, the mean LOS following hospital readmissions after 30 days post randomisation, and the mean number of contacts with hospital and personal social services, was similar between the randomised arms (Tables 26–28). The mean total costs of hospital and community health services were also similar between the randomised arms (Tables 29–31).

FIGURE 10
Flow chart for 12-month follow-up for service-use questionnaire.
TABLE 26
Levels of service use for questionnaire responders within 12 months post randomisation: whole study cohort
TABLE 28
Levels of service use for questionnaire responders within 12 months post randomisation: non-cardiac surgery subgroup
TABLE 29
Costs (£) of health and personal social services for questionnaire responders, between discharge from the index admission and 12 months post randomisation: whole study cohort
TABLE 31
Costs (£) of health and personal social services for questionnaire responders, between discharge from the index admission and 12 months post randomisation: non-cardiac subgroup
TABLE 27
Levels of service use for questionnaire responders within 12 months post randomisation: cardiac surgery subgroup
TABLE 30
Costs (£) of health and personal social services for questionnaire responders, between discharge from the index admission and 12 months post randomisation: cardiac surgery subgroup
Twelve-month total costs
Tables 32–34 report the total costs at 12 months across all the resource-use items recorded. The values presented are the results after using MI to handle missing values for health and community service costs at 12 months. Table 32 reports that, overall, the mean total costs were lower in the TGC than in the CM group, but with 95% CIs that encompass zero. For the cardiac surgery stratum, the mean total costs were similar between the groups (see Table 33), but, for non-cardiac patients, the mean costs were lower in the TGC than in the CM group, with an incremental cost of –£9865 (95% CI –£18,558 to –£1172) (see Table 34).
TABLE 32
Total costs (£) at 12 months post randomisation: whole study cohort
TABLE 34
Total costs (£) at 12 months post randomisation: non-cardiac surgery subgroup
TABLE 33
Total costs (£) at 12 months post randomisation: cardiac surgery subgroup
Figures 11–13 report SAs that investigate whether or not the base-case results are robust to alternative assumptions. The results show that the incremental costs under these alternative scenarios are similar to the base case. For example, in the SAs that include additional costs of staff time and tests associated with monitoring TGC, and further costs for managing hypoglycaemic episodes, the mean incremental costs of TGC overall and for the non-cardiac subgroup are similar to the base case (see Figures 11–13). Moreover, when alternative approaches were taken to unit costing, this had little impact on the results.
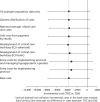
FIGURE 11
Sensitivity analysis reporting mean total costs at 12 months post randomisation according to alternative assumptions: whole study cohort.

FIGURE 13
Sensitivity analysis reporting mean total costs at 12 months post randomisation according to alternative assumptions: non-cardiac surgery subgroup.
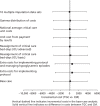
FIGURE 12
Sensitivity analysis reporting mean total costs at 12 months post randomisation according to alternative assumptions: cardiac surgery subgroup.
Lifetime cost-effectiveness results
The Kaplan–Meier survival curves show that when the time horizon was extended beyond 1 year, for those for whom survival data were available, the probability of survival remained similar between arms (Figure 14).

FIGURE 14
Kaplan–Meier survival curves. Overall cohort, TGC vs. CM.
Figure 15 considers alternative parametric extrapolations for both treatment arms combined, using the observed survival data after day 30. Of the alternative survival functions, the Gompertz function appears to fit the observed data best in that it reports the lowest Akaike and Bayesian information criterion (Table 35). The Gompertz function also offers the most plausible projections of future survival (Table 36), in that the levels of excess death compared with those for the age- and gender-matched general population remain constant over time from 2 years post randomisation onwards.
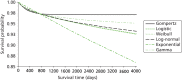
FIGURE 15
Comparison of alternative parametric extrapolations for survival from 12 months to 5 years post randomisation, across both randomised arms.
TABLE 35
Fit of alternative parametric survival functions applied to the CHiP study data after day 365
TABLE 36
Ratios of the death rates from applying alternative parametric extrapolations for all patients from the CHiP Study compared with the age- and gender-matched general population
Tables 37–39 present the resultant life-years, QALYs, lifetime costs and INBs according to the base-case assumptions. Overall, at a threshold of £20,000 per QALY, the INBs are positive, but with wide 95% CIs that include zero. For cardiac patients, the INBs are close to zero with wide CIs. For non-cardiac patients, the INBs are positive but with 95% CIs that include zero.
TABLE 37
Lifetime cost-effectiveness analysis: mean (SD) costs (£), life-years and INBs (£): whole study cohort
TABLE 39
Lifetime cost-effectiveness analysis: mean (SD) costs (£), life-years and INBs (£): non-cardiac surgery subgroup
TABLE 38
Lifetime cost-effectiveness analysis: mean (SD) costs (£), life-years and INBs (£): cardiac surgery subgroup
The cost-effectiveness acceptability curves consider alternative thresholds of willingness to pay for a QALY gain, and show that, overall and for the cardiac surgery stratum, it is highly uncertain whether or not TGC is cost-effective (Figures 16 and 17). For the non-cardiac stratum, the probability that TGC is cost-effective is relatively high. For example, at ceiling ratios of £10,000 to £30,000 per QALY, the probability that TGC is cost-effective ranges from 90% to 70% (Figure 18).

FIGURE 16
Probability that TGC vs. CM is cost-effective at alternative levels of willingness to pay for a life-year gained: whole study cohort.
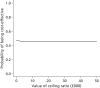
FIGURE 17
Probability that TGC vs. CM is cost-effective at alternative levels of willingness to pay for a life-year gained: cardiac surgery subgroup.

FIGURE 18
Probability that TGC vs. CM is cost-effective at alternative levels of willingness to pay for a life-year gained: non-cardiac surgery subgroup.
The SA on the lifetime results suggests that these findings are robust to alternative assumptions about the extrapolation of long-term survival, QoL for PICU survivors or long-term costs (Figures 19–21).

FIGURE 19
Sensitivity analysis reporting lifetime INBs (£) according to alternative assumptions: whole study cohort.

FIGURE 21
Sensitivity analysis reporting lifetime INBs (£) according to alternative assumptions: non-cardiac surgery subgroup.

FIGURE 20
Sensitivity analysis reporting lifetime INBs (£) according to alternative assumptions: cardiac surgery subgroup.
- Results - A clinical and economic evaluation of Control of Hyperglycaemia in Pae...Results - A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial
- Methods - Helping pregnant smokers quit: a multi-centre randomised controlled tr...Methods - Helping pregnant smokers quit: a multi-centre randomised controlled trial of electronic cigarettes versus nicotine replacement therapy
- List of abbreviations - Optimal pharmacotherapy pathway in adults with diabetic ...List of abbreviations - Optimal pharmacotherapy pathway in adults with diabetic peripheral neuropathic pain: the OPTION-DM
- List of abbreviations - Progesterone to prevent miscarriage in women with early ...List of abbreviations - Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT
- Assessment of cost-effectiveness - Contrast-enhanced ultrasound and/or colour du...Assessment of cost-effectiveness - Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: a systematic review and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...