Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cassell JA, Dodds J, Estcourt C, et al. The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care. Southampton (UK): NIHR Journals Library; 2015 Jan. (Health Technology Assessment, No. 19.5.)

The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care.
Show detailsScope of the economic evaluation
As a result of recruitment failure, we were unable to complete the economic evaluation originally planned. It was possible only to carry out a limited cost analysis of the pilot study and preliminary economic evaluation of the intensive recruitment phase.
The economic assessment presented in this chapter focuses on two main topics: (1) the costs of the PN model presented here; and (2) the costs of intensive recruitment as undertaken in phase 4.
Comparison of costs for alternative partner notification pathways
The success of any new strategy or intervention must be balanced by the resources required to achieve the intended outcome, and additional resources must be evaluated in terms of any additional benefit that can be attributed to them and whether or not the additional costs are justified given any additional benefit. Thus, the costs of achieving any PN success as a result of either a patient or provider referral pathway used in the pilot are integral to assessing the cost-effectiveness of each approach.
It was not possible to assess the success of the strategy properly in terms of outcomes compared with current practice owing to the recruitment issues in this trial. However, we can attribute costs to the patient and provider referral pathways used in the pilot and compare these with the costs that are likely to be incurred by other PN strategies that have been evaluated in the UK to date. Thus, the resulting analysis is not a full economic evaluation comparing costs and outcomes of two or more alternatives,67 but a partial evaluation which compares a number of alternative strategies in terms of costs only.
Economic objective
What are the costs of the patient and provider referral models of PN as used in the pilot and how do these costs compare with other proposed/existing pathways that might be used for the purpose of PN?
Methods
We compared the costs associated with the pilot PN pathways with two separate published studies that investigated the costs (and outcomes) associated with PN strategies compared with current practice in the UK.
The two studies with which we compared the pilot pathways are: (1) the NIHR-funded ClaSS project, which included a nested PN trial;23 and (2) the MRC-funded APT studies.33,68 In both studies, resource use data were collected alongside a primary clinical study and unit costs were applied. The average cost per case of PN as reported in the published ClaSS and APT studies is naturally affected by the success in terms of relative effectiveness of any particular strategy. For example, if a particular PN strategy successfully reaches and treats more sexual partners than an alternative strategy at the same cost, the cost per partner treated will be lower than the alternative, as more partner(s) have been treated. In the current study, it is not appropriate to compare across these different strategies in terms of effectiveness because the approaches apply to different individuals in different geographical settings and such a direct comparison will cause bias.
Furthermore, the ClaSS project represented a randomised controlled trial while the APT study was an exploratory trial and was not randomised. Instead we attempted to consider the typical pathway for one index case and their respective partners. We therefore assumed the average resource use (e.g. average time of consultation with the health-care professional relevant to that pathway) and applied unit costs to the average resource use likely to be incurred by one individual and their partners on that pathway. In order to provide a consistent approach to the following comparative analysis of costs it was necessary to make some assumptions prior to conducting the analysis.
Assumptions
- All pathways begin from the diagnosis of and discussion with the index case and include the costs and resources associated with the index case to ensure consistency across pathways. The inclusion of the index patient is required because some pathways include PN advice at this initial stage and fair disaggregation of associated resource use would not be possible.
- Based on results from the ClaSS study, we have assumed that each index case generates 1.5 partners.
- All index patients and their partners comply with all aspects of the pathway.
- All index cases and partners receive the same treatment and tests where required, and so the costs of tests and treatment are not included in any of the pathways. We included only resource use associated with trying to deliver PN (that is, the associated staff costs).
- The ClaSS project and APT study pathways assessed the success of PN in their respective studies by recording how many partners were consulted or treated as part of the outcome. The cost of follow-up telephone calls to the index to assess the outcome for the partner, if required, was assumed to be a cost of the research and not part of the intervention and, therefore, was not directly recorded. To achieve consistency with the current study, resource use and associated costs have been included for these studies to represent the required follow-up to index cases or partners to assess whether or not PN has been achieved. These costs are assumed to be the same as those incurred by the current study.
We present a brief summary of the ClaSS study, the APT study and the patient and provider pathways used in the pilot of the current study. For both pathways in each of the three studies we explain how we have determined resource use and applied unit costs.
Partner notification in the Chlamydia Screening Studies
The primary objective of the PN trial, which was an integral component of the ClaSS study, was to explore effectiveness of PN advice provided in a primary care setting compared with referral to genitourinary clinics. The latter was and still is the current practice.
The costs incurred by each PN strategy are presented from the perspective of the NHS. Costs were originally obtained in pounds sterling at 2003 prices and subsequently updated to 2005 prices for the ClaSS report. We have used reported resource use from the ClaSS study and applied wages and costs that apply for 201169 for the purpose of the current report. Practice nurses recorded the total duration of the consultation, which included the time taken to give results and treatment, explain the study, obtain consent, and conduct randomisation followed by either PN or referral. Published data on the duration of GUM clinic consultations for PN were used.
In the nested PN trial for the ClaSS project, nurse-led PN at the practice for the index patient while the patient is receiving their own result was estimated to add just a few minutes extra on to the consultation time. For individuals in the comparator arm, where the index was advised to seek PN advice at the GUM clinic, the appointment with the nurse at the general practice surgery was for the purpose of receiving treatment and the result only. The index patient then required an additional appointment at the GUM clinic for PN advice. Partner(s) were also required to attend the GUM clinic for treatment and advice.
A slight adjustment was made to the ClaSS project results which are presented here. The initial appointment in the ClaSS project was estimated to take almost 42 minutes for those individuals randomised to the nurse-led PN strategy and 38.8 minutes for those randomised to the GUM PN strategy, but both these timings included time for randomisation and consent to the study. In the current study, the explanation of the study and taking of consent were estimated to take between 10 and 15 minutes. We have assumed the mid-point of this range (12.5 minutes) and deducted this from all the timings.
Partner notification in the Accelerated Partner Therapy study
The objective of the APT study was to compare costs and outcomes associated with three alternative methods of expedited PN in the primary care setting. The cost analysis carried out alongside the exploratory trial was conducted from the perspective of the NHS and considered only direct health service costs.
Following the pre-determined criteria, eligible index patients who had given consent to participate in the study were offered one of three methods of PN: (1) APTHotline, telephone assessment of their sex partner by a clinic-based nurse-qualified HA; (2) APTPharmacy, assessment of their sex partner by a trained community pharmacist; or (3) routine clinic PN, patient referral which included infection-specific information, advice that the sex partner should attend the clinic for testing and treatment and, in one clinic, a standard letter detailing antibiotic treatment options for the sex partner to give to his/her GP if appropriate. Each index patient was asked to choose which method they preferred for each contactable sexual partner. The index was instructed to provide the partner with all relevant information. Once the partner engaged with the allocated PN method, the appropriate health-care professional explained the study and sought consent from the partner to participate. Any partner who did not like the method of PN chosen for them by the index patient could default to routine PN (PN at GUM clinic as per ClaSS study).
In the APTHotline group, either partners collected the treatment pack from the clinic reception or the index patient could take the pack to them, which occurred if the sex partner completed his/her telephone assessment before the index patient had left the clinic. Partners in the APTPharmacy group received their treatment packs from the trained community pharmacist at the time of their consultation.
Engagement in the APT process was apparent when the partner adhered to the method of PN chosen for them by the index case. In some cases it was necessary for the index case to be followed up for confirmation about where, if at all, the partner received APT or routine care. All resource use incurred as a result of the APT strategies was collected prospectively.
Routine partner notification
Resource use associated with routine PN in the APT study was based on the primary data collected in the ClaSS project, as described in the previous section.
- APTHotlineThe estimated cost of the APTHotline included the cost of the telephone equipment, the cost of the consultation and the cost of the receptionist’s time spent giving out the packs (for those partners who collected the APT pack themselves). In addition to the duration of the consultation, it was assumed that the nurse-qualified HA spent 10 minutes carrying out administrative work such as filling in forms and passing on relevant information to the receptionist.
- APTPharmacyThe duration of the consultation with the community pharmacist was recorded in the primary study and the cost per hour applied is based on recent published sources. In addition to the duration of the consultation, it was assumed, based on direct reports from the study, that the pharmacist spent 10 minutes carrying out administrative work such as filling in forms. The APT pack was collected on the spot, at the end of the consultation with the community pharmacist. Appropriate costs from secondary sources for 201169 have been applied to the resource use.
Patient and provider referral pathways as used in the pilot study
Two alternative approaches to PN were compared in the pilot: the patient referral pathway and the provider referral pathway. In both pathways, the first step is to notify the index patient of their positive diagnosis and invite them to attend the practice for treatment. At the appointment the general practice surgery nurse treats the patient and collects baseline information which is entered onto a custom-built web tool that sends an automatic email alert to the HA.
Index patients are randomised at general practice surgery level and the appropriate pathway is offered to the patient by the HA. The HA attempts to contact the index patient by telephone and a maximum of three attempts are applied to this stage. If the patient is not successfully contacted within three attempts they are not pursued further. The duration of all relevant contact is recorded on the web tool as far as possible by the HA.
- Patient referral pathway
- Under patient referral, patients are given information about their infection and asked to tell their partner about the problem and the need to be treated. This is carried out by the HA by telephone. During the initial call, the HA checks the patient’s baseline information and records additional information about the index’s sexual history and details of sexual partner(s). One point of follow-up is pursued with a maximum of three attempts made to contact the index. Information is recorded by hand on pro formas and transferred to the web tool.
- 1-week follow-up for index
- The HA calls the index to check their treatment and adherence. The HA asks if the index’s partner(s) has/have accessed testing or treatment and also asks about any new partners. Information is recorded by hand on pro formas and transferred to the web tool.
- Provider referral pathway
- Under the provider referral pathway, in addition to information and support given as per patient referral, the index patients are asked if they would prefer the HA to contact one or more of their partner(s) on their behalf, anonymously if required. Indexes may use patient referral, provider referral or a mixture of both referral methods. For instance, index patients might accept the offer of provider referral for their ex-partners and patient referral for their current partner.
- If the provider option is taken up, in addition to the details collected for patient referral, the HA takes partners’ contact information: name, telephone number and when there was sexual contact. The HA will then contact the partners. A maximum of three attempts are applied to this stage. If the patient is not successfully contacted within three attempts they are not pursued further. Information is recorded by hand on pro formas and transferred to the web tool. All partners contacted by the HA are followed up at 1 week.
- 1-week follow-up for partner
- The HA calls the partner to check attendance, diagnosis, treatment and adherence. Information is recorded by hand on pro formas and transferred to the web tool.
Where a mixture of both patient and provider referral methods are used, the HA would follow up the index at 1 week regarding any partners that the index has contacted themselves, as per patient referral.
Results
Pathways 1–6 present the pathways for each of the six strategies under comparison. Tables 18–20 present the resource use in terms of items such as length of appointment, number of telephone calls, etc. The unit costs applied to these resource items such as relevant staff salary or cost of call and equipment is appropriately applied to each item.
TABLE 18
Unit costs, resource use and total cost for ClaSS project pathways
TABLE 20
Unit costs, resource use and total cost for pilot study pathways
TABLE 19
Unit costs, resource use and total cost for APT project pathways
The cheapest strategy is the ClaSS project strategy of providing PN advice to the index at the practice when the index receives the result of their own test (strategy 1), with the partner(s) being assumed to attend the local GUM clinic to get their own PN advice and treatment. This strategy has an estimated cost of approximately £42.00 per index, assuming the index case has 1.5 partners.
The ClaSS project strategy of PN at the GUM clinic (strategy 2) is the next cheapest strategy. This strategy assumes that the index case is identified at the practice but that the index then attends the GUM clinic to receive PN advice and the partner also subsequently presents at the GUM clinic for assessment and treatment. Thus there are additional health service interactions required, adding to the costs for this strategy which are estimated as £48.31 per index (assuming 1.5 partners).
Strategy 5 (patient referral) and strategy 3 (the APT study strategy of PN via a hotline) are roughly equal in cost. In the patient referral strategy (strategy 5), the patient assumes the responsibility of contacting the partner and the average cost per index of this strategy is £52.53 (assuming 1.5 partners). In the APT strategy (strategy 3), the index is assumed to be identified as positive at the GUM clinic and the partner is requested (by the index) to telephone the study hotline for assessment and advice about how to get treatment (each index is assumed to have 1.5 partners). The average cost per index case for strategy 3 is approximately £52.80. In both these strategies the effort of contacting the partner is incurred by the index.
Strategy 4 (PN via pharmacy) assumes the index receives PN advice at the GUM clinic during diagnosis but requires the partner to attend the pharmacy for assessment and treatment. The additional cost of the pharmacist consultation contributes to this strategy being slightly more expensive than the preceding ones. This strategy costs approximately £56.38 per index case (assuming 1.5 partners).
Strategy 6 (provider referral) is the most costly strategy. Like strategy 2 (PN at GUM clinic), this strategy requires additional interactions with the health service in that after initial diagnosis of the index (irrespective of the setting) the index does not receive PN advice at that point, but a subsequent and entirely new interaction is required as the HA receives an alert to contact the index, provide them with an assessment and discuss PN advice. In the provider referral strategy all the costs of contacting the partner(s) are borne by the health service and not the patient.
Thus strategy 6 (provider referral) is the most costly strategy, with an estimated cost of approximately £85.98 per index (assuming 1.5 partners). For this reported strategy it is assumed that all partners are contacted by the provider at the wish of the index, although in the strategy index cases could potentially choose a mixture of patient and provider referral approaches for their partners. If a mixture was the preferred approach then it can be assumed the average cost for the strategy would be somewhere between the results of strategy 6, where it is assumed in this case that provider referral is applied to all partners, and the results of strategy 5, where patient referral is applied to all partners (Figures 10–12).

FIGURE 10
Chlamydia Screening Studies pathways. (a) PN at general practice surgery; and (b) PN at GUM clinic.

FIGURE 12
Pilot pathways. (a) Patient referral; and (b) provider referral.
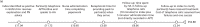
FIGURE 11
Accelerated Partner Therapy pathways. (a) PN via hotline; and (b) PN via pharmacy.
Discussion
The estimated costs of the alternative strategies of PN range from approximately £40 to £90 per index case, based on the assumptions that all index cases have 1.5 partners requiring PN and there is complete compliance of all index patients and their partners in adherence to whatever PN approach is indicated by the particular strategy, and excluding costs associated with the actual test and treatment for everyone involved.
The least costly strategy appears to be that of PN at the practice (strategy 1) as offered during the ClaSS project. This costs approximately £42.00 per index case. This strategy costs less than any other because the index receives PN advice at the same time as being diagnosed (i.e. receiving their own result) and the costs of contacting the potentially infected sexual partner(s) are borne entirely by the index, which is asked to inform their sexual partner that they should attend the GUM clinic for their own assessment and advice. There is no cost incurred by the health-care provider in this strategy related to contacting the partner, although the partner is ultimately assumed to attend the GUM clinic, and this cost is included.
Strategy 6 (provider referral) is the most costly strategy because the health service bears the costs of contacting the partners (or potentially some of them). If the index did not choose provider referral, even though offered, this strategy would cost no more than the patient referral strategy (strategy 5).
Strengths and weaknesses
The strength of this analysis is that it is the first to directly compare a full range of potentially feasible alternative strategies for PN advice to index patients and their partners, in terms of costs. The costs associated with some pathways were estimated as part of previous studies, and for the purpose of comparison some assumptions were required. However, in all cases, the assumptions were made in advance of any calculations and have not been adjusted in the light of the results.
A weakness in the analysis is that the comparison does not represent the costs associated with a ‘head to head’ comparison in a primary study. Furthermore, the costs presented are estimates and the detail of their differences should be interpreted with caution. The limited data available and the limitations imposed by the halting of the wider trial mean that the costs are estimates based on relatively small numbers and present an indication only of potential differences. Appropriate information with which to estimate confidence intervals around resource use, such as length of time for appointments, was available for some resources used but not others. Therefore, we have not presented confidence intervals for the estimated costs. Detailed scrutiny through in-depth sensitivity analysis would be inappropriate given the available data.
The major weakness is that very few positive index cases were identified to enable PN to be tested. We have compared the proposed pilot strategies with strategies from other studies, and it would not be appropriate to attempt to assess cost-effectiveness relative to other strategies, since the comparison was not head to head or randomised. Therefore, in order to facilitate the reporting of comparable costs for the pathways in this study, all strategies are assumed to be equally successful in achieving their objective. However, this is not likely to be the case and, as a result, costing studies alone cannot be helpful to decision-makers. Clearly the least costly strategies to administer will not necessarily save money if they are ineffective in achieving their desired objective. In contrast, the more costly strategies might be sufficiently more effective to justify the additional cost through a demonstrable increase in the success or effectiveness of the strategy. This has not been proved in this study. Indeed, the difficulty in trial recruitment and the failure to recruit enough infected individuals into the study was the reason that it is possible to present only an estimated comparison of costs. Thus, the major weakness of this study is that it has not been possible to assess the strategies in terms of their relative cost-effectiveness.
Conclusions
The results from this costing study suggest that alternative PN strategies are unlikely to differ much in terms of costs. However, the more responsibility is assumed by the health service to contact partners to facilitate PN strategies (and further follow index cases), the more costly the strategies will be.
Unanswered questions for future research
Whether or not the additional costs to the health service implied by strategies such as provider referral, which assume the most responsibility for contacting partners, are justified in terms of effectiveness and lead to a greater societal benefit by reducing the spread of the disease would need to be fully assessed in a cost-effectiveness study with appropriate population modelling.
Partner notification is an essential component of addressing and treating STIs. The conclusion from this study is that increasing the degree of responsibility assumed by the health service to reach partners will increase the cost. A justification for spending more of the health service’s limited resources to reach partners to achieve the outcome sought is required, which this study was unable to show.
Intensive recruitment to increase uptake of individuals being offered screening for chlamydia: is it effective and is it cost-effective?
Background
The planned economic data collection and analysis for this study was to be carried out during the main trial itself, and was not scheduled to have any role in the pilot trial. The objective of the pilot study was to explore the feasibility of recruiting individuals for chlamydia screening and PN in the general practice setting. When it became clear during the pilot that recruitment to the full trial may be problematic, the research team decided to explore a dedicated intensive recruitment approach.
Acknowledging that the full trial was unlikely to proceed without a better response to recruitment, and thus in an effort to salvage all possible information from the project’s pilot trial, the economic team considered it necessary to attempt to include a comparison to the intensive recruitment approach used in order to undertake an economic analysis. An economic evaluation is defined as a comparison of two or more interventions in terms of costs and outcomes.67 Thus, the only comparison that could be considered which was pragmatic and would not add any additional burden to staff in practices (and to the research team who would recruit in the practices, and for whom recruitment was the main priority) was a simple and pragmatic ‘before and after’ (intensive recruitment) study.
In the pilot trial, a specific aim was to intensively recruit patients for chlamydia screening in general practices by introducing a suitably trained researcher into a practice for a 3-week period. The costs and outcomes associated with this approach could be measured and the success of intensive recruitment could potentially be evaluated for the purpose of a preliminary economic analysis, if it could be compared with the costs and resources used by, and the outcomes achieved, prior to the intensive recruitment phase.
Objective
What is the additional cost and success of intensively recruiting patients in general practices compared with the existing approach within any practice?
The data from ‘before-and-after’ comparisons are more appropriately presented as cost–consequence analyses, where costs and outcomes are presented in a disaggregated manner but in which no further analysis to achieve a cost-effectiveness result is undertaken. This is appropriate in this case because the data for the comparison are not derived from a controlled experiment.
Estimating costs and outcomes used in general practices in the pilot with regard to chlamydia screening and identifying positive cases
General practices in the UK manage a diverse range of primary care issues which might include antenatal care and advice, treating patients for minor illness, identifying infection and chronic illness and where necessary referring patients on to secondary care, to mention but a few. Screening and/or testing individuals for infection with chlamydia is one of many tests and services practices can provide. Although the practice readily provides this wide variety of services it must be realised that it is a finite resource: when a nurse or doctor provides an appointment for the purpose of screening and treating an individual who is positive for an infection such as chlamydia, it means that that particular appointment is not available to another patient who has a different need. Thus, providing a chlamydia test to someone who is not likely to be positive for chlamydia (a low-risk individual) means that not only is there a waste of the time and resources used in that particular appointment, there is also a forgone benefit for the individual who would have liked an appointment but did not get one and was given a place in the queue instead. In health economics, this is referred to as ‘opportunity cost’. Opportunity cost is the value of the consequences/outcome/benefits forgone by choosing to deploy a resource in one way rather than in its next best alternative use.67 It is, therefore, appropriate in an economic analysis to estimate costs and outcomes associated with the intervention, even if the intervention is perceived to be a service that already exists and is already being paid for.
Methods
We provided practices with a short baseline questionnaire to explore their existing approach to chlamydia testing and PN. The questionnaire was developed in a relatively short time frame (when it was realised recruitment was poor) and was intended to be a pragmatic assessment of what the approach of a particular practice to chlamydia testing and PN was prior to the anticipated period of intensive recruitment. This was a one-off opportunity to assess the testing regime prior to the proposed intensive recruitment intervention, as the questionnaire was sent out just prior to planned intensive recruitment at the 11 practices. It was not possible given limited time and resources to monitor the exact throughput of testing and the result (positive or negative case of infection) and the questionnaire relied on the recall of the previous month of the member of staff completing the questionnaire. The data collection sheet used to assess the existing approach to testing is presented in Appendix 1.
The cost data applied to the resource use estimated by each practice are explained and presented in Appendix 2.
Results
Outcome data for existing approach to chlamydia testing
Eleven answered questionnaires were returned, all relating to the previous month. The questionnaires required the respondent for each practice to highlight the approximate range of the number of tests taken from choice groups of 10. The possible options for the response were ≤ 10, ≤ 20, ≤ 30, and so on – although in some cases the practice did report an exact number. Unless otherwise indicated, if a practice reported that they tested ≤ 10 individuals in a month it was assumed (as shown in Table 21) that they had carried out 10 tests (although it was acknowledged that less than or equal to 10 could mean only one, and it is also possible and perhaps more realistic to assume an average of five). Where they reported the ≤ 20 range, it was assumed the number of tests was 20 – and given there was a lower band that could have been chosen, that ‘less than or equal to 20’ meant at least more than 10 and so on. It is acknowledged that 15 could have been an appropriate estimate for the less than or equal to 20 band but we assume that if the true number of tests was really less than 10 the responder would have chosen the ‘less than or equal to 10’ choice band.
TABLE 21
Number of individuals screened and number of positive case identified as reported by individual practices directly prior to intensive recruitment
This assumption, based on the uppermost limit of the choice band, was used to avoid introducing a favourable bias towards intensive recruitment. For instance, if the lowest estimate or the mid-point in the range for the number of tests carried out in the previous month was presented, it might suggest that intensive recruitment was more effective than would be appropriate. So the assumption was a pragmatic attempt to avoid any bias towards the intensive recruitment strategy.
The result for the number of positive cases of chlamydia detected in the month prior to the intensive recruitment phase was reported on the questionnaire as an exact number.
The number of tests reported at each practice and the number of positive cases identified are presented for each practice in Table 21.
The costs associated with the existing approach to chlamydia testing for each practice are presented in detail in Appendix 2.
Discussion of existing approach
The results suggest that general practices believed there was reasonable activity in testing individuals for chlamydia in the month prior to intensive recruitment. It had been planned to estimate costs associated with the reported testing activity. These costs are reported in Appendix 2 as a result of our concern with regard to their reliability. We believe to present results in terms of the average cost per test carried out or average cost per positive case identified would be misleading. The limitation here is likely to result from recall bias of the staff completing the questionnaire. It is also acknowledged that the pragmatic design of the questionnaire, in asking respondents to report a general band of activity, may have led to inaccurate reporting.
Concern over some of the assumptions necessary to estimate the costs associated with testing and the reliability of the data provided on testing by each practice suggests they should be viewed with some caution. This is discussed further in the Discussion section in reference to some other data recently made available.
Intensive recruitment testing
The principal objective for the intensive recruitment process was to encourage all 16- to 24-year-olds to test for chlamydia and consent to participate in the trial. The use of external researchers, trained in the offer of chlamydia testing and consent, has been successful in a number of contexts.
The hypothesis was that for disease areas such as STIs, where there is much stigma and embarrassment associated with the disease, parachuting an expert into an environment to recruit and stimulate motivation within the practice might perhaps be an appropriate approach which could be both effective and potentially cost-effective.
The additional resource use and costs associated with intensive recruitment were all collected by the research team whilst undertaking the process in each practice. The number of tests carried out and the number of positive cases identified are also directly calculated by the trial manager and the research team.
A number of assumptions were required for the cost analysis to be completed.
Assumptions and framework for intensive recruitment
There are three different types of costs:
- General costs including training, recruitment and set-up. These are distributed across all participating practices as a lump sum cost. In addition, each practice would receive service support costs for participating in the research.
- Labour costs are already provided directly from the intensive recruitment team and not routine sources. The cost estimates for salary of the staff involved are specific to the grade of the researcher, the duration spent at the practice, and any equipment or disposables used.
- There are no PN costs included because no data are provided on PN or its success.
All costs are presented in pounds sterling in 2011/12 prices.
Data for intensive recruitment approach based on costs of research staff
Data from 10 clinics were available to assess the impact of intensive recruitment. The staff time involved and the costs associated with the process are presented in Table 22. The details of staff time and costs are presented in Appendix 3. The total costs are apportioned across the clinics. The average length of the intensive recruitment at each clinic is between 15 and 18 days (weekdays).
TABLE 22
Labour costs for intensive recruitment
The average numbers of days and hours spent at each clinic to carry out the process is presented in Table 23.
TABLE 23
Time taken for intensive recruitment
In Table 24, we present the costs for each clinic for the intensive recruitment period. In Appendix 3 we present the breakdown of these costs. The fixed costs of the process for set-up and training are apportioned equally and a fixed element applied to all practices. The time, resources and associated costs for each practice were recorded directly by the intensive recruitment team.
TABLE 24
Cost and outcomes reported for period of intensive recruitment
The variance seen between practices (from £1578 to £6408) is a result of the travel and accommodation costs for practices at a longer distance from the researchers’ base. The costs for practice 7 are considerably lower, as recruitment was managed by the local PCRN.
In Table 24, outcome data for the intensive recruitment period showing number of cases screened and number of cases identified as positive are presented. Compared with the ‘existing’ activity data as reported by the prior to intensive recruitment survey, there is an overall increase in the number of screening tests in 6 of the 11 practices. In 4 out of 11 practices intensive recruitment testing was lower than what was reported prior to the intensive recruitment period. One practice did not, in the event, undergo a period of intensive recruitment.
In April 2013, new data became available from the chlamydia testing activity data set (CTAD), which is a relatively new (set up in 2011) universal disaggregate data set for the reporting of chlamydia testing data from all NHS and NHS-commissioned laboratories in England. We accessed the data relevant to the practices in our survey for the month prior to intensive recruitment. These data are presented alongside the results from the ‘before’ intensive recruitment survey and alongside the activity that resulted from intensive recruitment. These are presented in Table 25.
TABLE 25
Self-reported, CTAD–reported and intensive recruitment results for the number of individuals screened and number of positive cases identified as reported by individual practices
We compared CTAD figures for the month prior to intensive recruitment with the testing rates reported by practices. Three practices provided a reasonable recalled estimate of their activity (practices 9, 10, and 11). Although practices 9 and 11 might appear to have overestimated their screening rates, this is an artefact of the questionnaire in which the upper limit of the range offered was assumed.
Three results based on the questionnaire cannot be verified by the CTAD data. Practices 2, 4, 8 and 10 all underestimated their screening rates, and two practices overestimated their screening rates (practices 1 and 3) in the pre-intensive recruitment survey questionnaire.
All the results suggest that intensive recruitment is costly, but importantly it does not seem to produce a respectable number of positive cases of chlamydia identified, given the cost and effort.
The results of this study suggest that intensive recruitment helped to increase the number of tests carried out in some practices but not others, given the result of the survey carried out before intensive recruitment and the results of the CTAD figures. It is clear that the additional tests being carried out are not effective at identifying individuals who have the disease. The results suggest that in some practices there was an increase in the number of people who were tested but this did not lead to an increase in the number of positive cases identified.
Discussion
Although a comparison of activity before and after a period of intensive recruitment to screening was attempted, the results of our study suggest that a full economic evaluation was not required and a cost and consequence analysis was the most appropriate approach. The results show that the number of tests assumed carried out prior to intensive recruitment and reported through recall when completing the questionnaire was lower in the majority of practices (6 out of 11) before intensive recruitment took place. Thus, intensive recruitment for these six practices did improve the screening rate based on the questionnaire results. However, a comparison with the CTAD figures makes any inference from the effectiveness of intensive recruitment less clear. In 3 out of 11 practices it was implied that the screening rate was higher in the period before intensive recruitment than during intensive recruitment.
The results suggest that the intensive recruitment period may have been successful in a few practices in increasing the number of screening tests carried out in the general practice setting. The results suggest that the recalled number of positive cases detected in any practice in the month prior to intensive recruitment is likely to be an optimistic estimate.
However, it is clear that while intensive recruitment may have increased the number of tests carried out (although this is not convincing given the CTAD data), it did not lead to an improvement in the number of positive cases of infection being identified.
Costs associated with screening prior to the period of intensive recruitment were estimated and are reported in Appendix 2. The results of the costing exercise should be interpreted with caution, as a result of some of the assumptions relating to the number of tests carried out, who carried them out and the time devoted to them being carried out. However, these results suggest much higher costs associated with intensive recruitment.
The strength of this analysis is that it was the first primary study to collect cost and outcome data associated with active intensive recruitment of STI screening in a trial that was failing to recruit. All cost and resource use data, as well as all the clinical data on numbers of individuals who were screened and detected as positive and treated, have been collected in a primary research study. A limitation is that no robust comparator data exist with which to conduct an economic evaluation, and a further limitation is that these data were not collected as part of a controlled study. Furthermore, to estimate costs some assumptions were made about the time and resource use of the staff carrying out the tests.
The inferences of this study are that intensive recruitment was actually not effective at detecting positive cases. Intensive recruitment is shown to be costly and there is no clear improvement in terms of a resounding effect on numbers of positive chlamydia cases identified, as the positive cases identified remained zero in the majority of practices. The number of individuals undergoing a screening test may increase as a result of intensive recruitment, although the results of this study do not provide strong support for this. But it is clear that intensive recruitment is costly and does not achieve the desired objective of finding positive cases and treating them.
- Cost analyses and preliminary economic evaluation - The relative clinical effect...Cost analyses and preliminary economic evaluation - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Glossary - Diagnostic accuracy of the Thessaly test, standardised clinical histo...Glossary - Diagnostic accuracy of the Thessaly test, standardised clinical history and other clinical examination tests (Apley’s, McMurray’s and joint line tenderness) for meniscal tears in comparison with magnetic resonance imaging diagnosis
- Summary and protocol for the randomised controlled trial as originally planned -...Summary and protocol for the randomised controlled trial as originally planned - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Phase 2: identifying improvements - The relative clinical effectiveness and cost...Phase 2: identifying improvements - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Phase 4: intensive recruitment by external researchers - The relative clinical e...Phase 4: intensive recruitment by external researchers - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
Your browsing activity is empty.
Activity recording is turned off.
See more...