Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cassell JA, Dodds J, Estcourt C, et al. The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care. Southampton (UK): NIHR Journals Library; 2015 Jan. (Health Technology Assessment, No. 19.5.)

The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care.
Show detailsPartner notification outcomes
During all phases of the pilot a total of 25 (3.2%; n = 783) patients were diagnosed positive for a STI (24 for chlamydia and one for genital herpes) across eight practices. They are summarised in Figure 13. The majority of patients (n = 18) came through patient referral practices, five through provider and two through contract (Figures 14–17). The majority of patients diagnosed with a STI were female (22 females vs. 3 males). The HA was able to contact successfully 11 (44%) of the patients diagnosed with a STI, six (24%) of whom were followed up at 1 week and two (8%) subsequently followed up at the 6-week and 3-month periods.
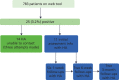
FIGURE 13
Follow-up of positive respondents.
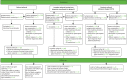
FIGURE 14
Consent at diagnosis practices recruited in phase 1 and continuing (n = 3) (8 November 2010–31 July 2012).

FIGURE 17
Intensive recruitment practices recruited in phase 4 (n = 8) (2 February 2012–3 August 2012).
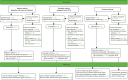
FIGURE 15
Consent at test practices recruited in phase 1 and continuing (n = 3) (November 2010–31 July 2012).
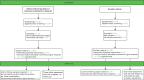
FIGURE 16
Consent at test practices newly recruited in phase 3 and continuing (n = 2) (11 July 2011–31 July 2012).
Partners were known to have been tested for four patients; three partners were diagnosed positive for chlamydia and for two of these the HA was told by the index patient that they had been treated. The two participants who were followed up at the 3-month period were retested for chlamydia, with both testing negative.
Given the small numbers, it is not possible to present outcome data for the range of primary and secondary outcomes.
Consolidated Standards of Reporting Trials diagrams
Summary recruitment data have been given at the end of each chapter describing a pilot phase, with a complete summary at the end of phase 4. Here we present for completeness the Consolidated Standards of Reporting Trials (CONSORT) flow diagrams for the various phases and categories of practice (Figures 14–18).

FIGURE 18
Consent at diagnosis practice recruited in phase 4 (n = 1) (3 February 2012–31 July 2012).
- Results of all phases - The relative clinical effectiveness and cost-effectivene...Results of all phases - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Cost analyses and preliminary economic evaluation - The relative clinical effect...Cost analyses and preliminary economic evaluation - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Glossary - Diagnostic accuracy of the Thessaly test, standardised clinical histo...Glossary - Diagnostic accuracy of the Thessaly test, standardised clinical history and other clinical examination tests (Apley’s, McMurray’s and joint line tenderness) for meniscal tears in comparison with magnetic resonance imaging diagnosis
- Summary and protocol for the randomised controlled trial as originally planned -...Summary and protocol for the randomised controlled trial as originally planned - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Phase 2: identifying improvements - The relative clinical effectiveness and cost...Phase 2: identifying improvements - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
Your browsing activity is empty.
Activity recording is turned off.
See more...