Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cassell JA, Dodds J, Estcourt C, et al. The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care. Southampton (UK): NIHR Journals Library; 2015 Jan. (Health Technology Assessment, No. 19.5.)

The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care.
Show detailsOverview of the proposed randomised controlled trial
The technologies compared were three different interventions in PN (Figure 1):
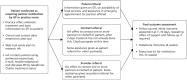
FIGURE 1
Summary of PN interventions and outcome assessment.
- PATIENT REFERRAL, where patients are given information about their infection and asked to tell their partner about the problem and the need to be treated.
- PROVIDER REFERRAL, where, in addition to (i), patients are asked to agree to a specialist HA (contact tracing expert) contacting one or more of their partner(s) at the time of diagnosis.
- CONTRACT REFERRAL, where, in addition to (i), patients are asked to agree to a specialist HA informing partner(s) if this has not been done after a verbally agreed period of time (usually no more than 7 days).
The null hypothesis
Provider referral and contract referral offer no advantage over patient referral alone in PN for curable STIs in the primary care setting.
In order to answer the research question for the main trial, the following objectives and outcomes were established.
Objectives
- To standardise, appropriate for the primary care setting, three contemporary and evidence-based models of PN for STIs (patient referral, provider referral and contract referral).
- To compare the clinical effectiveness of these three models.
- To compare the cost-effectiveness of these three models.
- To determine the acceptability to patients of each approach to PN, and to identify means of improving PN rates for ‘highly connected’ partnerships.
- To enhance the efficiency of the trial through mathematical modelling of the potential impact of each modality of PN on outcomes for different types of partner (main, casual and ex-partners) and for men who have sex with men (MSM).
- To provide comprehensive, definitive evidence for policy-makers and public health practitioners on the implementation of clinically effective and cost-effective PN for patients diagnosed with STIs in the primary care setting.
Outcomes
Primary outcomes of the randomised controlled trial
- Number of partners per index patient treated for chlamydia and/or gonorrhoea/non-specific urethritis/pelvic inflammatory disease.
- Proportion of index patients testing negative for the relevant STI at 3 months.
Secondary outcomes of the randomised controlled trial
- Number of partners per index patient presenting for treatment.
- Proportion of index patients having at least one partner treated.
- Number of main, casual and ex-partners per index patient tested for the relevant STI.
- Number of main, casual and ex-partners testing positive for the relevant STI.
- Number of current partners tested for a human immunodeficiency virus (HIV) infection by 3 months.
- Number of index patients tested for a HIV infection by 3 months.
- Time to definitive treatment of index patient for the relevant STI.
- Time to definitive treatment of current partner for the relevant STI.
- Uptake by index patients of ‘contract’ and ‘provider’ referral for one or more partners, within the relevant randomised groups.
- Patient-related factors impacting on PN or STI disclosure to main, casual and ex-partners.
Identification and recruitment of eligible individuals
Target population
The target population was as follows:
- all 16- to 24-year-olds testing positive for chlamydia after chlamydia screening
- all patients (aged ≥ 16 years) diagnosed with a curable STI following clinical presentation
- all patients (aged ≥ 16 years) diagnosed elsewhere and attending the practice for antibiotic treatment.
Exclusion criteria
- Any patient aged < 16 years.
- Any patient unable to fully understand the trial information.
- Any patient who required translation of trial information.
- Any patient who required advocacy.
Setting
The setting of the trial was a diverse sample of primary care practices, both in specific localities [primary care research network (PCRN) practices in the South East region of England] and nationally [recruited via the Medical Research Council (MRC) General Practice Research Framework and the PCRN].
Patient and public involvement
A focus group of six sixth-form college students were consulted regarding their opinion on the participant information resources and recruitment methods. In addition, two University College London medical students who were members of the ‘Sexpression’ group (a network of student-led projects that teaches about relationships and sex education in local communities across the UK) agreed to sit on our Trial Steering Committee and advise on participant information resources and recruitment methods.
Approach to recruitment
Participants were to be recruited through letters of invitation, and personal invitation on attending the practice as follows:
- Practices were asked to mail out letters to 300 randomly selected 16- to 24-year-olds at the beginning of the pilot (taken from the practice list), inviting them for a chlamydia test (as part of the NCSP). The invitation letter also mentioned the trial.
- Clinical staff (usually practice nurses) approached potential participants, either (a) at the time of first attending the practice either with symptoms of a suspected or presumptive STI (symptomatic patients) or (b) at the time of chlamydia testing (asymptomatic patients, most of whom are tested as part of the NCSP) (Figure 2). The clinic staff were asked to explain that the practice was taking part in a study, as part of which additional assistance might be given to patients needing PN for a STI. It was explained that the practice had been randomly allocated to a group which would help either with providing care for STI patients up to the recommended national standard or with additional options for support. Patients were told the allocation of the practice. It was also explained that, should the patient be diagnosed with a STI, participation in the trial would mean, in all cases, that they would be contacted by an experienced HA. This HA would, depending on the trial allocation of the practice, assist them in their plans and actions to inform and obtain care for both current and ex-partners. Patients agreeing to be recruited at this stage provided personal contact details through which the study HA could communicate with them.

FIGURE 2
Process of enrolling a participant.
Randomisation
Cluster randomisation at practice level was chosen for two reasons. First, there was a strong likelihood that clinical practice would be influenced by participation in the trial – with randomisation at patient level, practitioners who considered that ‘provider referral’ or ‘contract referral’ had advantages might more readily suggest that patients randomised to ‘patient referral’ attended a specialist clinic. Second, randomisation of patients who attend unpredictably in the middle of a busy surgery is more challenging than randomisation of patients with chronic disorders, while practice randomisation reduces this difficulty.
Consent at time of test versus consent at time of diagnosis
Two approaches to consent were piloted: consent at time of test (CAT) and consent at time of positive diagnosis (CAD). It was anticipated that CAD would reduce the workload for the health professional. However, a previous failed trial demonstrated the difficulty of achieving recruitment at the same time as a positive diagnosis is communicated to the patient.32
We obtained written informed consent from all adults (aged ≥ 16 years). Where written informed consent was not possible (i.e. the patient had been tested for chlamydia, but no researcher was immediately available to take consent), consent was taken over the telephone soon after the patient had taken the test. In this case, potential participants were asked to agree to a researcher calling them to take consent. A ‘reason-for-test’ form included information on why patients were testing, whether or not they were interested in participating in the trial, contact information and signature (if they were happy for consent to be taken over the telephone).
Partner notification process
Following the diagnosis of a STI in general practice surgeries, patients were identified as in need of antibiotic treatment and PN (see Figure 1). The practice offered antibiotic treatment and basic information on STIs to the patient. Minimal clinical and contact data were then recorded on the web tool (see Chapter 8 for details). If the patient received a definite STI diagnosis, practice staff entered this information on the web tool, which automatically sent the research study HAs the basic contact information needed to contact and manage the patient, and information on the randomisation status of the practice (Figure 3). The HA then contacted the patient using their preferred means of contact (e.g. text, e-mail or mobile phone call). During the initial consultation the HA discussed PN with the patient and checked their treatment status. The patient was then followed up at 1 week, 6 weeks and 3 months for their PN outcome assessment (Box 1). The HA sent test kits for patients to be tested for reinfection at the 3-month period (testing with a NAAT). The patient then returned the tests to the HA based at Barts Health NHS Trust Sexual Health Services at The Royal London Hospital (Barts) for them to send to the laboratory, which subsequently returned the results to the HA (Figure 4).

FIGURE 3
Initial management of infection and referral to HA.
BOX 1
Outcome assessment with HA HA records additional index details and baseline information for partner(s).
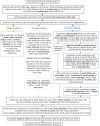
FIGURE 4
Health adviser flow chart.
Development and implementation of a web tool for clinical referral and outcome measurements
A key aspect of the trial was the development of a web-based referral system, the ‘web tool’. This system enabled us to collect the recruitment and trial data and assist with the management and communication between the HA and general practice. It was also envisaged that this referral tool could be used in a number of settings where PN can be carried out remotely by a professional (Figure 5). We report further on the development of this system in Chapter 8.
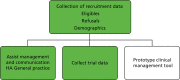
FIGURE 5
Web tool functions.
Data collection
There were a number of data collection points and Table 1 summarises the data captured from each stage of the research process. The data specification was adapted from the accelerated partner therapy (APT) trial specification in order to allow for direct comparison between trials.33
TABLE 1
Data collection points
Substudies
Three substudies were included in the PN programme to enhance the trial. We proposed to explore patient-related determinants of PN with a view to advising on targeting different approaches of PN. The economic evaluation proposed to determine the cost-effectiveness of alternative methods of PN, and the mathematical modelling study proposed to address the problem of the right skew in the number (and nature) of sexual partnerships.
- Summary and protocol for the randomised controlled trial as originally planned -...Summary and protocol for the randomised controlled trial as originally planned - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Phase 2: identifying improvements - The relative clinical effectiveness and cost...Phase 2: identifying improvements - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Phase 4: intensive recruitment by external researchers - The relative clinical e...Phase 4: intensive recruitment by external researchers - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Method - Clinical and cost-effectiveness of an adapted intervention for preschoo...Method - Clinical and cost-effectiveness of an adapted intervention for preschoolers with moderate to severe intellectual disabilities displaying behaviours that challenge: the EPICC-ID RCT
- Definition of the decision problem - Total hip replacement and surface replaceme...Definition of the decision problem - Total hip replacement and surface replacement for the treatment of pain and disability resulting from end-stage arthritis of the hip (review of technology appraisal guidance 2 and 44): systematic review and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...