Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cassell JA, Dodds J, Estcourt C, et al. The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care. Southampton (UK): NIHR Journals Library; 2015 Jan. (Health Technology Assessment, No. 19.5.)

The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care.
Show detailsIntroduction
As reported above, despite additional evidence-based interventions, we had not been able to deliver acceptable opportunistic testing rates needed to underpin this PN trial either in initially recruited practices or in the phase 3 newly recruited practices.
Following advice from our Trial Steering Committee, we considered an alternative approach currently being used in the pilot phase of the ACCEPt primary care chlamydia screening study currently taking place in Australia.
Methods
We had a teleconference with investigators from this study, including a member of our Trial Steering Committee. The ACCEPt team was undertaking a prevalence study in Australian general practice, prior to the implementation of a randomised trial of chlamydia screening based on primary care. The ACCEPt team advised us that for this prevalence study they were using a system in which researchers external to the general practice surgery (although occasionally an in-practice nurse) would undertake a 2- to 3-week period of intensive recruitment. This would involve approaching every young person in the waiting room and seeking their consent for chlamydia testing. The team had found that beyond 2–3 weeks practices found the presence of a recruiter burdensome, and that the proportion of reattenders rose, causing concerns about repeated approaches. Australian colleagues had also experienced similar recruitment issues for chlamydia testing in primary care. Australian practice staff believed they did not see many young people, yet data showed that young people do attend general practice surgery at least once a year. They also reported similar barriers to testing (e.g. not enough time, discomfort about raising sexual health testing) (Professor J Hocking, Melbourne School of Population and Global Health, 2011, personal communication).
A review of the literature found that this approach had been successful in a UK study undertaken by Grun et al.50 in a London general practice surgery prior to the implementation of the NCSP. There were also informal reports of similar approaches within the NCSP in England [NCSP regional facilitators (RFs), 2011, personal communication].
With the support of the Trial Steering Committee and our funders we moved to phase 4 of the trial, the final phase, using external researchers for an intensive recruitment period in each practice. It was expected that this would potentially boost recruitment into the trial. We sought to assess the feasibility and effectiveness of this approach, which has implications for the feasibility in general of intervention studies targeting young people attending general practice.
The overall aim for phase 4 of the trial was to recruit patients using a trained external researcher for a brief intensive recruitment period in each practice.
The objectives for phase 4 of the trial were:
- to recruit eight new practices and retain two already involved for this phase
- to assemble and manage a team of external researchers
- to set up practices for intensive recruitment
- to boost recruitment into the trial.
Practice selection and recruitment
Ten practices were involved in the intensive recruitment phase. Two practices from the previous pilot phase agreed to participate in the intensive recruitment phase: one from Yorkshire and Humberside (a new practice from phase 3) and one from the South East (an original pilot practice from phase 1). Eight additional new practices were recruited: four from the South East, one from Yorkshire and Humberside, two from London and one from the South West.
We sought to identify practices capable of large screening numbers that were capable of hosting research, using the sample frame of the NCSP data set on number of tests and positives by practice and supported by PCRN data on practice characteristics and research capability. In selecting practices, we prioritised the following desirable characteristics:
- capacity to conduct research (Are they able to conduct research? Do they have the space personnel and time?)
- number of potential NCSP cases on the basis of the number of positive tests per year from NCSP data set
- number of 16- to 24-year-olds seen in the practice over the last 6 months (estimated footfall based on practice size and age composition)
- research experience of the practice (number of years, number of studies, types of studies)
- specific sexual health sessions run in the practice including contraception (if ‘yes’, details of experience) which are associated with concentration of large numbers of young people in a single session and which can be targeted for recruitment
- level of enthusiasm of practice (as reported by PCRN or trial team)
- adoption of a practice team-based approach
- number of GPs/nurses/health-care workers able to consent.
Practices were approached by the trial team or a member of the PCRN. Each practice was asked to complete an eligibility criteria form. If the initial contact was from the PCRN, the trial team contacted the practice following an expression of interest if they fitted at least two or more of the eligibility criteria. The trial team was looking for a mixture of practices (practices seeing a high number of patients diagnosed with a STI and those with high enthusiasm for the trial and a whole-team approach). Once selected, the practice was asked to complete a pre-trial questionnaire.
An additional practice was also recruited at its request because of the keen interest of the practice GP, who was previously a national NCSP GP champion (the GP champion’s role was to provide peer support to other local GPs and motivate practices to take on and offer chlamydia screening to their patients). The GP in this practice wished to do all the consenting personally and requested that it be a CAD practice.
Overview of intensive recruitment processes within practices
Figure 6 summarises the processes of recruitment within practices. In order to achieve these, we needed to develop operational guidelines and training procedures, recruit and train a cohort of external researchers, and ensure that all governance requirements were met prior to commencing recruitment. These processes are also described below.
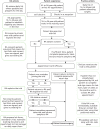
FIGURE 6
Intensive recruitment flow chart. CT, chlamydia testing; RA, research assistant; RN, research nurse.
Recruitment and training of external researchers
We engaged a total of five researchers. Two were members of the trial team and had non-clinical research backgrounds, and three were employed on sessional contracts. One of these researchers was from a general nursing background and unfamiliar with research, while two were from a community sexual health background and experienced in research and working with young people. In addition, a PCRN chose to provide (unremunerated) recruitment in one of its practices, and this was done by three individuals.
A researcher was placed in-house (at the practice) for a maximum of 3 weeks. In the case of two practices the researcher was required to stay in accommodation near the site to ensure that clinics would be covered in full. An additional three practices required 2–4.45 hours’ travel each day.
Research passports and Criminal Records Bureau checks were undertaken as necessary. These were required for most individuals.
The research team conducted training sessions for the researchers. Box 2 shows the content covered in these sessions. Researchers also undertook a role-play session where the researcher acted out approaching a patient in the waiting room using different scenarios. Researchers were also given a training manual which included the topics covered on the training day.
BOX 2
Training for external researchers An introduction to the trial (explanation of chlamydia and the importance of timely treatment of the index and their partners).
When recruiting in practices, the external researcher wore a T-shirt bearing the word ‘researcher’ and their first name on the front; this made it easy for patients to identify them. The trained researcher was situated in the waiting room (in some cases moving around waiting rooms, depending on the number of waiting areas) and gained consent in a private area. The researcher could use the trial posters or message screen as an aid when approaching patients. The researcher asked the patients if they had seen the posters and explained that the practice was taking part in an important research study and that all young people between the ages of 16 and 24 years were being asked to take part, minimising any concerns that they were being chosen specifically to take part.
The aim was to recruit with minimum disruption to the practice and achieve high participation. Once the researcher had made initial contact in the waiting room, the patient was taken into a private room to check eligibility and go through the consent process. The process could be interrupted if the GP or nurse was ready to see the patient. The researcher reassured the patient that they would not miss their appointment and that they could complete the consenting process after their appointment if they were called in. When gaining consent from patients the researcher took the patient through the patient information leaflet, taking them through each point and highlighting why the trial was important (i.e. to find the best way of providing PN, that the NHS recommends a yearly test and a test after a change of partner). Once consented, the patient went to the toilet to collect a sample and returned it to the researcher (or reception/drop box/GP/nurse if the researcher was busy with another patient).
When gaining consent from patients the researcher took the patient through the patient information leaflet, taking them through each point and highlighting why the trial was important (i.e. to find the best way of providing PN, that the NHS recommends a yearly test and a test after a change of partner). All chlamydia tests were documented on a chlamydia test list to be uploaded onto the web tool by a trained practice member of staff (normally at the end of each day) (see Figure 6).
For patients using self check-in it was recommended that patient information leaflets be highly visible by the check-in screen. This message on the check-in screen duplicated the message on the posters asking all 16- to 24-year-olds to pick up a leaflet and help with the study.
Each researcher was required to complete a tracker form, to track the recruitment of patients. Data included individuals approached, age, gender, consent, if the study was mentioned by clinical staff, whether consent took place before or after the appointment (or both), if the patient refused to take part and the reason for refusal.
Practice set-up and training
Each practice was set up by a member of the trial team and the external researcher. The trial team member was present for the first 1 or 2 days of the trial. The lead GP, research nurse and practice manager were required to liaise directly with the researcher and trial team member to establish the most effective way of identifying and approaching 16- to 24-year-old patients whilst in the practice waiting room and how best to manage the chlamydia testing and laboratory process within the practice.
Any GPs or nurses who might take consent or follow up patients diagnosed with a STI were trained on site by the trial member. Where possible, the trial team also attended a practice meeting to highlight the trial to all practice staff.
Role of the practice staff in intensive recruitment
A revised information leaflet for the GP and primary care team was provided. This included a background to the trial, trial objectives, myths in practice, what practice participation would involve (including details of intensive recruitment and encouraging all staff to direct eligible patients to the researcher), benefits for the practice, ethics, feedback and trial team contact information.
All clinicians were instructed to ask their patients if they had been approached by the in-house researcher. If they had already been approached and consented, the clinician was asked to reinforce the importance of the study. If their patient had not yet been approached, clinicians were asked to mention the importance of the trial and, if agreeable, encourage the patient to take a chlamydia test. If the patient took a test at this point they were directed to contact the researcher directly after their appointment. If the researcher was not available they were asked to leave their completed test at reception or in a clearly marked deposit box along with a signed reason-for-test form. The researcher was then able to obtain consent over the telephone. Once the result was sent back to the surgery, the research nurse or GP dealt with the patient’s treatment and referral to the HA.
Results in intensive recruitment practices during phase 4
A total of 1444 potential participants were identified through the intensive recruitment phase (Figure 7) Of these, 88 were not approached; some because the researcher recalled they had been approached previously (30.7%), 15.9% were reported to be too ill or with someone too ill and 11.4% were not approached as they were with children (Table 11).
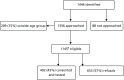
FIGURE 7
Intensive recruitment in 10 practices (data recorded by intensive recruitment researchers). a, Two missing data.
TABLE 11
Reason for the patient not being approached by the researcher (n = 88)
Researchers did not approach some patients accompanied by their parents because they considered it inappropriate to do so. For example, some patients accompanied by their parents would not make eye contact with the researcher or appeared uncomfortable in the waiting room. There was no difference between gender and approach by researcher [93.4% (806/863) of females were approached compared with 92.6% (339/366) males, χ2 = 0.241; p = 0.62].
In total, 1356 patients were approached, of whom 209 (15%) were outside the relevant age group. Of the remaining patients, 492 were tested and consented (43%) and 653 refused to participate (57%) (missing data n = 2).
The largest proportion of patients who refused to participate (36.6%) did so because they had already been tested elsewhere, while a smaller proportion of patients said that they did not want to participate (21.6%). Other reasons specified included not being sexually active, no time, too unwell, did not need a test, parent refused, they were already in a trial and they had recently passed urine (Table 12).
TABLE 12
Reason for refusal (n = 653)
There was no difference in recruitment rates by the type of researcher recruiting. Externally employed researchers recruited 42.9% (237/553) of those approached, the trial team recruited 44.6% (150/336) and the PCRN team recruited 41.0% (105/256) (PCRN staff recruited in one practice based in Yorkshire and Humberside). Recruitment was more successful when the trial had been mentioned to the patient by a member of the practice staff (Table 13), with 68% (98/144) of patients consenting when practice staff mentioned the trial compared with 39% (394/1001) consenting when they did not. There was no difference in consent rate by gender [41.7% (335/804) of females consented compared with 46.3% (157/339) of males, χ2 = 2.100; p = 0.15].
TABLE 13
Mention of trial by clinic staff
- Introduction
- Methods
- Practice selection and recruitment
- Overview of intensive recruitment processes within practices
- Recruitment and training of external researchers
- Practice set-up and training
- Role of the practice staff in intensive recruitment
- Results in intensive recruitment practices during phase 4
- Summary of all recruitment during phase 4
- Phase 4: intensive recruitment by external researchers - The relative clinical e...Phase 4: intensive recruitment by external researchers - The relative clinical effectiveness and cost-effectiveness of three contrasting approaches to partner notification for curable sexually transmitted infections: a cluster randomised trial in primary care
- Method - Clinical and cost-effectiveness of an adapted intervention for preschoo...Method - Clinical and cost-effectiveness of an adapted intervention for preschoolers with moderate to severe intellectual disabilities displaying behaviours that challenge: the EPICC-ID RCT
- Definition of the decision problem - Total hip replacement and surface replaceme...Definition of the decision problem - Total hip replacement and surface replacement for the treatment of pain and disability resulting from end-stage arthritis of the hip (review of technology appraisal guidance 2 and 44): systematic review and economic evaluation
- Clinical effectiveness results - Levetiracetam as an alternative to phenytoin fo...Clinical effectiveness results - Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT
- Discussion - Levetiracetam as an alternative to phenytoin for second-line emerge...Discussion - Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...