Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Edwards SJ, Barton S, Thurgar E, et al. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: a systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2015 Jan. (Health Technology Assessment, No. 19.7.)

Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: a systematic review and economic evaluation.
Show detailsMethods for reviewing effectiveness
Evidence for the clinical effectiveness of topotecan, PLDH, paclitaxel, trabectedin and gemcitabine was assessed by conducting a systematic review of published research evidence. The review was undertaken following the general principles published by the Centre for Reviews and Dissemination (CRD).39 The protocol for the systematic review was registered on PROSPERO (registration number CRD42013003555).
Identification of studies
The literature search for this review was designed to update and expand the systematic search carried out in TA91, which evaluated the clinical effectiveness and cost-effectiveness of topotecan, PLDH and paclitaxel.13 Medical subject heading (MeSH) and text terms for ovarian cancer, topotecan, PLDH and paclitaxel were taken from the search strategy presented in TA91, and text terms added for the interventions trabectedin and gemcitabine. To ensure the capture of all potentially relevant studies to inform a network meta-analysis (NMA), the decision was taken not to restrict the start date of the update search to the end date of the search (2004) reported in TA91.
As a result of the large number of studies retrieved from the scoping search, the decision was taken to implement search filters for RCT. Filters developed and validated by Scottish Intercollegiate Guidelines Network were used.40 The identified RCTs facilitated construction of three distinct networks for the outcomes of OS and PFS for both the platinum-sensitive (two networks) and platinum-resistant/-refractory (PRR) (one network) subgroups. In an attempt to identify a study to link the discrete networks for the platinum-sensitive subgroup, the retrieved abstracts were re-examined to consider interventions that were outside the scope of this review. Owing to time constraints, the decision was taken not to search for non-randomised trials. Bibliographies of previous reviews and retrieved articles were searched for additional studies. Clinical trial registries were also searched to identify planned, ongoing and finalised clinical trials of interest. In addition, clinical experts were contacted with a request for information on any additional studies of which they had knowledge. The manufacturer submissions (MSs) were assessed for unpublished data. Electronic databases searched were EMBASE, MEDLINE® and Cochrane Central Register of Controlled Trials (CENTRAL). Although the protocol stipulates that the Index of Scientific and Technical Proceedings would be searched to identify relevant conference proceedings, owing to time constraints this was not undertaken. However, based on the conference abstracts retrieved from the search of the prespecified electronic databases, the Technology Assessment Group (TAG) considers it likely that the key conference abstracts have been identified. Conference abstracts that were reviewed and found not to report additional results to those presented in the relevant full publication were excluded.
Electronic databases were initially searched on 18 January 2013 and results uploaded into Reference Manager version 11.0 (Thomson Research Soft, San Francisco, CA, USA) and deduplicated. An update search was carried out on 23 May 2013. No papers or abstracts published after this date were included in the review. Full details of the strategies are presented in Appendix 1.
Titles and abstracts returned by the search strategy were examined independently by two researchers (SB and TK) and screened for possible inclusion. Disagreements were resolved by discussion, or involvement of a third reviewer (SJE) in cases for which consensus could not be achieved. Full texts of potentially relevant studies were ordered. Full publications were assessed independently by two reviewers (SB and TK/AS) for inclusion or exclusion against prespecified criteria, with disagreements resolved by discussion or input from a third reviewer when consensus could not be achieved.
Inclusion and exclusion criteria
For the review of clinical effectiveness, only RCTs were considered for inclusion in the review. Systematic reviews and non-randomised studies were excluded, as were studies that considered drugs administered as ‘maintenance therapy’ following directly on from first-line therapy without evidence of disease progression. Inclusion criteria were based on the decision problem outlined in Chapter 2 (see Decision problem) (presented as a whole in Table 6). No restrictions were imposed on language or date of publication. Reference lists of identified systematic reviews were used as a source of potential additional RCTs, as well as a resource to compare studies retrieved from the systematic literature search.
TABLE 6
Inclusion criteria (based on the decision problem) for studies evaluating clinical effectiveness
As in TA91,13 second-line chemotherapy was defined as the second chemotherapy regimen, administered either as a result of relapse after first-line therapy or immediately following on from first-line therapy in patients with progressive disease (PD) or stable disease (SD). The definition applied in cases where the second-line regimen comprised the same treatments as the first-line regimen.
For the purposes of this review, based on expert opinion, supportive care was defined as treatment for recurrent ovarian cancer that does not have an anti-tumour mode of action.
Data abstraction strategy
Data pertaining to study design, methodology, baseline characteristics, and clinical outcomes efficacy were extracted by two reviewers (TK/AS) into a standardised data extraction form and validated by a second (SB). Discrepancies were resolved by discussion when necessary. Authors of reports published as meeting abstracts only, where insufficient methodological details were reported to allow critical appraisal of study quality were contacted with a request for additional information. If no additional information was obtained, the studies were excluded. Data abstraction forms for the included studies are provided in Appendix 2.
Critical appraisal strategy
The quality of the clinical effectiveness data was assessed by two independent reviewers (TK and SB) and checked for agreement. The study quality was assessed according to recommendations by the NHS CRD39 and Cochrane Handbook for Systematic Reviews of Interventions41 and recorded using the Cochrane risk of bias tool.41
Methods of data synthesis
Details of results on clinical effectiveness and quality assessment for each included study are presented in structured tables and as a narrative summary. The possible effects of study quality on the clinical effectiveness data and review findings are discussed. The 16 RCTs identified evaluated 14 different pairwise comparisons. Therefore, there were insufficient data for most comparisons to carry out a standard pairwise meta-analysis. However, the TAG determined that the data identified were sufficiently homogeneous to investigate comparative effectiveness of interventions via a NMA. The methods used for the NMA followed the guidance described in the NICE Decisions Support Units (DSUs) Technical Support Documents (TSDs) for Evidence Synthesis. In essence, a NMA assumes that each trial included in the network could have potentially included all treatments of interest but that some of these treatments are missing completely at random (MCAR). To illustrate this further, in a simple indirect comparison of three treatments A, B and C, the trials of A compared with B and of B compared with C are assumed to have been potentially trials of A compared with B compared with C but where one arm from each trial is MCAR. In this example, an estimate of the relative treatment effect of A compared with C can be inferred using treatment B as a common comparator.
The TAG conducted a NMA for each network using a Bayesian Markov chain Monte Carlo simulation in WinBUGS version 1.43 (MRC Biostatistics Unit, Cambridge, UK). The following were implemented for each analysis:
- Uninformed priors (also called ‘flat’ priors) were used.
- All outcomes were considered independent. For example, although OS and PFS might be correlated in advanced ovarian cancer, the degree of correlation is unlikely to be derived from summary trial estimates provided in published papers.42 As such, in the absence of individual patient data (IPD), the TAG took the pragmatic approach of assuming all efficacy and safety outcomes were independent.
- Results for all efficacy outcomes analysed were based on 50,000 iterations after a ‘burn-in’ of 30,000 iterations. For safety outcomes all analyses had a ‘burn-in’ of 30,000 iterations, with results based on 100,000 iterations.
- Summary effect estimates for OS and PFS were hazard ratios (HRs), whereas ORR and all safety outcomes used odds ratios (ORs) as summary effect estimates.
- As a result of disparity in HRs reported in the identified trials, in terms of unadjusted HRs compared with adjusted HRs, together with variation in adjustment factors, for consistency the TAG used only unadjusted HRs in the NMA.
However, the ability of the TAG to conduct NMAs was limited by the low number of trials identified (typically only one trial per treatment comparison). The constraints imposed by the limited number of trials available for analysis were:
- Implementation of a fixed-effects model for all analyses. Although it was planned that fixed- and random-effects models would be explored and the model with the lowest deviance information criterion selected as the preferred model, the sparse number of trials available necessitated the use of a fixed-effects model. Using an uninformed prior for the between trial heterogeneity in a random-effects model ‘overwhelmed’ the influence of the available data for analysis with the posterior estimation of tau approximating the prior value used. Identification of an alternative source for the prior, for example from an existing systematic review, was explored but no suitable review was identified.43 As such, despite the potential clinical heterogeneity from two studies, which are discussed in detail later (see Comparability of baseline characteristics), the TAG made the pragmatic decision to use a fixed-effects model in the absence of any reliable estimate available.
- Disconnected networks identified for each outcome assessed. The trials identified in the clinical systematic review were unable to populate a single network for any of the outcomes assessed. A wider selection of treatments was assessed, as the systematic review was conducted in such a way as to identify all trials with at least one intervention of interest present. Unfortunately this did not uncover trials that could link the disconnected networks together.44 In addition, the TAG’s clinical advisors did not consider any of the suggested assumptions to link the disconnected networks together to have face validity.
- Heterogeneity and inconsistency. The networks constructed, typically ‘linear’ in nature, and the sparse number of trials available, typically only one per pairwise comparison, prevented the TAG from exploring any potential heterogeneity or inconsistency in each analysis.
The potential impact of these limitations is discussed where the results are reported.
Manufacturer submissions
All data submitted by the manufacturers were assessed. Data presented that met the inclusion criteria, and had not been identified in another published source, were extracted and quality assessed in accordance with the procedures outlined in this protocol. Economic evaluation included in the MSs, which complied with NICE’s advice on presentation, was assessed for clinical validity, reasonableness of assumptions and appropriateness of the data used in the economic model (see Description and critique of manufacturer submitted evidence).
Interpreting the results from the clinical trials
Clinical effectiveness
For the outcomes of OS and PFS/TTP, which are time-to-event outcomes, most trials identified evaluated comparative clinical effectiveness using a HR, which is the ratio of the hazard (e.g. death or progression) rate between two groups. Typically, a reported HR of < 1 indicates that the event of interest is occurring more slowly in the experimental group compared with the control group. In some trials identified, HR of > 1 (i.e. event occurs more frequently in the experimental group) is reported to favour a treatment. In these instances, the event recorded is not the hazard but the opposite event, i.e. survival or no progression over time. For the purposes of this review, PFS and TTP have been reported and evaluated under the outcome heading of PFS. Many trials identified also assess the extent to which a tumour shrinks compared with initial size, which is the response rate. Response rate is a dichotomous event (i.e. patients either respond or do not respond) and is reported as the proportion of patients achieving a response according to prespecified criteria.
Adverse effects
Many trials evaluating chemotherapeutic treatments categorise the severity of adverse effects based on criteria developed by the National Cancer Institute (NCI), one aim of which was to standardise reporting of adverse effects.45 According to the National Cancer Institute Common Toxicity Criteria (NCI-CTC),46 adverse effects are graded from 0 to 5, with increasing grade indicating more severe adverse effects (Table 7). The NCI-CTC also provides a detailed list of adverse effects commonly occurring in oncology trials, together with clinical descriptions on grade of severity that are specific for each adverse reaction.
TABLE 7
The NCI-CTC for adverse effects
Results
The RCTs meeting the inclusion criteria are discussed in the sections that follow. Initially, a summary of the quantity and quality of the evidence is provided, together with a table presenting an overview of the included trials. Additionally, a more detailed narrative description, together with an overview of trial quality, for each included trial is presented, including those trials previously identified in TA91.13 A narrative description of population baseline characteristics and potential imbalances are discussed for each trial. Owing to the number of trials identified, baseline characteristics are not tabulated within the main body of the report but are provided within the data abstraction forms in Appendix 2. Instead, baseline characteristics for key prognostic factors in recurrent ovarian cancer (age, number of prior lines of chemotherapy, interval since last chemotherapy, and performance score) are presented for included trials in a summary table (see Table 10).
TABLE 10
Population baseline characteristics of the included trials
Clinical effectiveness results are reported by outcome (OS, PFS, ORR, QoL and adverse effects). Within the efficacy outcomes of OS, PFS and ORR, results are presented separately based on platinum sensitivity. Results by population are ordered: platinum sensitive, which is broken down further to fully platinum sensitive (FPS) and PPS, when data are available; PRR; and the overall population (when trial includes patients with platinum-sensitive disease or PRR disease). Results for QoL and adverse effects are presented for the overall population, irrespective of sensitivity to platinum. Within the outcome, results are initially presented separately for each trial reporting data, and are supplemented with the findings from the NMA, including a description of assumptions made and potential bias across the trials included in the network.
Quantity and quality of research available
The searches retrieved a total of 5993 records (post deduplication) that were of possible relevance to the review (Figure 3). These were screened and 104 full references were ordered. Of these, five had to be cancelled because they were unobtainable. Of the full references evaluated, 28 papers describing 16 studies were included in the review.

FIGURE 3
A PRISMA flow diagram for studies included and excluded from the clinical effectiveness review.
The full list of studies included in the review is given below (see Table 8), whereas a list of the papers screened but subsequently excluded (with reasons for exclusion) from the review is presented in Appendix 3.
TABLE 8
Summary of studies included in the review of the clinical effectiveness literature
Included studies
Sixteen RCTs reported in 15 primary publications, with 13 accompanying publications, were included in the review. One RCT from TA9113 was included, which was identified in the literature search only as an abstract and the results of which have not been published in full elsewhere (referred to hereafter as Trial 30–57).47 An overview of the identified trials is provided in Table 8. Of the 16 RCTs identified, five evaluated the intervention and comparator within their licensed indication, and dose and route of administration.13,21,48–50 The remaining 11 RCTs evaluated the intervention or comparator outside the parameters specified in the licence, in terms of, for example, dose or route of administration. No RCT identified evaluated interventions specifically in a population who were allergic or intolerant to platinum-based treatments. Of the nine RCTs identified in TA91, only one RCT51 has been excluded from this update. Cantu et al.51 evaluated paclitaxel alone compared with a combination of cyclophosphamide, doxorubicin and cisplatin (CAP). Doxorubicin administered in the trial is the non-pegylated formulation and is outside the scope of this review, which specifies PLDH as the intervention of interest.
Two manufacturers [Eli Lilly and Company (gemcitabine); PharmaMar (trabectedin)] submitted clinical evidence for consideration for this multiple technology appraisal (MTA).
Eli Lilly (gemcitabine) did not carry out a systematic review of the literature; instead, the manufacturer reported clinical data from three studies:
- a Phase III study comparing gemcitabine plus carboplatin with carboplatin monotherapy in patients with platinum-sensitive, recurrent ovarian cancer (study JHQJ)
- a single-arm, Phase II study of gemcitabine plus carboplatin in platinum-sensitive, recurrent ovarian cancer (study JHRW)
- a single-arm, Phase I/II dose-finding study of gemcitabine plus carboplatin in platinum-sensitive, recurrent ovarian cancer (study SO026).
The data provided by the manufacturer for JHQJ, the Phase III study comparing gemcitabine plus carboplatin with carboplatin monotherapy, are reported in the full publication of the trial,50 which was identified and included as part of the systematic review of the literature on clinical effectiveness (see Results, above).
The two additional studies (JHRW and SO026) are single-arm trials and as such do not meet the criteria for inclusion in the review (see Results, above).
PharmaMar (trabectedin) carried out a systematic search of the literature. Specifically, the manufacturer updated the review carried out for the STA TA222,15 which evaluated the use of trabectedin plus PLDH in the treatment of platinum-sensitive ovarian cancer. The manufacturer searched the following databases: EMBASE; MEDLINE; MEDLINE® In-Process & Other Non-Indexed Citations; and The Cochrane Library. Studies were included if:
- the study type was a RCT
- the population of interest was relapsed platinum-sensitive ovarian cancer
- outcome data for PFS, OS or AEs were included
- the interventions and comparators of interest included at least one of trabectedin, PLDH, paclitaxel, topotecan, etoposide, or best supportive care.
The manufacturer limited the comparators within searches to the comparators outlined in the NICE pathway for patients who are unsuitable for platinum-based chemotherapy; this represents the target population for the MS.
The manufacturer identified two additional relevant studies relating to OVA-301,30 which were identified as part of the review of the clinical effectiveness literature and are discussed in a subsequent section (see subsequent text).
Study characteristics
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
Two RCTs29,31 were identified for this comparison. The RCTs were of similar design but one was a Phase II RCT29 and one was a Phase III RCT.31 In addition, the dose of PLDH evaluated differed between the trials, with 45 mg/m2 and 30 mg/m2 used by Bafaloukos et al.29 and Pujade-Lauraine et al.,31 respectively. The licence for PLDH does not recommend a dose of PLDH for use in combination with platinum-based chemotherapy. Bafaloukos et al.29 note in the discussion that, at the time of initiation of the trial, limited information was available on the optimal dose for PLDH in combination with carboplatin. As highlighted by Bafaloukos et al.,29 retrospective analyses suggest that lower dose intensities of PLDH (30–40 mg/m2) are as clinically effective but with improved tolerance. Clinical experts have fed back that, in UK clinical practice, PLDH would most likely be used at a dose of 30 mg/m2 when combined with carboplatin.
Bafaloukos et al.29 report the results of a randomised study in which 204 patients with histologically confirmed recurrent ovarian cancer were randomised to either PLDH (45 mg/m2) plus carboplatin (AUC 5) every 28 days or paclitaxel (175 mg/m2) plus carboplatin (AUC 5) every 21 days. Patients recruited had disease that had recurred at least 6 months after platinum-based chemotherapy, i.e. women with platinum-sensitive disease. Women with only elevated CA125 levels (twice the ULN or more) as an indicator of disease were also included.
The primary aim of the study was to evaluate the comparative clinical effectiveness of the two treatment regimens in terms of response rate and toxicity in women with platinum-sensitive ovarian cancer relapsing after first-line platinum-based therapy. OS and TTP were analysed as secondary outcomes. Subsequent to randomisation, 15 patients were found to be ineligible (reasons provided). Therefore, analyses presented are based on data from 189 eligible patients (96 in the paclitaxel plus carboplatin group vs. 93 in the PLDH plus carboplatin group). The reported power calculation indicates that 201 patients were needed to identify a 20% difference in response rate between the groups. The study might have been underpowered to detect a difference between groups in response rate.
Randomisation (1 : 1) was performed at the central Hellenic Cooperative Oncology Group (HeCOG) Data Office in Athens but details on the method of randomisation were not reported. Stratification criteria were not applied at randomisation. Tumour response was evaluated using World Health Organization (WHO) criteria for patients with measurable disease and CA125 based on Rustin’s criteria for patients without measurable disease. Median duration of follow-up was reported as 43.6 months (95% CI 0.1 to 74.8 months), but the range of follow-up was not reported either for the full trial population or for the individual treatment groups.
All patients received standard premedication of dexamethasone, diphenhydramine and ranitidine (Zantac®, GSK) prior to paclitaxel. In the group receiving paclitaxel, premedication was administered twice, orally 12 hours before and again intravenously 30 minutes before paclitaxel infusion. In the group receiving PLDH, premedication was administered only intravenously prior to PLDH infusion. Six cycles of chemotherapy were administered, unless disease progression or unacceptable toxicity occurred. A maximum of 2 weeks’ delay was allowed for toxicity and treatment was discontinued if longer toxicity-related delays occurred. For grade 3 and grade 4 thrombocytopenia, a 25% and a 50% dose reduction, respectively, was recommended for all drugs.
A median of six cycles of paclitaxel plus carboplatin (range 1–9) and six cycles of PLDH plus carboplatin (range 1–8) were administered. Most patients in each group completed the planned treatment (68% in paclitaxel plus carboplatin and 70% in PLDH plus carboplatin).
In the second RCT identified for this comparison, Pujade-Lauraine et al.31 report the results of a randomised international, multicentre, open-label, Phase III non-inferiority trial (CALYPSO) in which 976 patients with platinum-sensitive (disease progression > 6 months after prior treatment) relapsed/recurrent ovarian cancer received a combination of PLDH plus carboplatin (n = 467) or carboplatin plus paclitaxel (n = 509). Prior treatment must have included a taxane and no more than two previous platinum-based regimens (i.e. patients had failed first- or second-line treatment). Patients with measurable [according to Response Evaluation Criteria in Solid Tumours (RECIST)] and CA125 assessable [according to Gynecologic Cancer Intergroup (GCIG)] criteria were eligible.
The primary publication presents results on PFS. Accompanying publications were identified that present results on mature OS data,56 clinical effectiveness results in the subgroup of patients with PPS ovarian cancer (relapse at between 6 and 12 months since receipt of last cycle of chemotherapy)57 and QoL.59
The trial was of a non-inferiority design with the aim of determining whether PLDH (30 mg/m2) plus carboplatin (AUC 5) every 4 weeks was non-inferior to the standard treatment of paclitaxel (175 mg/m2) plus carboplatin every 3 weeks.31 The goal was to evaluate the comparative effectiveness of the treatments in terms of efficacy and toxicity. The primary outcome of the trial was PFS, with OS, QoL and toxicity as prespecified secondary outcome measures. Determination of disease progression was based on RECIST and GCIG criteria modifications, and included any of the following: occurrence (clinically or by imaging) of any new lesion; increase in measurable and/or non-measurable tumour defined by RECIST; CA125 elevation defined by GCIG criteria; health status deterioration attributable to disease; and death from any cause before progression was diagnosed. Assessments were independently reviewed. All patients were observed for at least 5 years from random assignment to assess OS.
Randomisation was in permuted blocks of six in a 1 : 1 ratio, and patients were stratified based on therapy-free interval from last chemotherapy (6–12 months vs. 12 months), measurable disease (yes vs. no) and centre. Despite randomisation, an imbalance in treatment allocation was noted (467 randomised to PLDH plus carboplatin vs. 509 randomised to paclitaxel plus carboplatin).
All patients received antiemetics, including a serotonin antagonist and corticosteroid. Patients randomly assigned to paclitaxel plus carboplatin received premedication to prevent hypersensitivity reactions. Dose delay and dose reduction were allowed for haematological and non-haematological toxicity. In the absence of unacceptable toxicity or disease progression, patients were treated for a total of six courses of therapy; if SD or PR was achieved after six courses of therapy, patients were allowed to remain on therapy until progression.
To assert non-inferiority of PLDH plus carboplatin, it was estimated that a sample size of 898 evaluable patients (estimate of 745 progression) would be required.31 The calculation was based on non-inferiority margin with a HR of 1.23 at 15 months or a 7.9% absolute difference at 12 months (90% power and a one-sided CI of 95%).
Median follow-up was 22 months; median follow-up in the individual treatment groups not reported.31 The median number of cycles was six in each treatment group, with a range of cycles from 1 to 14 in the PLDH plus carboplatin group and 1 to 12 in the paclitaxel plus carboplatin group. A significantly larger proportion of patients in the PLDH plus carboplatin group completed at least six cycles of treatment (85% vs. 77%; p < 0.001).
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with carboplatin alone
Alberts et al.28 reported the results of a randomised study in which 61 patients from the USA with recurrent stage III or i.v. epithelial or peritoneal ovarian carcinoma were randomised to PLDH (i.v. infusion of 30 mg/m2) plus carboplatin (AUC 5) once every 4 weeks (31 patients) or carboplatin (AUC 5) alone once every 4 weeks (30 patients). A follow-up study reporting final OS results was also identified.55
To be eligible for enrolment, patients had to have histologically diagnosed stage III or IV disease that was determined to be progressive based on RECIST or GCIG CA125 criteria. Patients also had to have a progression-free interval and a PFI of 6–24 months after first-line platinum-based chemotherapy, which indicates that the study focused on women with platinum-sensitive disease. Patients were excluded if Zubrod performance status was > 1. Prior treatment with up to 12 courses of a non-platinum containing consolidation treatment during the 6- to 24-month PFI was allowed on the proviso that treatment had been completed at least 28 days prior to registration.
The primary aim of the study was to evaluate the comparative clinical effectiveness of the two treatment regimens in terms of OS in women with platinum-sensitive ovarian cancer. PFS, confirmed CR rate and time to treatment failure were analysed as secondary outcomes. Objective response and disease progression were defined according to standard RECIST criteria.69 GCIG CA125 progression criteria were also implemented in defining disease progression.70
Details on the method of randomisation were not reported, but randomisation was 1 : 1 to each group and was reported to be equal between the groups. Randomisation was stratified by disease measurability, number of disease sites and serous histology. The power calculation reported indicates that the study had initially planned to recruit 900 patients over a period of 4.5 years. However, as a result of slow patient accrual, the study closed early with only 61 patients enrolled. Initially designed as a Phase III RCT, results were reported as for a Phase II RCT. Median duration of follow-up was reported as 22.4 months, but the range of follow-up was not reported either for the full trial population or for the individual treatment groups. Markman et al.55 reported a longer follow-up of the same trial. However, the duration of follow-up in this study is unclear.
Each treatment was given until progression, intolerable toxicity or a request from either the clinician or the patient to be removed from the study. Dose modifications were allowed, based on toxicity to PLDH. The maximum cumulative dose of PLDH was 600 mg/m2. Any patient with a compromised left ventricular ejection fraction (LVEF) (< 45% or decreases by a relative 20% from baseline) was removed from PLDH and continued on the carboplatin treatment. Carboplatin dose modifications were allowed for gastrointestinal and neurological toxicity. Patients with persistently greater than or equal to grade 2 peripheral neuropathy, despite dose reduction, were permanently taken off carboplatin treatments. The median number of treatment cycles given was 7 (range 1–18) for patients in the PLDH plus carboplatin group and 6 (range 2–16) for those in the carboplatin alone group. No major protocol violations were reported.
Trabectedin plus pegylated liposomal doxorubicin hydrochloride compared with pegylated liposomal doxorubicin hydrochloride alone
Monk et al.30 report the results of an open-label, randomised, multicentre (124 centres in 21 countries), Phase III trial involving 672 women with recurrent ovarian cancer after failure of first-line platinum-based chemotherapy (OVA-301). Patients with platinum-resistant ovarian cancer (PFI < 6 months) or platinum-sensitive ovarian cancer (PFI ≥ 6 months) were eligible, but those who experienced progression during first-line therapy (platinum refractory) were excluded. Measurable disease by RECIST criteria69 was also an inclusion criterion. Related publications identified were a follow-up study reporting mature OS analysis,64 clinical efficacy results for the subgroup of patients with PPS65 and full results for QoL.67
The aim of OVA-30130 was to compare the efficacy and safety of PLDH (30 mg/m2) plus trabectedin (1.1 mg/m2) every 21 days (n = 337) compared with PLDH (50 mg/m2) alone every 28 days (n = 335). The primary outcome was PFS, which was defined as time from randomisation to disease progression or death. Primary analysis of PFS was based on independent radiology review (radiological evaluation alone) by radiologists who were masked to treatment allocation. Secondary end points included OS, ORR (response maintained ≥ 4 weeks by RECIST69), and duration of response (calculated from date of first documentation of response to date of PD or death from PD). QoL was a tertiary outcome and was evaluated using the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire C30 (QLQ-C30) and ovarian cancer-specific QLQ-OV28.71 All efficacy analyses were based on the intention-to-treat (ITT) principle.
Randomisation was by a permuted block method (1 : 1 ratio) and patients were stratified by performance status [Eastern Cooperative Oncology Group (ECOG) score of 0 or 1 vs. 2] and platinum sensitivity (sensitive vs. resistant). After enrolment of 440 patients, and before central radiology review, the study was amended, changing the two primary efficacy end points, OS and PFS, to a single primary end point, PFS. OS became a secondary end point; the sample size remained unchanged. The sample size calculation indicated that 415 PFS events were required to test statistical difference between treatment groups with at least 90% power; it is reported that approximately 650 patients were to be randomised over 2 years.
Treatment was continued until disease progression or confirmation of CR and could be continued for two or more cycles beyond confirmed CR. A maximum of two dose reductions for each drug was allowed (in the trabectedin plus PLDH group, trabectedin could be reduced to 0.9 mg/m2 and subsequently to 0.75 mg/m2, and PLDH to 25 mg/m2, then to 20 mg/m2; in the PLDH group, PLDH could be reduced to 37.5 mg/m2 and then to 28 mg/m2). Median cumulative trabectedin dose was 5.6 mg/m2 (range 1–23 mg/m2). For PLDH, median cumulative PLDH dose was 154.4 mg/m2 (range 15–630 mg/m2) and 216 mg/m2 (range 3–1061 mg/m2) when administered in combination with trabectedin and as a monotherapy, respectively. Incidence of dose reductions was similar between groups, whereas cycle delays were less frequent with PLDH alone than trabectedin plus PLDH.
Median duration of follow-up in the initial publication was not reported30 but median follow-up in the longer-term study was 47 months.64
The authors report that, despite stratification before randomisation, there was an imbalance between groups in mean baseline PFI that favoured PLDH alone (13.3 months with PLDH alone vs. 10.6 months with trabectedin plus PLDH; p = 0.009). Post hoc hypothesis-generating analyses on the influence of PFI on OS were carried out (discussed in Assessment of effectiveness).
It should be noted that use of trabectedin plus PLDH as an intervention in patients with PRR is not covered by the scope of this review. Clinical effectiveness data for only platinum-sensitive patients are presented.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
Gordon et al.49 report the results of a Phase III randomised study comparing PLDH compared with topotecan in 474 women with histologically proven recurrent epithelial ovarian carcinoma that recurred after or did not respond to first-line platinum-based chemotherapy. The RCT was open label in design and was carried out at multiple centres (104 sites) in the USA and Europe. Patients with either measurable or assessable disease were included, where measurable disease was defined as presence of bidimensionally measurable lesions with clearly defined margins based on imaging scans and assessable disease was defined as unidimensionally measurable lesions by imaging scan in conjunction with serum CA125 levels of > 100 U/ml. A follow-up publication reported data on more mature OS, together with subgroup analyses based on platinum sensitivity.54
Patients were randomised to receive either PLDH 50 mg/m2 as a 1-hour infusion every 28 days (239 patients) or topotecan 1.5 mg/m2 daily for five consecutive days every 21 days (235 patients).49 In the absence of disease progression, treatment in each group could be continued for up to 1 year. Treatment could also continue if the patient demonstrated sustained clinical benefit. Patients who discontinued treatment after 6 months (six cycles of PLDH or eight cycles of topotecan) were considered protocol completed.
The study was described as randomised, but details on the method of randomisation were not reported. Patients were stratified for platinum sensitivity and for the presence or absence of bulky disease (tumour mass > 5 cm). Patients were classified as platinum sensitive if they had a PFI of > 6 months after first-line platinum-based chemotherapy and platinum refractory if they had SD, progressed during initial platinum-based therapy or relapsed within 6 months after completion of therapy. In the subsequent publication,54 analyses for OS and PFS for the subgroups of patients with PPS disease (PFI > 6 to ≤ 12 months) and FPS disease (PFI > 12 months) are presented. The authors report that the main outcome measures of efficacy were PFS and OS. Overall response rate (confirmed CR plus PR), time to response, duration of response, QoL, and safety and toxicity were also assessed. The study was designed with 80% power to demonstrate statistical equivalence between the two treatment groups. The initial sample size calculation found that a total of 350 assessable patients, 175 patients in each treatment group, would need to be randomised. To accommodate two interim analyses (necessitating 5% more patients) and anticipated loss of 20% of randomised patients who might not be assessable for efficacy end points, the sample size was increased to 460.
Protocol deviations included: (1) failure to meet entry criteria (seven patients receiving PLDH, two patients receiving topotecan); (2) patients who continued on study after first clinically significant change in LVEF (13 patients receiving PLDH); (3) patients who continued treatment after documented disease progression (40 patients receiving PLDH, 42 patients receiving topotecan); and (4) patients who completed fewer than eight cycles of treatment but were deemed protocol completed by the investigator (20 patients receiving topotecan).
Dose modifications were permitted. Reasons for reduction in PLDH dose included PPE, haematological toxicity, elevated bilirubin, stomatitis, or all other grade 3 and grade 4 events until resolution to grade 2 or lower. In the event of severe neutropenia during any cycle with topotecan, the dose was reduced by 0.25 mg/m2 for subsequent courses. Treatment with either drug was temporarily suspended or discontinued in cases of: disease progression; serious or intolerable AEs precluding further treatment; inability to tolerate study drug despite dose modification; LVEF of < 45% or a 20% decrease from baseline; and patient’s decision to withdraw participation or patients requiring radiation.
Median duration of follow-up was not reported in either publication.49,54 In addition, information on mean or median number of cycles received in each treatment group was not provided. However, the mean cycle dose and cycle length for each treatment group were reported to be close to those specified in the protocol, indicating good compliance in following the dosing guidelines.
Pegylated liposomal doxorubicin hydrochloride compared with paclitaxel
In a publication available as only a conference abstract, O’Byrne et al.47 provided a brief overview of a trial comparing PLDH compared with paclitaxel. The search did not retrieve a full publication of this study. However, as part of TA91,13 the manufacturer of PLDH (Schering-Plough) provided a full trial report as part of the industry submission.72 The description of trial methodology and results for OS and adverse effects have been adapted from TA91.13
The trial by Schering-Plough was a Phase III, randomised, open-label study involving 216 women with epithelial ovarian carcinoma after failure of first-line platinum-based chemotherapy. Additionally, to be eligible, women had to have measurable disease and be taxane naive. The trial was designed to compare the clinical effectiveness and safety of PLDH (50 mg/m2) every 28 days compared with paclitaxel (175 mg/m2) every 21 days.
Randomisation was carried out in a 1 : 1 ratio, with patient stratification by platinum sensitivity and bulky disease. No details on the method of randomisation are reported.
TA9113 reports that the planned enrolment was for 438 patients but only 216 were randomised (108 in each treatment arm), with the trial closing early due to poor accrual. It is thought that poor accrual was associated with the approval of Taxol for use in combination with platinum-based therapy for the first-line treatment of ovarian cancer by the European Agency for the Evaluation of Medicinal Products.
Patients were assessed weekly for haematological toxicities, and radiological imaging was repeated every 7–8 weeks to assess disease status. Patients achieving either a CR or PR were re-evaluated 4 weeks later to confirm the initial observation of response. All participants were to have been followed for a minimum of 1 year for survival and disease progression.
At baseline, the two treatment groups were balanced in terms of age, treatment-free interval (TFI), disease bulk, the number of previous chemotherapy regimens, the type of previous chemotherapy agents received, histology and performance status.
As a result of the low recruitment rate, efficacy analysis in TA9113 was limited to OS. AEs were also described.
Topotecan compared with paclitaxel
ten Bokkel Huinink et al.21 report the results of an open-label, Phase III randomised study involving 235 patients with stage III/IV ovarian cancer, who had progressed during or after treatment with one platinum-based chemotherapy. The study was designed to compare the effectiveness and toxicity of topotecan (1.5 mg/m2) for five consecutive days every 21 days compared with paclitaxel (175 mg/m2) every 21 days. Enrolled patients had at least one bidimensionally measurable lesion as evidenced by CT or magnetic resonance imaging (MRI) scan, ultrasound or physical examination. Patients who had received more than one prior chemotherapy, or who had been previously treated with topotecan or paclitaxel, were ineligible. A second publication reporting more mature OS data was also identified.52 A related study reports results from an analysis of patients who received third-line treatment during the trial, and specifically crossover therapy with the treatment received in the other group.53
The primary outcome measures were response rate, duration of response and TTP. Response rate included CR or PR as a best response as determined by WHO criteria, with all responses independently reviewed by a radiologist who was masked to treatment allocation. Secondary outcome measures were time to response and OS. Of the 235 patients randomised, nine patients did not receive treatment and were excluded from analyses. An additional 24 patients were not evaluated for response, but were included in the calculation of response rate.
Randomisation was reported to be carried out by telephone, but details on the method of randomisation were not available. Patients were stratified by age (< 65 vs. ≥ 65 years), ascites (present vs. absent) and prior response to platinum-based therapy (resistant vs. early vs. interim vs. late response). Resistant disease was defined as no response to initial chemotherapy or having an initial PR or CR with subsequent progression while still receiving treatment. Early, interim and late response were defined as initial CR or PR with subsequent relapse within 3 months (early), 3–6 months (interim) or > 6 months (late) after cessation of chemotherapy.
Patients with a CR or PR continued treatment until either progression or 6 months past the maximal response; those who progressed were removed from the study. Those whose best response was SD after six cycles could be removed from the study or switched to the alternative regimen.
Patients on paclitaxel received premedication with dexamethasone and H1- and H2-receptor antagonists to prevent hypersensitivity. No premedication was initially given to those on topotecan but was allowed in subsequent cycles if nausea or vomiting occurred. Dose reductions in each group were permitted for toxicity. The minimum dose allowed was 1.0 mg/m2 per day for topotecan and 135 mg/m2 for paclitaxel; the dose of topotecan could also be escalated to a maximum of 2 mg/m2 per day. Patients were withdrawn from treatment if there was a > 2-week delay in treatment at the minimum dose of either medication because of toxicity. The target dose was achieved in 90% of cycles of topotecan and 98% of cycles of paclitaxel. Median number of cycles received was five in each group, with patients treated with topotecan receiving between 1 and 17 cycles compared with between 1 and 12 cycles for patients treated with paclitaxel.
A sample size calculation was not reported. Median duration of follow-up at the time of the first publication was unclear.21 Median follow-up at the time of the publication reporting more mature OS data was reported in TA91 to be 58.5 weeks in the topotecan group (range 0–86 weeks) and 52.6 weeks in the paclitaxel group (range 0–117 weeks).13
Gemcitabine plus carboplatin compared with carboplatin alone
Pfisterer et al.50 report the results of a Phase III international, open-label randomised study assessing the comparative clinical effectiveness of gemcitabine (1000 mg/m2) plus carboplatin (AUC 4) (n = 178) compared with carboplatin alone (AUC 5; n = 178) in patients with platinum-sensitive recurrent ovarian cancer, with recurrence occurring at least 6 months after completion of first-line platinum-based therapy. Patients were enrolled with measurable or assessable lesions according to Southwest Oncology Group (SWOG) criteria. Exclusion criteria included an ECOG score of > 2, inadequate bone marrow or kidney function or serious concomitant conditions, or life expectancy of < 12 weeks.
The primary outcome of the trial was PFS, with OS, response rate, duration of response, QoL and toxicity measured as secondary outcomes. It should be noted that the study was not powered to detect a difference between treatments in OS. Randomisation was carried out through the central Arbeitsgemeinschaft Gynaekologische Onkologie (AGO)-Ovarian Cancer Study Group (OVAR) office (method of randomisation not reported), with patients randomised at a 1 : 1 ratio. Patients were stratified by PFI (6–12 months vs. ≥ 12 months), first-line therapy (platinum plus paclitaxel vs. other platinum-based therapy) and bidimensionally measurable disease (yes vs. no).
Median duration of follow-up was reported as 17 months, but the range of follow-up was not reported either for the full trial population or the individual treatment groups. Treatment cycles in each group were repeated every 21 days for six cycles, in the absence of PD or unacceptable toxicity. At the investigator’s discretion, benefiting patients could receive a maximum of 10 cycles of therapy. The median number of cycles administered was six cycles in each group. Cycles could be postponed up to 2 weeks owing to toxicity, and longer toxicity-related delays led to treatment discontinuation. For grade 3 non-haematological toxicities (excluding nausea/vomiting), dose modifications and/or study discontinuation were at the investigator’s discretion. Patients in the gemcitabine plus carboplatin arm received 75.6% of the planned mean dose of gemcitabine (92.8% on day 1 and 63.4% on day 8) and 96.2% of the planned dose of carboplatin. Patients in the carboplatin arm received 98.2% of the planned dose.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
Two RCTs were identified for this comparison.48,61 One RCT was a collaboration between the International Collaborative Ovarian Neoplasm (ICON) group and the AGO group and hereafter is referred to as ICON4/AGO-OVAR 2.2 (Parmar et al.61). The RCTs identified were of similar design, but one was a Phase II RCT (Gonzalez-Martin et al.48) and the other was a Phase III RCT (ICON4/AGO-OVAR 2.261).
The ICON4/AGO-OVAR 2.2 trial61 comprised results from two randomised trials that were run in parallel. ICON4/AGO-OVAR 2.261 was an international multicentre trial enrolling 802 patients in 119 hospitals across five countries. ICON4 was co-ordinated by the Istituto di Recerche farmacologiche Mario Negri (IRFMN), and the Medical Research Council’s Clinical Trials Unit (MRC CTU), and AGO-OVAR 2.2 was co-ordinated by AGO. Each co-ordinating unit had its own protocol, with minor differences in eligibility criteria.
All centres enrolled patients with relapsed epithelial ovarian cancer who had previously received platinum-based chemotherapy and had been treatment free for at least 6 months; patients in IRFMN were required to have been treatment free for a minimum of 12 months. The IRFMN and AGO-OVAR 2.2 protocols specified that women were to have received only one prior chemotherapy treatment to be eligible for enrolment, whereas the MRC-CTU protocol permitted women to have received more than one previous line of chemotherapy. Measurable disease at baseline was an entry criteria for patients randomised in centres co-ordinated by the IRFMN, but not MRC CTU or AGO co-ordinated centres. The IRFMN and MRC CTU protocols required that patients have had previous platinum-based chemotherapy, with or without paclitaxel. By contrast, the AGO protocol specified that patients must have previously received cisplatin plus paclitaxel or carboplatin plus paclitaxel. Patients with concomitant or previous malignant disease were ineligible.
The trial compared the clinical effectiveness of paclitaxel plus platinum-based chemotherapy with platinum-based chemotherapy alone. Patients were randomised to receive paclitaxel [175 (ICON4) or 185 (AGO-OVAR 2.2) mg/m2 as a 3-hour infusion] plus platinum chemotherapy (392 patients) or conventional platinum-based therapy (410 patients). Platinum-based therapy comprised carboplatin (AUC 5) or cisplatin (minimum 75 mg/m2 as monotherapy or 50 mg/m2 in combination therapy). In all protocols, cycles were administered every 21 days.
The aim of the study was to evaluate whether paclitaxel should be given in addition to platinum-based chemotherapy in patients with platinum-sensitive disease, who would otherwise be treated with conventional platinum-containing regimens. Randomisation used a computer minimisation method (1 : 1 ratio) and patients were stratified by multiple factors that were determined by the protocol of the assigned centre. In ICON4 protocols, patients were stratified by age, centre, last chemotherapy received, time since last chemotherapy completed and intended platinum treatment. In AGO-OVAR 2.2, patients were stratified by whether the patient had undergone secondary debulking surgery and time since completion of last chemotherapy.
The primary outcome measure of all protocols was OS; secondary outcomes were PFS and QoL. Progression required clinical or radiological evidence of disease (not only raised CA125 level). The sample size calculation found that 800 patients would be sufficient to detect an 11% difference between the groups if the control group survival was 50% at a power of 90% and a 5% significance level.
Median follow-up was 42 months. Of the full trial population, 72% of patients received a minimum of six cycles of assigned chemotherapy; reasons for not completing six cycles included disease progression or death, toxicity or patient preference.
Gonzalez-Martin et al.48 reported the results of a Phase II study, in which 81 patients with platinum-sensitive recurrent ovarian cancer, who had received no more than two previous chemotherapy lines, were randomised to receive carboplatin alone (AUC 5; 40 patients) every 21 days or paclitaxel (125 mg/m2 of > 3 hours) plus carboplatin (AUC 5; 41 patients) every 21 days. Patients had to have measurable disease as measured by CT or clinically evident but non-measurable disease that was evaluable by CA125 level, based on Rustin’s criteria. Patients who had an ECOG performance status of > 2, life expectancy of < 12 weeks or inadequate bone marrow, liver or kidney function were ineligible.
The primary outcome measure was ORR (CR or PR), which was evaluated using WHO criteria in those with measurable disease, or by CA125 level, according to Rustin’s criteria, in those without measurable disease. OS, TTP and QoL were reported as secondary outcome measures.
Both treatments were administered for a minimum of six cycles unless there was progression, unacceptable toxicity or a patient refused treatment. After six cycles, patients could continue for three further cycles if clinical benefit could be expected. All patients randomised to receive paclitaxel were treated with standard premedication 30 minutes before infusion, which comprised dexamethasone, diphenhydramine and ranitidine. In cases of grade 4 neutropenia or thrombocytopenia, doses were reduced to carboplatin AUC 4 (both groups) and paclitaxel 150 mg/m2.
Randomisation was reported to have been carried out by a central data centre (no further details reported). Patients were stratified by PFI [6–12 months (PPS) compared with > 12 months (FPS)] and number of previous chemotherapy lines (one vs. two). The trial was an unusual ‘pick up the winner’ design. The authors of the trial comment that this type of design has a ‘90% chance of selecting the better treatment if the difference is at least 15% and the smaller response rate is assumed to be 30%’. A sample size calculation is not presented, but the authors state that the trial was not designed or powered to detect differences in survival. The authors go on to comment that ‘no formal statistical comparison between the two arms was planned’, but a selection of statistical comparisons are reported ‘for exploratory purposes only’.
Patients in both treatment groups received a median of six cycles of treatment, with between two and nine cycles of carboplatin alone administered and between one and eight cycles of paclitaxel plus carboplatin administered. Three patients in the paclitaxel plus carboplatin arm did not receive one cycle of treatment. The proportion of patients requiring a dose reduction was small and was similar between the groups (4.7% with carboplatin alone vs. 6.6% with paclitaxel plus carboplatin). By contrast, a significantly larger proportion of patients required a dose delay in the carboplatin alone group (34.4%) compared with the paclitaxel plus carboplatin group (21%; p-value for difference < 0.006). The difference was attributed to the absence of haematological recovery by day 21 in the group receiving carboplatin alone.
The three patients who received no treatment in the paclitaxel plus carboplatin group were included in the ITT analysis of overall response but were excluded from other analyses. Median duration of follow-up was 67.7 weeks. At this time point, 32 patients had died and median OS has not been reached in the paclitaxel plus carboplatin group. The range of follow-up was not reported either for the full trial population or the individual treatment groups.
Paclitaxel plus carboplatin compared with paclitaxel alone
Lortholary et al.62 reported the results of a Phase II, multicentre, open-label, three-armed randomised trial (CARTAXHY) in patients with PRR recurrent ovarian cancer. Eligible patients were those who had received at least one prior therapy, with the most recent regimen combining platinum with a taxane agent. In addition, patients were required to have either measurable (according to RECIST criteria) or CA125 assessable disease, an ECOG performance status of ≤ 2 and a life expectancy of > 12 weeks. Patients with measurable disease (according to RECIST criteria) or evaluable disease (CA125) were enrolled. Patients who had previously been treated with weekly paclitaxel were excluded.
In total, 165 patients were randomised (1 : 1 : 1 ratio) to treatment with weekly paclitaxel (80 mg/m2 administered on days 1, 8 and 15 of a 4-week cycle; 57 patients), weekly paclitaxel plus carboplatin (AUC 5 administered on day 1 of a 4-week cycle; 51 patients) or weekly paclitaxel plus weekly topotecan (3 mg/m2 administered on days 1, 8 and 15 of a 4-week cycle; 57 patients). The combination of paclitaxel plus topotecan in the treatment of patients with PRR ovarian cancer is not covered by the scope of this review, and the efficacy results for this group are not presented.
The primary outcome was PFS. Secondary end points were response rate, OS, QoL and toxicity. QoL was assessed using the EORTC Quality of Life Questionnaire (EORTC-QLQ) and toxicity was assessed according to NCI-CTC. The efficacy analyses were based on the ITT principle.
Randomisation was carried out at the Group d‘Investigateurs Nationaux pour I’Etude des Cancers Ovariens (GINECO) data centre but details on the method of randomisation are not available. Patients were stratified according to centre, TFIs (progression during treatment vs. relapse between 0 and 3 months vs. relapse at > 3 months and ≤ 6 months), and presence of a measurable lesion at baseline.
Treatments were administered for six to nine cycles or until progression or unacceptable toxicity. On progression, patients treated with weekly paclitaxel or weekly paclitaxel plus weekly topotecan received carboplatin (AUC 5) and patients treated with weekly paclitaxel plus carboplatin went on to receive treatment of physician’s choice. One patient in the weekly paclitaxel group did not receive any treatment. Patients received a median three cycles in each group.
Dose reductions for toxicity of one level were to paclitaxel 65 mg/m2, carboplatin AUC 4 mg/ml/minute, and topotecan 2.4 mg/m2. Dose reductions of two levels were to paclitaxel 5 mg/m2, carboplatin AUC 3.5 mg/ml/minute, and topotecan 2 mg/m2. In cases in which there was a treatment delay of > 2 weeks, patients were discontinued from the study.
The sample size calculation indicated that 165 patients would be required for adequate power to detect a difference among groups with 80% power. Median duration of follow-up was 15 months.
Paclitaxel compared with oxaliplatin
Piccart et al.63 report the results of a multicentre (17 European centres across six countries), open-label, randomised, Phase II study. Patients were enrolled who had histologically or cytologically proven advanced ovarian cancer that had progressed or stabilised after prior treatment, with relapse occurring within 12 months of the last platinum-based chemotherapy regimen. No more than two prior cisplatin- and/or carboplatin-containing chemotherapy regimens were permitted. Patients were also ineligible if they had prior treatment with platinum derivatives other than cisplatin and carboplatin or with paclitaxel, docetaxel, or high-dose chemotherapy with haematopoietic stem cell support.
The primary aim of the trial was to evaluate the clinical effectiveness of oxaliplatin (Eloxatin®, Sanofi) (130 mg/m2 over 2 hours) every 21 days (n = 45) compared with paclitaxel (175 mg/m2 over 3 hours) every 21 days (n = 41).
Patients were assigned to their study group by the EORTC. No details on the method of randomisation are reported in the full publication. Patients were stratified by centre, performance status (0 vs. 1 vs. 2), PFI (0–6 months vs. 6–12 months) and number of prior platinum-based regimens (1 vs. 2). The primary outcome measure was the objective confirmed response rate, which was assigned as per WHO criteria and verified by two independent radiologists. Secondary outcome measures were TTP, OS, time to treatment failure and QoL.
For patients randomised to receive paclitaxel infusion, premedication included oral dexamethasone (20 mg) 12 and 6 hours before infusion, and diphenhydramine (50 mg intravenously) plus cimetidine (300 mg; Tagamet®, GSK) or ranitidine (50 mg intravenously) 30 minutes before the infusion. Antiemetic therapy before oxaliplatin infusion was a serotonin antagonist (5-HT3), with a single dose of corticosteroid (e.g. dexamethasone 20 mg).
Treatment in each group was continued until disease progression, unacceptable toxicity or patient refusal. The initial paclitaxel and oxaliplatin doses could be reduced in subsequent cycles, or the cycles could be delayed by 1 or 2 weeks, depending on toxicity. Dose reduction of below 90 mg/m2 for paclitaxel and 75 mg/m2 oxaliplatin per cycle was not permitted, and patients requiring these or lower doses went off study. The median number of cycles of treatment was six (range 1–8) in the paclitaxel group and four (range 1–8) in the oxaliplatin group. Most patients had a delivered relative dose intensity of at least 95%.
Median duration of follow-up was not reported. A total of five patients were not assessable for response (two in the paclitaxel arm and three in the oxaliplatin arm): four were ineligible because of eligibility deviations and one died 6 days after the first oxaliplatin cycle, as a result of a massive pulmonary thromboembolism (unrelated to treatment).
A sample size calculation was not reported. The authors comment that, despite the use of several centres, as a result of wider use of paclitaxel as a first-line treatment at the time the trial was initiated, accrual of paclitaxel-naive patients became slow in the later stages of the trial. It is unclear whether the trial was adequately powered to detect a difference between treatments.
Topotecan oral compared with topotecan intravenous
Gore et al.24 report the results of a multicentre, international (Europe, South Africa and North America) randomised trial of open-label design that compared topotecan administered orally (2.3 mg/m2 daily for five consecutive days; n = 135) with intravenously (1.5 mg/m2 daily for five consecutive days; n = 131). Both treatment regimens were given on a 21-day cycle. Patients were enrolled who had relapsed epithelial ovarian cancer (histological diagnosis) that was measurable at baseline and was of FIGO stage III or IV (266 patients randomised). To be eligible, patients were also required to have an ECOG score of ≤ 2. Patients had either progressed during or relapsed within 12 months of completing first-line chemotherapy, and only one prior chemotherapy regimen was permitted. Initial treatment must have been platinum based and could have been given in conjunction with a taxane.
The aim of the study was to compare the efficacy, safety and tolerability of oral topotecan compared with standard i.v. topotecan in patients with relapsed ovarian cancer. Randomisation (1 : 1 ratio) was carried out by telephone (no further details reported) and stratified by prior taxane exposure, interval from previous platinum therapy and tumour diameter (< 5 cm vs. ≥ 5 cm). Three categorisations of response to first-line chemotherapy were defined: platinum refractory (PD or SD during initial chemotherapy); platinum resistant (initial response followed by relapse within 6 months); and platinum sensitive (initial response with subsequent relapse at > 6 months).
Outcomes assessed included response rate (as per WHO criteria), time to response, TTP, survival and toxicity. Median follow-up was not stated. Although open label in design, all responses that were claimed to be confirmed or partial were subject to independent, blinded radiological review. The only outcome evaluated for the subgroups categorised by extent of sensitivity to platinum was response rate.
Duration of treatment with topotecan was determined by response to therapy and was at the discretion of the clinician. It was recommended that patients with SD receive a minimum of four cycles of treatment and that patients responding to treatment receive at least two cycles of treatment beyond response. Patients assigned to oral topotecan received a median of four (range 1–23) cycles and those assigned i.v. topotecan received a median of six (range 1–26) cycles. Dose reductions were permitted for grade 3 or 4 AEs, with about 10% of patients in each group requiring a reduction in dose.
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
Sehouli et al.23 report the results of a randomised, multicentre, Phase II trial in Germany involving 194 patients with platinum-resistant recurrent epithelial ovarian or primary peritoneal cancer after radical surgery and at least one platinum-containing chemotherapy. Patients with disease measurable by CT or MRI, or disease evaluable by CA125 according to GCIG criteria, were eligible. Platinum resistance was defined as clinical disease progression after a TFI of < 6 months after a platinum-based regimen. Inclusion criteria with regards to number of previous lines of chemotherapy were not specified.
The primary goal of the trial was to compare weekly administration of topotecan at a dose of 4.0 mg/m2 each week, applied on days 1, 8 and 15 of a 28-day cycle (n = 97) compared with the conventional regimen of 1.25 mg/m2 for five consecutive days (n = 97). The rationale for the trial was that weekly administration of topotecan is considered to be less toxic and is widely used in clinical practice, despite the lack of an evidence base of effectiveness. It should be noted that the dose used in the ‘conventional’ 5-day regimen is lower than the dose recommended in the Summary of Product Characteristics (SmPC) for topotecan (1.5 mg/m2).
Randomisation was central with permutated blocks in a 1 : 1 ratio and was carried out by phone and facsimile. However, the level of masking in the trial is unclear. The primary outcome evaluated was the clinical benefit rate, which was defined as the composite of CR, PR and SD. Response was determined according to RECIST for measurable disease or GCIG criteria for serum CA125 levels. Use of the CA125 marker or scans to evaluate response was at investigators’ discretion, with all responses confirmed by a second examination. Secondary end points were toxicity, PFS and OS; QoL was also explored. All analyses were based on the ITT principle. No sample size calculation was reported but it is stated that the study was not powered for a direct comparison between the dosing schedules or to reveal differences in response rates.
Treatment in each group was continued until intolerable toxicity or disease progression or until the patient refused further therapy, with maximum treatment duration of 12 months. Dose of topotecan could be reduced by 25% for any grade 3 or 4 adverse effects according to the NCI-CTC.
Median follow-up was 23.4 months (range 12.7–41.4 months). Patients in the weekly topotecan group received statistically significantly fewer cycles of chemotherapy than the group receiving topotecan at the conventional dosing regimen (3.5 with weekly topotecan vs. 4.8 with conventional topotecan; p = 0.002).
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
Omura et al.68 conducted a Phase III, randomised, multicentre trial comparing two doses of paclitaxel (250 mg/m2 vs. 175 mg/m2) involving patients with recurrent or persistent histologically confirmed epithelial ovarian cancer despite prior platinum therapy. A third group, paclitaxel 135 mg/m2, was closed early because of inadequate patient accrual. Eligible patients had received not more than one prior platinum-based regimen, had adequate bone marrow, kidney and liver function; and a Gynecologic Oncology Group (GOG) performance status of 0, 1 or 2.
The aim of the trial was to evaluate whether increasing dose of paclitaxel was associated with an increase in response. The primary outcome measures were PFS and OS. Objective response (CR or PR) rates were recorded in patients with measurable disease (pleural effusion or elevated CA125 level were not regarded as measurable disease). The study also assessed whether prophylactic filgrastim 10 µg/kg was more effective than filgrastim 5 µg/kg at reducing the incidence of febrile neutropenia in patients receiving paclitaxel 250 mg/m2. The TAG considers that the administration of filgrastim is unlikely to influence comparative clinical effectiveness.
Sequential, permuted block randomisation was used to assign patients to paclitaxel 175 or 250 mg/m2 by 24-hour i.v. infusion every 3 weeks. Both treatments were administered for a minimum of six cycles. Patients could continue treatment indefinitely if there was no clinical progression or excessive toxicity after six cycles. Paclitaxel dose intensity could be reduced for some grade 3 or greater toxicities (not otherwise specified). Patients experiencing neutropenic fever while receiving paclitaxel 175 mg/m2 were allowed filgrastim during subsequent therapy cycles.
Based on the sample size calculation, it was estimated that 540 patients, followed until approximately 80% had died, would provide an 80% chance of detecting a true HR of 1.4 between paclitaxel 135 mg/m2 and either of the more intense regimens (type I error p = 0.025 for one-tailed test). However, the study failed to enrol sufficient patients in the paclitaxel 135 mg/m2 arm and a decision was made to ‘allocate all of the type I error to the comparison of the two higher-dose regimens’. Initially designed to evaluate effects of the two paclitaxel regimens in platinum-resistant clinically measurable disease, owing to slow accrual, after commencement of the trial, the eligibility criteria were expanded to include patients with platinum-sensitive disease and without clinically measurable disease.
Of the 184 women randomly assigned to paclitaxel 175 mg/m2 and the 188 to paclitaxel 250 mg/m2, 164 (89%) and 166 (88%), respectively, were eligible. Ten eligible women (three in the paclitaxel 175 mg/m2 group and seven in the paclitaxel 250 mg/m2 group) were not assessed for tumour response because of death, toxicity or withdrawal but were classified as not responding for an ITT analysis among eligible patients. The primary survival outcomes were restricted to eligible patients.
Median duration of follow-up is not reported. The proportion of women receiving six or more cycles of therapy was similar between the two groups, with 58% and 55% of patients in the paclitaxel 175 mg/m2 and paclitaxel 250 mg/m2 group, respectively, receiving six or more cycles. One patient refused to take any dose of the allocated treatment.
Paclitaxel weekly compared with paclitaxel every 3 weeks
Rosenberg et al.60 report the results of a randomised bifactorial multicentre study carried out at sites in Sweden and Finland. The aim of the study was to assess the efficacy and toxicity of paclitaxel given at the same dose intensity administered either weekly or every 21 days. Patients were randomised to paclitaxel 67 mg/m2 every 7 days or paclitaxel 200 mg/m2 every 21 days. Enrolled patients (n = 208) had advanced ovarian cancer (histologically proven) that had progressed during or relapsed after administration of a platinum-based regimen. To be eligible, patients had to have measurable disease that had been documented clinically and/or radiologically. Only one prior platinum-containing regimen was permitted. In addition, all patients were taxane naive.
The RCT was of a bifactorial design. In addition to randomisation to either paclitaxel weekly or every 21 days, patients were also randomised to oral dexamethasone (20 mg) taken 12 hours and 6 hours before paclitaxel infusion or administration of i.v. dexamethasone (20 mg) 30 minutes before paclitaxel infusion. Results in the full publication cited here focus on treatment with paclitaxel. Premedication with clemastine 2 mg (Tavegel®, Novartis) and cimetidine 300 mg (or ranitidine 50 mg) was given intravenously to all patients 30 minutes prior to paclitaxel infusion.
The primary endpoint of the study was clinical response rate as per WHO criteria, with TTP and OS evaluated as secondary outcomes. Randomisation was reported to have been carried out at the BMS office in Stockholm and patients were randomised in a 1 : 1 ratio. Patients were stratified by platinum resistance, with a differentiation at 6 months (randomisation strata: relapse at ≤ 6 months vs. > 6 months after primary platinum-based treatment). No further details on the method of randomisation are reported. The level of masking in the trial is unclear.
Median duration of follow-up was 27 months (range 7–47+ months). Patients to whom paclitaxel was administered weekly at a dose of 67 mg/m2 received a median of 5.7 courses of treatment (range 1–16 courses) compared with a median of seven courses in the group receiving paclitaxel 200 mg/m2 every 21 days (range 1–17 courses). More patients in the paclitaxel weekly arm (32 vs. 20) were taken off the study early (within 9 weeks) owing to either early progression or for administrative reasons. The difference in early progressions could be because of a low initial weekly dose or some patients may have had a more aggressive tumour biology.
The sample size calculation estimated that 318 patients would be required to detect the prespecified relative difference between groups of 54% with 80% power. To ensure a sufficient number of evaluable patients, it had been planned to recruit a total of 350 patients. Owing to slow recruitment of taxane-naive patients with recurrent disease, the study closed early after inclusion of 208 patients. The study may therefore have been underpowered to detect a difference between the two regimens.
Quality assessment of studies included in clinical effectiveness review
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
The trial carried out by Bafaloukos et al.29 is generally well designed with the primary analysis based on the ITT population. However, limited details on trial methodology are provided in the full publication. Randomisation is reported to have been carried out at the central HeCOG Data Office in Athens but a description of the method of randomisation is not reported. The level of masking within the trial is unclear. The primary outcome is response rate, which is determined by radiological scan or CA125 level. Assessment of response is associated with disparity in interpretation of scan results, both across different assessors and within categorisation (CR or PR) by an individual assessor. It is unclear whether radiological scans were evaluated by an independent review panel. In addition, TTP was measured from date of treatment initiation rather than date of randomisation, which is a more commonly used definition for TTP in clinical trials. The evaluation of the quality of the trial is presented in Table 9.
TABLE 9
Summary of quality assessments of studies included in review of clinical effectiveness
The CALYPSO trial31 is a well-designed and well-conducted trial. Progression and response were reviewed independently. Although the methods indicate that analyses are based on the ITT principle, three randomised patients (one in the PLDH plus carboplatin group and two in the paclitaxel plus carboplatin group) were judged to be ineligible because of absence of evidence of ovarian cancer post randomisation and were excluded from analyses of clinical effectiveness. Thus, the analyses are not strict ITT analyses. The evaluation of the quality of the trial is presented in Table 9.
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with carboplatin alone
The Alberts et al.28 trial seems to be generally well designed, although limited details on the methodology of the trial are provided in the full publication. The method of randomisation and level of masking are unclear. As the primary outcome is OS, masking, or lack of masking, is unlikely to introduce bias into the evaluation of treatment effect. The key issue associated with trial design is that the study is likely to have been underpowered as a result of early closure owing to slow patient accrual (61 patients recruited out of a planned 900 patients). The authors identify several factors that could have contributed to slow accrual, including dissolution of the SWOG Gynecologic Cancer Committee after initiation of the trial and publication of results from the larger ICON4/AGO-OVAR 2.2 trial.61 The evaluation of the quality of the trial is presented in Table 9.
Trabectedin plus pegylated liposomal doxorubicin hydrochloride compared with pegylated liposomal doxorubicin hydrochloride alone
The OVA-301 trial30 was a well-conducted trial. Methodologically, the design of the trial was robust, with clinical effectiveness analyses based on the ITT population, and progression and response reviewed by an independent radiologist who was masked to treatment allocation. A secondary analysis of the primary outcome of PFS was carried out based on review by an independent oncologist (radiological assessment in conjunction with prespecified clinical data) who was also masked to treatment allocation. The methods of the trial are well reported. As noted in the Final Appraisal Determination (FAD) for the assessment of trabectedin plus PLDH as part of the Technology Appraisal process (TA222),73 one potential area that affects the external validity of the trial is the omission of a platinum-based chemotherapy as a comparator, particularly as a large proportion of patients enrolled had platinum-sensitive disease. The authors commented that the inclusion of platinum-resistant patients contributed to the decision against use of a platinum-based control, as platinum-based therapy would have been inappropriate in this setting. The evaluation of the quality of the trial is presented in Table 9.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
The trial carried out by Gordon et al.49 was generally a well-designed trial. Although open label in design, scans for assessment of disease response and progression underwent independent radiological review. Although the methods state that analyses are based on the ITT principle, in the first publication, results are based on patients who received at least a partial dose of study drug (474 patients out of 481 randomised), which is a modified ITT analysis. However, in the publication describing longer-term follow-up of OS, analysis of OS is based on the ‘all randomised’ population and, as such, is a true ITT analysis. The evaluation of the quality of the trial is presented in Table 9.
Pegylated liposomal doxorubicin hydrochloride compared with paclitaxel
Technology appraisal no. 9113 reports that the study carried out by Schering-Plough (Trial 30–57) was a reasonably good-quality, randomised, open-label comparative trial. The key issue noted was that approximately 50% of the planned number of patients were recruited (216 recruited out of planned 438 patients). It is therefore likely that the trial is underpowered to detect a difference between PLDH and paclitaxel in treatment effect. TA91 also notes that the results of the trial ‘are likely to be preliminary and the longer term implications of any differences observed in the treatment effect at the time of data analysis are unclear’. The evaluation of the quality of the trial is presented in Table 9.
Topotecan compared with paclitaxel
A key strength of the trial evaluating topotecan compared with paclitaxel (ten Bokkel Huinink et al.21) is that, for the primary outcome of response rate, all claimed responses were evaluated by an independent radiologist who was masked to treatment allocation. As a sample size calculation was not reported, there is uncertainty whether the trial was adequately powered to detect a difference between treatments. Furthermore, results are not based on the ITT principle, with only patients who received at least one dose of study drug being included in the final analysis. The trial design allowed patients to cross over to the alternative treatment should they fail to respond to their allocated treatment. The switch in treatment during the trial generates confounding in the final analysis of OS. The evaluation of the quality of the trial is presented in Table 9.
Gemcitabine plus carboplatin compared with carboplatin alone
The trial carried out by Pfisterer et al.50 is generally a well-designed and well-conducted trial, with efficacy analyses based on the ITT principle. With PFS as a primary outcome and an open-label design, there is potential for bias. It is unclear from the full publication whether radiological assessment of progression was reviewed by an independent panel. The evaluation of the quality of the trial is presented in Table 9.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
ICON4 and AGO-OVAR 2.2 are well-conducted parallel trials.61 Comprehensive details on most aspects of trial methodology are provided in the full publication.61 The level of masking is unclear but OS is the primary outcome and therefore awareness of treatment allocation is unlikely to influence results of this outcome. Analyses of clinical effectiveness are based on the ITT population. The evaluation of the quality of the trial is presented in Table 9.
The trial carried out by Gonzalez-Martin et al.48 was a Phase II trial of a ‘pick-the-winner’ design, which the authors state has a ‘90% chance of selecting the better treatment if the difference is at least 15% and the smaller response rate is assumed to be 30%’. Therefore, no sample size calculation was carried out. A ‘pick-the-winner’ trial is designed as a screening trial to facilitate a selection between promising experimental regimens in a Phase II setting, and, as such, do not typically include the standard of care. Trials with a ‘pick the winner’ design are underpowered for hypothesis testing or comparisons of treatment effect on the outcomes of interest, such as survival.74 Therefore, as the authors comment, all reported statistical analyses are exploratory and reported p-values should be interpreted with caution. Limited details on trial methodology are reported and the level of masking in the trial is unclear. Although it is reported that randomisation was carried out in a central data centre, the method of randomisation is not described. The evaluation of the quality of the trial is presented in Table 9.
Paclitaxel plus carboplatin compared with paclitaxel alone
Limited details of the methodology of the CARTAXHY trial62 are available in the publication presenting the results of the trial. A key strength of the trial is that clinical efficacy analyses were based on the ITT principle. Although it is stated that the study is randomised, details on the method of randomisation are not reported. As an open-label trial, there is potential for bias in the assessment of progression and response. It is unclear whether radiological scans underwent independent radiological review. The evaluation of the quality of the trial is presented in Table 9.
Paclitaxel compared oxaliplatin
The trial reported by Piccart et al.63 is generally a well-designed trial. The primary outcome was objective confirmed response. As an open-label design, the outcome of confirmed response could potentially be open to bias. The descriptions of the methods states that response was verified by two independent radiologists. However, it is unclear whether the independent radiologists were truly independent and masked to treatment allocation. Although limited details are reported on the method of randomisation, it is reported that the treatment allocation was assigned by the EORTC. The key issue associated with the trial is the uncertainty around the power of the trial. The evaluation of the quality of the trial is presented in Table 9.
Topotecan oral compared with topotecan intravenous
The trial reported by Gore et al.24 is generally well designed, with analysis based on the ITT population. In addition, although open label in design, assessments of CR and PR were validated by masked independent radiological review.
It is stated that randomisation was carried out by telephone but no details on the method of randomisation are reported. No power calculation is reported and thus it is unclear whether the study is adequately powered. The population is clinically homogeneous in that all patients randomised had measurable disease at baseline and also had received only one prior chemotherapeutic treatment. The evaluation of the quality of the trial is presented in Table 9.
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
There are several factors that impact on the quality of the design and conduct of the trial carried out by Sehouli et al.23 Although it is stated that all analyses are carried out based on the ITT principle, the analysis of clinical benefit does not include all patients randomised. There is no discussion of the omission of patients from this analysis. The dose used for the ‘conventional’ regimen for topotecan is lower than that recommended in the SmPC. The authors comment that the reduced dose is widely accepted by many international cancer societies but go on to highlight that there are no RCTs evaluating the comparative effectiveness of 1.25 mg/m2 compared with 1.5 mg/m2 of topotecan. In addition, use of radiological scans or CA125 level to determine response was at the discretion of the investigator. It is widely accepted that CA125 level is not sufficient to confirm response to treatment. Examination of the results for response indicates that a large proportion of patients were evaluated by CA125 level alone (80.1%). Moreover, it is unclear whether the investigator was masked to treatment allocation. Although responses had to be confirmed by a second examination, it is unclear whether response was confirmed by the same investigator or by independent review. The trial was not adequately powered to detect a difference between groups. These factors potentially limit the comparison of the results from this trial with similar trials in ovarian cancer. The evaluation of the quality of the trial is presented in Table 9.
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
Limited methodological details were reported in Omura et al.68 The method of randomisation was robust, with treatment regimens sequentially assigned from stratified, permuted blocks. The level of masking in the trial is unclear. Although the methods state that the analyses are based on the ITT principle, patients identified post randomisation to be ineligible for participation in the trial were excluded from all analyses, and, therefore, analyses are not based on the ITT population. A key issue with the trial is the sample size, with only 265 patients recruited from a planned 540, even after expansion of the protocol to include platinum-sensitive patients and those with measurable disease. Thus, the study is likely to be underpowered to detect a true difference between the treatment regimens for which results are reported.
Paclitaxel weekly compared with paclitaxel every 3 weeks
The trial carried out by Rosenberg et al.60 is of reasonable quality. Efficacy analyses are based on the ITT principle. Limited details are reported on trial methodology in terms of method of randomisation and level of masking. The key issue with the trial is that it is potentially underpowered to detect a difference in the primary outcome of response rate between the paclitaxel regimens evaluated. The evaluation of the quality of the trial is presented in Table 9.
Comparability of baseline characteristics
Within most of the trials identified, the treatment groups were well matched in terms of population baseline characteristics, including age, TFI, the number of previous chemotherapy agents received, disease measurability (for those trials including patients with measurable and non-measurable disease), and performance status. Differences between groups that were reported to be significant are described below; imbalances that were reported to be non-significant or for which the significance of the difference was not assessed in the trial are not discussed. Detailed baseline characteristics of the individual trials are available in the data abstraction forms presented in Appendix 2.
An unanticipated imbalance in PFI was noted in a retrospective analysis of OVA-301.30 Patients in the PLDH monotherapy group had a significantly longer mean PFI than patients in the trabectedin plus PLDH group (mean PFI: 13.3 months with PLDH alone vs. 10.6 months with trabectedin plus PLDH; p = 0.009). Longer PFI is correlated with increased likelihood of response to treatment. Therefore, the potential direction of bias in analysis of treatment effect is against trabectedin plus PLDH. To account for this imbalance, the authors carried out additional exploratory analyses based on PFI as a continuous covariate (discussed in Chapter 4, Trabectedin for the treatment of patients with relapsed platinum-sensitive ovarian cancer). The analyses were not prespecified and as such were hypothesis generating.
Baseline characteristics of key prognostic factors (based on expert advice) are summarised in Table 10. Also, based on expert advice, the TAG has focused on the subgroups of platinum-sensitive ovarian cancer and PRR ovarian cancer rather than the full trial population. Baseline characteristics are considered in terms of comparability within platinum-sensitive patients and PRR patients.
Considering patients with platinum-sensitive disease, a potential source of heterogeneity within the trials is the proportion of patients with FPS (relapse at > 12 months after last platinum-based treatment) ovarian cancer compared with PPS (relapse at ≥ 6–12 months after last platinum-based treatment) at baseline. The greater the duration of PFI, the more favourable the prognosis. In trials involving patients with only platinum-sensitive disease,28,29,31,48,50,61 the proportion of patients with PPS ovarian cancer ranges from 28.6% to 43.0%. Considering the large trial ICON4/AGO-OVAR 2.2,61 the proportion of patients with PPS compared with FPS is 74.7% and 25.3%, respectively. ICON4/AGO-OVAR 2.261 has been reported to have longer median PFS and OS for both groups compared with other trials involving platinum-sensitive patients, which is thought to be attributable to the comparatively larger proportion of patients with FPS ovarian cancer who have an improved prognosis compared with those who are PPS. Given that the NMA is based on relative treatment effects (HR), and that most trials are well balanced between groups in FPS ovarian cancer compared with PPS, the TAG considered the trials sufficiently clinically homogeneous to compare treatments in a NMA.
Number of prior lines of chemotherapy is another source of potential heterogeneity. Increasing number of previous chemotherapy regimens is associated with a decrease in response to treatment. Of the 16 trials identified, seven included patients who had received two or more prior lines of chemotherapeutic treatment. In trials involving only patients with platinum-sensitive disease, the proportion of patients with more than one line of prior chemotherapy in each trial is generally small, ranging from 4% to 15.5%. By contrast, as could be expected, in trials involving patients with PRR ovarian cancer, the proportion of patients with two or more chemotherapy regimens is larger, at about 30% in all trials. In all trials, the number of patients with multiple lines of prior chemotherapy is well balanced within the trial. It is possible that inclusion of trials in which patients received two or more chemotherapy regimens is likely to underestimate the effects of the evaluated treatments in patients with first recurrence of disease, and thus potentially bias the results of an indirect comparison towards treatments that are given as second line. Again, as the HR used in the NMA is a relative treatment effect, the impact of these trials on the overall result could be minimal.
Scales evaluating performance status are used to assess disease progression and how a patient’s daily living abilities are affected by their disease. On the ECOG scoring system (also referred to as the Zubrod or WHO score), the lower a patient’s performance score, the greater their capacity for physical activity; a score of ‘0’ or ‘1’ indicates that the patient is ambulatory. In the Karnofsky scale, which scores from ‘100 to 0’, higher performance score is favourable: a score of > 80 indicates that a patient is able to carry on normal activity and to work with no special care required. Good performance status has been shown to be an important prognostic factor in several types of cancer.75
In the identified trials, the proportion of patients with unfavourable baseline performance score (ECOG/Zubrod/WHO score of ≥ 2; Karnofsky score of < 80) is small, ranging from 0% to 16% across the trials. Including patients with less favourable performance scores is likely to underestimate the effect of the treatments. For example, in Gonzalez-Martin et al.,48 the 12.3% increase in proportion of people with ECOG score 2 in the platinum treatment group may limit the benefit received by people receiving platinum monotherapy when compared with paclitaxel plus platinum (i.e. paclitaxel plus platinum may have less benefit over platinum monotherapy). In addition, in Rosenberg et al.60 the 9% increase in WHO score 2 in the three-weekly paclitaxel group may limit the benefit received by three-weekly paclitaxel monotherapy compared with weekly paclitaxel monotherapy (i.e. the benefit of paclitaxel weekly may have less benefit over three-weekly paclitaxel). However, in those trials that include patients with a less favourable performance score, the proportion of patients in each treatment group is well balanced and thus the impact on the overall result could be minimal.
Diagnosis of recurrent disease based on raised CA125 levels alone has been found to predate evidence of disease progression from clinical examinations or radiological scans by a median of 4 months in 70% of patients with ovarian cancer.76 Thus, there is uncertainty whether patients diagnosed as having recurrent disease by only CA125 level would have the same diagnosis on radiological scan. In addition, it is also possible that the degree of sensitivity to platinum could differ. For example, based on CA125 level alone, a patient could be categorised as PPS at baseline but as FPS 4 months later with radiological confirmation. Of the trials identified, seven RCTs23,28,29,31,48,62,68 reported that patients with only CA125 level as an indicator of recurrent disease were enrolled. In trials in patients with platinum-sensitive disease, there was considerable variation across the trials in the proportion of patients with non-measurable disease at baseline, ranging from 8.5% to 38.2%. In some trials, patients with non-measurable disease were not included in analyses of response rate. Despite the identified disparity in methods used to diagnose recurrent disease at baseline, as the proportion of patients in each group within the individual trials was well balanced, the TAG considered that the heterogeneity could have a minimal impact on the NMA.
Considering heterogeneity among treatments evaluated, it is important to note that ICON461 evaluated the efficacy of adding paclitaxel to ‘conventional’ platinum-based chemotherapy compared with platinum-based therapy alone. A large proportion of patients in each treatment group received carboplatin as the platinum component of their regimen (80% in the paclitaxel plus platinum-based therapy group vs. 29% in the platinum-based chemotherapy alone group). Of the remaining 20% of patients in the paclitaxel plus platinum group, 10% were administered cisplatin, and 5% received paclitaxel plus carboplatin or cisplatin, switching between the two platinum monotherapies. In the conventional platinum-based monotherapy group, 4% of patients received cisplatin alone, and a further 2% received either carboplatin or cisplatin monotherapy, switching between the two platinum monotherapies. Moreover, 17% of patients in the conventional chemotherapy group received the triple therapy of cyclophosphamide, doxorubicin and cisplatin, which the ICON investigators had compared against carboplatin in an earlier trial and found no statistically significant difference between the treatments in effect on OS.61 Although a small proportion of patients received platinum treatment other than carboplatin, there is evidence that the regimens received have similar efficacy.
Although differences in key prognostic factors across the trials have been identified, when considering the trials that would inform the NMA for platinum-sensitive disease and for PRR disease, the TAG considers the trials sufficiently clinically homogeneous to compare clinical effectiveness of treatments.
Assessment of effectiveness
Based on clinical expert advice, the TAG has focused on the clinical effectiveness of interventions in populations defined by degree of platinum sensitivity [i.e. platinum sensitive (i.e. recurrence ≥ 6 months after last platinum-based treatment) and platinum resistant (i.e. recurrence < 6 months after last platinum-based treatment) or refractory (progression during platinum-based treatment)]. When it was not possible to extract data for the prespecified populations, for completeness, the TAG presents data for the full population of the study.
Overall survival
Overall survival is universally accepted as a measure of benefit in trials evaluating treatments for cancer, and is generally considered to be the most reliable end point.77 However, the large number of patients required to ensure adequate power to detect a difference between treatments and long follow-up periods can hinder the collection and analysis of survival data. The FDA and other regulatory authorities define OS as the time from randomisation until death from any cause.77 It should be noted that some of the trials reported here define OS as the time from administration of first cycle of study drug until death from any cause. As the event recorded is all-cause mortality, there is no bias associated with measurement of the end point.
A potential area of confounding with measurement of OS derives from the use of post-progression therapies. It has been proposed that subsequent lines of therapy are likely to be more effective in the less clinically effective group than in the treatment group, and is more likely to be considered when there is no significant difference in OS between the treatment and the control. Confounding from post-progression therapy is most likely to be an issue in trials in which most patients cross over to the alternative group after progression or in trials in which the ‘new’ therapy is available as a post-progression treatment in the control group.78
Summary of results for overall survival
Most trials identified reported results for the outcome of OS. No trial was identified evaluating treatments in a population solely comprising patients who were allergic or intolerant to platinum-based chemotherapy. Here, results for patients with platinum-sensitive or PRR disease are summarised. For trials not limited to either platinum-sensitive or PRR patients (i.e. includes a mix of PFI), results for the full trial population are presented in the main body of the text.
Results for overall survival for the subgroup of patients with platinum-sensitive (relapse at ≥ 6 months after last platinum-based chemotherapy) ovarian cancer
Ten RCTs evaluating eight different head-to-head comparisons of interventions and comparators of interest were identified (Table 11).
TABLE 11
Overall survival for patients with platinum-sensitive ovarian cancer
To inform the decision problem, a NMA was carried out. Based on trials identified, it was not possible to construct a complete network. Two discrete networks were generated: one evaluating platinum-based therapies and the second comparing non-platinum-based regimens. It should be stressed that results from the two discrete networks are not directly comparable.
In the network evaluating platinum-based chemotherapies, PLDH plus carboplatin and paclitaxel plus carboplatin were found to significantly improve OS compared with platinum monotherapy (Table 12). However, no statistically significant differences in OS were identified between the remaining treatments considered in the network.
TABLE 12
Results from NMA for OS of platinum-based chemotherapies
Analysis of non-platinum-based regimens indicates that PLDH monotherapy and trabectedin plus PLDH are both significantly more effective at prolonging OS than topotecan monotherapy (Table 13). No other significant OS differences were identified.
TABLE 13
Results from NMA for OS of non-platinum-based chemotherapies
Platinum-free interval is a prognostic factor for response. To investigate any potential differences in clinical efficacy between treatments with PFI, when data were available, OS was analysed for the subgroups of patients with FPS (relapse at > 12 months after last platinum-based treatment) and PPS (relapse at ≥ 6 to ≤ 12 months after last platinum-based treatment) ovarian cancer. Few trials involving platinum-sensitive patients evaluated treatment effect in these two subgroups: four trials54,56,61,64 afforded data on both FPS and PPS ovarian cancer; two trials56,61 evaluated platinum-based regimens and two trials54,64 non-platinum-based regimens.
Results in patients with fully platinum-sensitive ovarian cancer
Three of the four trials15,54,56 reported a HR as a measure of treatment effect (Table 14). The difference between treatment groups was not statistically significant in any trial. The fourth trial61 did not report a HR, but the proportion of people having an event was similar in each treatment group.
TABLE 14
Overall survival for the subgroup of patients with FPS ovarian cancer
HR for OS was not available from ICON4/AGO-OVAR 2.261 and so it was not possible to carry out a NMA.
Results in patients with partial platinum sensitivity
In patients with PPS ovarian cancer, PLDH monotherapy has been found to significantly prolong OS compared with topotecan (Table 15). Furthermore, trabectedin plus PLDH has been found to be significantly more effective than PLDH alone at increasing OS. The trial comparing platinum-based regimens did not report a HR for OS in this subgroup of patients. However, a similar proportion of patients in each group had had an event at the time of analysis.
TABLE 15
Overall survival for the subgroup of patients with PPS ovarian cancer
The results of the NMA are in agreement with the results of the individual trials (Table 16). Trabectedin plus PLDH was found to be significantly more effective at increasing OS than PLDH monotherapy and topotecan monotherapy. The difference between PLDH monotherapy and topotecan monotherapy remained significant and favoured PLDH monotherapy.
TABLE 16
Results from NMA for OS in patients with PPS ovarian cancer
Results in overall survival for the subgroup of patients with platinum-resistant/-refractory ovarian cancer
Platinum-resistant disease has been defined as disease that initially responds followed by relapse at < 6 months after last platinum-based chemotherapy. Platinum-refractory indicates disease does not respond to or progresses during first-line platinum-based chemotherapy.
Five RCTs13,23,52,54,62 reporting results for five different head-to-head comparisons involving PRR patients were identified (Table 17). Two RCTs enrolled only patients with PRR, with the remaining three RCTs reporting results from a subgroup of patients within the trial. None of the trials identified a significant difference in OS between the two treatment groups evaluated.
TABLE 17
Overall survival for the subgroup of patients with PRR ovarian cancer
Four of the five identified trials were included in the network13,23,52,54 (Table 18); the treatment regimens evaluated in the trial reported by Lortholary et al.62 did not inform the network. Trabectedin plus PLDH is outside of the scope for this review for the population of PRR patients; data have been included within the network to capture all the available evidence but are not included in the economic analysis. The results of the NMA are in alignment with the results of the individual trials, with no statistically significant differences in OS among the treatments evaluated.
TABLE 18
Results from NMA for OS in patients with PRR ovarian cancer
Platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
In the trial carried out by Bafaloukos et al.29 OS was calculated from the initiation of treatment until the date of last follow-up or the patient’s death. Analysis of OS was carried out on the ITT population when 122 patients were known to have died. It is important to note that the study was not powered to detect differences in OS. Median OS was 24.7 months in the PLDH plus carboplatin group and 29.4 months in the paclitaxel plus carboplatin group, with no statistically significant difference between the groups (HR 1.15, 95% CI 0.78 to 1.66; p = 0.455; see Table 23). The proportion of patients receiving post-progression therapy was similar between the groups [61/93 (65.6%) patients in the PLDH plus carboplatin group vs. 61/96 (63.5%) patients in the paclitaxel plus carboplatin group].
TABLE 23
Summary of results for OS in the platinum-sensitive recurrent ovarian cancer
The authors carried out a univariate and multivariate analysis based on the Cox proportional hazards model to evaluate the influence of prespecified prognostic factors on survival. Results indicated that performance status score of zero and longer PFI (> 12 months) were important independent prognostic factors for survival (Table 19).
TABLE 19
Results from analysis of influence of proposed prognostic factors on OS by baseline characteristics
Wagner et al.56 report mature OS data from CALYPSO.31 Based on a median follow-up of 49 months (range 0–68 months) and a total of 663 deaths, median OS was 30.7 months in the PLDH plus carboplatin group and 33.0 months in the paclitaxel plus carboplatin group. The accompanying HR of 0.99 (95% CI 0.85 to 1.16; p = 0.94; see Table 23) indicates that there was no statistically significant difference between treatments in OS; HR reported is for paclitaxel plus carboplatin compared with PLDH plus carboplatin. It should be noted that OS was not defined. Analysis of crossover treatment identified an imbalance between treatment groups, with a significantly larger proportion of patients randomised to paclitaxel plus carboplatin receiving PLDH (68%) compared with the alternative scenario of patients randomised to PLDH plus carboplatin receiving subsequent paclitaxel (43%; p < 0.001).
In a multivariate analysis, TFI of ≥ 12 months, ECOG performance status of 0, CA125 level of < 100 U/ml, non-measurable disease and one involved disease site were identified as factors significantly correlated with OS (Table 20).
TABLE 20
Results from univariate and multivariate analysis of influence of OS by baseline characteristics. Reprinted with permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: British Journal of Cancer 107(4), Copyright (2012)
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with carboplatin alone
Data from Alberts et al.28 were immature in terms of OS (based on data for 32 patients who had died). Longer-term data (evaluating 50 patients who had died) reported by Markman et al.55 found a median OS of 31 months in the PLDH plus carboplatin group and 18 months in the carboplatin alone group, giving a median OS gain of 8 months with PLDH plus carboplatin (p = 0.20). Markman et al.55 did not report the HR for the comparison between groups. Using the methods presented by Tierney et al.,79 the TAG calculated a HR of 0.70 (95% CI 0.40 to 1.21; see Table 23), for which HR < 1 favours PLDH plus carboplatin.
Trabectedin plus PLDH compared with PLDH alone
At the time of first publication of analysis of PFS from OVA-301,30 OS data were immature. Final OS analysis was reported in a follow-up study,64 in which OS analysis was based on 522 events (analysis planned once 520 deaths had occurred). Various subgroup analyses of OS are reported, including platinum-sensitive disease compared with platinum-resistant disease.
In the subgroup of patients with platinum-sensitive disease (relapse at > 6 months after last platinum-based treatment), of 430 patients randomised, 316 had died (156 in the trabectedin plus PLDH group vs. 160 in the PLDH alone group). Median OS in the trabectedin plus PLDH group was 27.0 months compared with 24.1 months in the PLDH alone group. The difference between groups did not reach statistical significance (HR 0.83, 95% CI 0.67 to 1.04; p = 0.106; see Table 23).
Observation of an unexpected, and statistically significant, difference in mean baseline PFI that favoured the PLDH group prompted the authors to carry out a post hoc analysis based on three categorisations of PFI (6 months vs. 6–12 months vs. > 12 months). The analysis suggested that patients with a longer PFI have longer OS, with median OS in each category of:
- < 6 months PFI: 13.6 months (95% CI 11.7 to 14.8)
- 6–12 months PFI: 20.3 months (95% CI 17.7 to 21.7)
- > 12 months PFI: 32.5 months (95% CI 28.4 to 38.5).
It should be noted that the analysis carried out (log-rank) stratified by dichotomous PFI and could not account for the observed imbalance between treatment groups in baseline PFI.
In the MS, PharmaMar present the results of a multivariate analysis Cox regression performed to provide a result for treatment effect adjusting for prespecified key prognostic factors (including PFI). The HR for OS from this analysis for the platinum-sensitive population was 0.78 (95% CI 0.62 to 0.98; p = 0.0319; taken from the PharmaMar submission), which suggests a 22% reduction for death in patients randomised to trabectedin plus PLDH. In this analysis, median OS was 28.4 months with trabectedin plus PLDH compared with 24.1 months with PLDH monotherapy. As noted earlier, as a result of variation in the reporting of adjusted and unadjusted HRs, the TAG has used unadjusted HRs in the NMA.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
Data for OS for patients with platinum-sensitive disease from Gordon et al.49 are based on 46% of the full trial population. OS results are based on a modified ITT population, and OS was defined as the time from the start of study drug administration to death. In the longer-term follow-up study (Gordon et al.54), for the full population, OS results are also reported based on the ITT population and the more commonly used definition of OS of time from date of randomisation until date of death (presented in Interpreting the results from clinical trials, Clinical effectiveness). At the time of analysis, 87% of patients had died and 13% of observations were censored.
In platinum-sensitive patients, Gordon et al.54 found a median OS of 107.9 weeks in the PLDH group compared with 70.1 weeks in the topotecan group. The difference between groups was statistically significant and favoured PLDH (HR 1.432, 95% CI 1.066 to 1.923; p = 0.017; see Table 23); in this analysis, HR of > 1 favours PLDH. The gain in OS corresponded to a 30% reduction in the risk of death for patients treated with PLDH. Survival rates at 1, 2 and 3 years are presented in Table 21. Results for PPS, FPS and PRR patients are discussed in subsequent sections.
TABLE 21
Survival rates in platinum-sensitive patients in PLDH and topotecan groups
Pegylated liposomal doxorubicin hydrochloride compared with paclitaxel
Trial 30–5713 evaluated OS as the primary outcome. TA9113 presents results for the subgroup of patients with platinum-sensitive disease (44 patients in the PLDH group vs. 41 patients in the paclitaxel group). Median OS was 65.4 weeks (range 3.9–263.7+ weeks) with PLDH and 57.0 weeks (range 14–172.3 weeks) with paclitaxel. The corresponding HR of 1.051 (95% CI 0.663 to 1.667; see Table 23) indicates that the difference between treatments is not statistically significant; HR of > 1 favours PLDH.
Topotecan compared with paclitaxel
ten Bokkel Huinink et al.21 defined OS as time from initial drug administration to death. Analysis of OS for the subgroup of patients with platinum-sensitive (late relapse) disease is not reported in either publication by ten Bokkel Huinink et al.21,52 reporting OS data. TA9113 found no statistically significant difference between topotecan and paclitaxel in OS, reporting an unadjusted HR of 1.010 (95% CI 0.663 to 1.541; see Table 23) in platinum-sensitive patients, for which HR < 1 favours topotecan. It should be noted that interpretation of OS results are potentially confounded by the permitted crossover to the alternative treatment should a patient not respond to their allocated treatment. In the full population, 43.8% (49/112) and 53.5% (61/114) in the topotecan and paclitaxel groups, respectively, crossed over to the alternative treatment during the trial.
Gemcitabine plus carboplatin compared with carboplatin alone
In the trial carried out by Pfisterer et al.,50 OS was measured from the date of randomisation to the date of death from any cause. It should be noted that the trial was not powered to detect a difference between treatments in OS. At the time of analysis, 71% of patients had died. The RCT found no statistically significant difference between gemcitabine plus carboplatin and carboplatin alone in median OS (HR 0.96, 95% CI 0.75 to 1.23; p = 0.7349). Median OS was 18.0 months in the gemcitabine plus carboplatin group and 17.3 months in the carboplatin alone group.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
ICON4/AGO-OVAR 2.261 defined OS as the time from randomisation to death from any cause. Patients known to be alive at the time of analysis were censored at the time of their last follow-up. At the time of analysis (median follow-up of 42 months), 530 patients (66%) had died. Median OS was significantly prolonged in the paclitaxel plus platinum-based chemotherapy compared with platinum-based chemotherapy alone (HR 0.82, 95% CI 0.69 to 0.97; p = 0.02; see Table 23). The difference between groups translates into an absolute difference in 2-year survival of 7% in favour of adding paclitaxel to platinum-based chemotherapy (57% vs. 50%). Paclitaxel plus platinum-based chemotherapy was associated with a gain in median OS of 5 months (median OS: 29 months with paclitaxel plus platinum-based chemotherapy vs. 24 months with platinum-based therapy alone).
The authors of ICON4/AGO-OVAR 2.261 also carried out an exploratory analysis to investigate the effect of randomisation strata on OS (summarised in Table 22).61 No statistically significant difference between treatment groups was identified for any of the subgroups analysed but, as the authors noted, many of the subgroups were small and may have lacked the power to detect any real differences between the groups. A non-significant trend was noted within the subgroups of age (< 55 years vs. 55–65 years vs. > 65 years) and the number of previous lines of chemotherapy (1 vs. 2 vs. > 2).
The data reported by Gonzalez-Martin et al.48 for OS are immature. At the time of analysis, median OS had not been reached in the paclitaxel plus carboplatin group. Of the 81 patients randomised, 32 patients had died, 23 in the carboplatin-alone group and nine in the paclitaxel plus carboplatin group. Analysis of available OS data found that median OS was prolonged in the paclitaxel plus carboplatin group, being significantly longer than the median OS of 72.7 weeks in the carboplatin alone group (HR 0.31, 95% CI 0.14 to 0.68; p = 0.0021; Table 23). OS was defined as time from date of randomisation to death. It should be noted that the study was not powered to identify a difference between groups in OS and that the statistical comparative analysis was exploratory.
Network meta-analysis (platinum sensitive)
The RCTs available for inclusion in the NMA evaluating OS in patients with platinum-sensitive recurrent ovarian cancer are summarised in Table 23. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Figure 4.
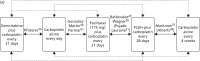
FIGURE 4
Networks for OS for people with platinum-sensitive recurrent ovarian cancer. (a) Network 1; and (b) network 2.
Network 1 (see Figure 4a) consisted of the following comparators:
- paclitaxel plus carboplatin
- gemcitabine plus carboplatin
- PLDH plus carboplatin
- platinum as a monotherapy.
Paclitaxel plus carboplatin was chosen as the baseline treatment as this would best help inform the economic evaluation conducted by the TAG (see Chapter 4, Treatment effectiveness). However, results are reported in Table 24, sequentially covering all possible comparisons. Overall, there was no significant difference (at the 5% level) for any of the doublet chemotherapies assessed compared with paclitaxel plus carboplatin. Platinum monotherapy was associated with a significant reduction in OS compared with all doublet chemotherapies, with the exception of gemcitabine plus carboplatin, for which no significant difference was found.
TABLE 24
Results of the NMA for OS for people with platinum-sensitive recurrent ovarian cancer
Network 2 (see Figure 4b) consisted of the following comparators:
- PLDH monotherapy
- trabectedin plus PLDH
- paclitaxel monotherapy
- topotecan monotherapy.
Pegylated liposomal doxorubicin hydrochloride monotherapy was chosen as the baseline treatment, as this would best help inform the economic evaluation conducted by the TAG (see Chapter 4, Treatment effectiveness). However, results are reported in Table 24, sequentially covering all possible comparisons. Overall, there was no significant difference (at the 5% level) for trabectedin plus PLDH or paclitaxel monotherapy compared with PLDH monotherapy. Topotecan monotherapy was associated with a significant reduction in OS compared with all other chemotherapy regimens assessed, with the exception of paclitaxel monotherapy, where no significant difference was found (albeit with a non-significant trend in favour of paclitaxel monotherapy).
Fully platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
Mature OS data from CALYPSO are reported in a follow-up publication to that of Pujade-Lauraine et al.31,56 A univariate Cox regression analysis was carried out in prespecified patient subgroups, one of which was based on TFI of 6–12 months (PPS) compared with ≥ 12 months (FPS). A total of 631 patients (305 patients in the PLDH plus carboplatin group and 326 patients in the paclitaxel plus carboplatin group) had a TFI of ≥ 12 months. The univariate analysis identified no statistically significant difference between PLDH plus carboplatin and paclitaxel plus carboplatin in OS in this subgroup of patients (HR 0.99, 95% CI 0.81 to 1.21; p = 0.90). It should be noted that OS was not defined.
Trabectedin plus PLDH compared with PLDH alone
Neither the long-term follow-up study of OVA-30164 nor the accompanying publication presenting results for the subgroup of patients with PPS disease report data on OS in the FPS subgroup.65 Although TA22215 reports OS data for patients with FPS disease, data are based 81% of the planned 520 deaths for the full trial population and are therefore immature. Data are reported here for completeness but have not been included in the NMA. In TA222,15 median OS in the FPS subgroup is reported as 31.7 months in the PLDH alone group. Median OS had not been reached in the trabectedin plus PLDH group. Accompanying HR of 0.89 (95% CI 0.58 to 1.35; p = 0.5746) indicates that there is no statistically significant difference between treatments in OS in this subgroup of patients. In addition to being based on immature data, this is a post hoc analysis, and as such is exploratory and hypothesis generating.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In the subgroup of patients with FPS ovarian cancer (PFI of > 12 months; 97 patients), Gordon et al.53 found no statistically significant difference between PLDH and topotecan in OS, with a HR of 1.15 (95% CI 0.71 to 1.85; p = 0.057; see Table 25), for which HR of > 1 favours PLDH. The median OS in each group was not reported. It should be noted that the number of patients with FPS ovarian cancer in each treatment group was not reported. Furthermore, although randomisation was stratified by platinum sensitivity (sensitive vs. resistant/refractory), patients were not stratified based on PPS compared with FPS, and these subgroup analyses were not prespecified. As subgroup analyses, the results should be interpreted with caution.
TABLE 25
Summary of results for OS in the FPS recurrent ovarian cancer
Paclitaxel plus carboplatin compared with platinum-based therapy alone
ICON4/AGO-OVAR 2.261 carried out a subgroup analysis to determine the effect of paclitaxel plus platinum chemotherapy on OS in various subgroups, including time since completion of last chemotherapy regimen (≤ 12 months vs. > 12 months). Most patients had received only one prior regimen of chemotherapy (92%) and therefore TFI is akin to PFI. In the subgroup of patients with FPS ovarian cancer (599 patients), a similar proportion of people in each treatment group had died at the time of analysis [180/300 (60.0%) with paclitaxel plus carboplatin vs. 187/299 (62.5%) with carboplatin alone]. Median OS in each group for this population, or an accompanying HR or p-value for the difference between groups was not reported.
Network meta-analysis (fully platinum sensitive)
The trials identified for potential inclusion in the NMA for OS in patients with FPS recurrent ovarian cancer are detailed in Table 25. Of the three RCTs identified, only two trials reported the required data for analysis49,56 and as they did not contain a common comparator it was not possible perform an indirect comparison.
Partially platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
A univariate Cox regression analysis of data from CALYPSO based on TFI of 6–12 months (PPS) included 344 patients (161 patients in the PLDH plus carboplatin group and 183 patients in the paclitaxel plus carboplatin group).56 The univariate analysis identified no statistically significant difference between PLDH plus carboplatin and paclitaxel plus carboplatin in OS in this subgroup of patients (HR 1.01, 95% CI 0.80 to 1.28; p = 0.92; see Table 26). It should be noted that OS was not defined.
TABLE 26
Summary of results for OS for people with PPS recurrent ovarian cancer
Trabectedin plus PLDH compared with PLDH alone
An accompanying publication to OVA-30130 reports results for the subgroup of patients with PPS ovarian cancer (relapse within 6–12 months of completion of platinum-based chemotherapy). OS data presented by Poveda et al.65 (419 deaths) are not as mature those in the long-term study reported by Monk et al.64 (522 deaths) and therefore are not reported here.
In the subgroup of patients with PPS ovarian cancer (relapse at 6–12 months after last platinum-based treatment), trabectedin plus PLDH significantly prolonged OS compared with PLDH alone (22.4 months with trabectedin plus PLDH vs. 16.4 months with PLDH alone; HR 0.64, 95% CI 0.47 to 0.86; p = 0.0027; see Table 26).64 The authors highlight that this is a post hoc analysis, and as such is exploratory and hypothesis generating.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In the subgroup of patients with PPS ovarian cancer (PFI of > 6–≤ 12 months; 122 patients), Gordon et al.54 found that PLDH significantly prolonged OS compared with topotecan (HR 1.58, 95% CI 1.07 to 2.34; p = 0.021; see Table 26), for which HR of > 1 favours PLDH. The median OS in each group was not reported. It should be noted that the number of patients with PPS ovarian cancer in each treatment group was not reported. Furthermore, although randomisation was stratified by platinum sensitivity (sensitive vs. resistant/refractory), patients were not stratified based on PPS ovarian cancer compared with FPS ovarian cancer and these subgroup analyses were not prespecified.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
In ICON4/AGO-OVAR 2.2,61 to be eligible for randomisation in the MRC CTU and AGO-OVAR protocols, patients had to have been treatment free for > 6 months. Thus, the subgroup of patients with a TFI of ≤ 12 months are, by the definition used in this review, PPS (213 patients). A similar proportion of people in each treatment group had died at the time of analysis [75/92 (81.5%) with paclitaxel plus carboplatin vs. 88/111 (79.3%) with carboplatin alone]. Median OS in each group for this population, or an accompanying HR or p-value for the difference between groups were not reported.
Network meta-analysis (PPS)
The RCTs available for inclusion in the NMA evaluating OS in patients with platinum-sensitive recurrent ovarian cancer are summarised in Table 26. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Figure 5.
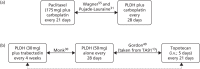
FIGURE 5
Networks for OS for people with PPS recurrent ovarian cancer. (a) Network 1; and (b) network 2.
Only Wagner et al.56 were able to provide data for network 1 (see Figure 5) and the results are presented in Table 27. The trial demonstrated no significant difference in OS for PLDH plus carboplatin compared with paclitaxel plus carboplatin.
TABLE 27
Results for NMA for OS for people with PPS recurrent ovarian cancer
Network 2 (see Figure 5) consisted of the following comparators:
- PLDH monotherapy
- trabectedin plus PLDH
- topotecan monotherapy.
The results of this NMA are presented in Table 27. Trabectedin plus PLDH was associated with significantly greater OS than PLDH monotherapy or topotecan monotherapy. Topotecan monotherapy was associated with a significant reduction in OS compared with all other chemotherapy regimens assessed.
Platinum resistant/refractory
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In the subgroup of patients with PRR ovarian cancer (254 patients), Gordon et al.54 found a median OS of 38.3 weeks in the PLDH group and 42.1 weeks in the topotecan group (median OS taken from TA9113). There was no statistically significant difference between the groups in OS (HR 1.07, 95% CI 0.82 to 1.39; p = 0.618; see Table 29); HR of > 1 favours PLDH. Survival rates at 1, 2 and 3 years are presented in Table 28.
TABLE 29
Summary of results of OS for people with PRR recurrent ovarian cancer
TABLE 28
Survival rates in PRR patients in PLDH and topotecan groups
Pegylated liposomal doxorubicin hydrochloride compared with paclitaxel
TA9113 presents results for the subgroup of patients with PRR disease (64 patients in the PLDH group vs. 67 patients in the paclitaxel group).11 There was no statistically significant difference between PLDH and paclitaxel in this subgroup of patients, with a HR of 0.87 (95% CI 0.61 to 1.24), for which HR of > 1 favours PLDH. Median OS was 36.7 weeks [range 2.3–241.1 weeks (upper limit includes a censored observation)] for PLDH and 54.3 weeks [range 1.7–211.4 weeks (upper limit includes a censored observation); see Table 29] for paclitaxel.
Topotecan compared with paclitaxel
Analysis of OS for the subgroup of patients with PRR (refractory, early and interim relapse) disease is not reported in the publications by ten Bokkel Huinink et al.21,52 TA9113 found no statistically significant difference between topotecan and paclitaxel in OS, reporting an unadjusted HR of 0.74 (95% CI 0.5 to 1.09; see Table 29) in PRR patients, for which HR < 1 favours topotecan.13 It should be noted that interpretation of OS results are potentially confounded by the permitted crossover to the alternative treatment should a patient not respond to their allocated treatment.
Paclitaxel plus carboplatin compared with paclitaxel alone
OS (not defined) was evaluated by Lortholary et al.62 as a secondary outcome and was reported not to differ among treatment groups, with median OS of 19.9 months, 15.2 months and 18.6 months for weekly paclitaxel, weekly paclitaxel plus carboplatin and weekly paclitaxel plus weekly topotecan, respectively. The number of events at the time of analysis is unclear. As discussed earlier, results from the weekly paclitaxel plus topotecan group are not of interest to this systematic review. The authors of the study were contacted with a request for the HR for the comparison of weekly paclitaxel compared with weekly paclitaxel plus carboplatin. The authors helpfully provided the requested information, which indicates that there is no significant difference between the two treatment groups in median OS (HR 1.07, 95% CI 0.86 to 1.34; p = 0.53; see Table 29).
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
OS was not defined by Sehouli et al.23 After a median duration of follow-up of 23.4 months, 55 (28.4%) patients remained alive. Median OS in the weekly topotecan group was 9.6 months compared with 9.3 months in the conventional topotecan group. The difference between groups did not reach statistical significance, with a HR of 1.04 (95% CI 0.74 to 1.44; p = 0.83; see Table 29). The authors carried out a multivariate regression analysis that identified the factors listed below as independent predictors of OS:
- duration of chemotherapy (HR 0.99, 95% CI 0.99 to 1.00; p < 0.001)
- baseline ECOG score (HR 1.47, 95% CI 1.16 to 1.86; p = 0.001)
- administration of follow-up chemotherapy (HR 0.53, 95% CI 0.37 to 0.76; p = 0.001).
Network meta-analysis (PRR)
The RCTs available for inclusion in the NMA evaluating OS in patients with PRR recurrent ovarian cancer are summarised in Table 29. The network of trials constructed for this outcome is depicted in Figure 6 and contains the following comparators:

FIGURE 6
Networks for OS for people with PRR recurrent ovarian cancer.
- PLDH monotherapy
- trabectedin plus PLDH
- paclitaxel monotherapy
- topotecan monotherapy, i.e. topotecan 1.25 or 1.5 mg/m2 daily for 5 days every 21 days
- topotecan monotherapy (weekly); i.e. topotecan 4.0 mg/m2 (weekly) on days 1, 8 and 15 of a 28-day cycle.
The results from this NMA are presented in Table 30. Overall, there was no significant difference in OS (at the 5% level) for any of the chemotherapies assessed compared with PLDH monotherapy (or with each other).
TABLE 30
Results of NMA for OS for people with PRR recurrent ovarian cancer
A RCT that provided results for this population but which did not share a common comparator within the network compared low-dose paclitaxel (80 mg/m2) with low-dose paclitaxel (80 mg/m2) plus carboplatin.62 However, Lortholary et al.62 identified no significant difference in OS between the two different treatment regimens (see Table 29). Trabectedin plus PLDH is outside of the scope for this review for the population of PRR patients; data have been included within the network to capture all of the available evidence but are not included in the economic analysis.
Full population (mixed platinum-free intervals)
Trabectedin plus PLDH compared with PLDH alone
Based on 522 deaths (analysis planned at 520 deaths), OVA-301 found no significant difference in OS between the two treatments, with median OS of 22.2 months in the trabectedin plus PLDH group and 18.9 months in the PLDH alone group (HR 0.86, 95% CI 0.72 to 1.02; p = 0.084; see Table 34).64 Survival rates in the two treatment groups at various time points are presented in Table 31.
TABLE 34
Summary of results of OS for a population of mixed PFIs
TABLE 31
Survival rates in the full trial population of OVA-301 reported by Monk et al.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
As noted earlier, data for OS from Gordon et al.49 are based on a modified ITT population and OS was defined as the time from the start of study drug administration to death. In the longer-term study,54 additional analyses are presented in which OS results for the full trial population are based on the ITT population and the more commonly used definition of OS of time from date of randomisation until date of death. At the time of analysis, 87% of patients had died and 13% of observations were censored. For completeness, both results are reported here.
Based on the modified ITT population (n = 474) and the original definition of OS, Gordon et al.54 found that PLDH significantly prolonged median OS compared with topotecan, with a median gain of 3.0 weeks (median OS 62.7 weeks with PLDH vs. 59.7 weeks with topotecan; HR 1.22, 95% CI 1.00 to 1.48; p = 0.05; see Table 34); in this analysis, HR of > 1 favours PLDH. The gain in OS associated with PLDH corresponded to an 18% reduction in the risk of death. Similar results were observed in the analysis of all patients randomised (n = 481), with a median gain of 6.6 weeks associated with PLDH (median OS 63.6 weeks with PLDH vs. 57.0 weeks with topotecan; HR 1.23, 95% CI 1.01 to 1.50; p = 0.038; see Table 34); in this analysis, HR of > 1 favours PLDH. Survival rates in the two treatment groups at various time points are presented in Table 32.
TABLE 32
Survival rates in the full trial population of the trial reported by Gordon et al.
To investigate the influence of multiple putative prognostic factors on OS, the authors carried out a multivariate Cox regression analysis.54 Variables evaluated were treatment, platinum sensitivity (sensitive vs. resistant/refractory), bulky disease (yes vs. no), baseline Karnofsky performance status (KPS) (< 80 vs. ≥ 80). The adjusted HR for OS was similar to that of the primary analysis, which led the authors to conclude that the results were not affected by potential prognostic factors (summarised in Table 33). Results suggest that age of < 65 years, platinum-sensitive disease and absence of ascites at baseline are associated with improved survival.
Pegylated liposomal doxorubicin hydrochloride compared with paclitaxel
In the full trial population of Trial 30–5713 (216 patients), there was no statistically significant difference between PLDH and paclitaxel in OS, with a HR of 0.93 (95% CI 0.70 to 1.23; see Table 34); HR of > 1 favours PLDH. Median OS was 46.6 weeks [range 2.3–263.7 weeks (includes censored observation)] with PLDH compared with 56.3 weeks (range 1.4–211.4 weeks) with paclitaxel.
Topotecan compared with paclitaxel
Data reported here are taken from the longer-term follow-up study reported by ten Bokkel Huinink et al.52 in which data had been collected for > 4 years. For analysis of OS, 20.5% of patients in the topotecan group and 12.3% of patients in the paclitaxel group were censored. There was no statistically significant difference between topotecan and paclitaxel in median OS (63 weeks with topotecan vs. 53 weeks with paclitaxel; p = 0.44; see Table 34).52 An accompanying HR was not reported in the full publication. However, TA9113 reported a HR of 0.91 (95% CI 0.68 to 1.23) for OS, for which HR < 1 favours topotecan.13 The HR had been adjusted for stratification factors. It should be noted that interpretation of OS results are potentially confounded by the permitted crossover to the alternative treatment should a patient not respond to their allocated treatment.
Paclitaxel compared with oxaliplatin
Piccart et al.63 evaluated OS as a secondary outcome measure, with OS defined as the time from day 1 of treatment to death. At the time of analysis, of the 86 patients randomised, 45 had died [52%; 25/41 (61.0%) in the paclitaxel group vs. 20/45 (44.4%) in the oxaliplatin group; see Table 34]. Median OS was 37 weeks in the paclitaxel group compared with 42 weeks in the oxaliplatin group. Statistical significance was not assessed in the full publication. Neither an accompanying HR nor a p-value for the difference between groups was reported.
Topotecan oral compared with topotecan intravenous
In the full trial population, Gore et al.24 found that median OS was significantly prolonged with i.v. topotecan compared with oral topotecan, with a median OS of 51 weeks with oral topotecan compared with 58 weeks with i.v. topotecan (risk ratio of death 1.36, 95% CI 1.00 to 1.85; p = 0.033; see Table 34). It should be noted that OS was not defined in the full publication.
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
Omura et al.68 defined OS as the time from randomisation until the date of death, or last contact if the date of death was unknown. Estimated median OS for the paclitaxel 175 mg/m2 and the 250 mg/m2 regimens were 13.1 and 12.3 months, respectively. The accompanying HR of 0.97 (95% CI 0.77 to 1.22; ratio of 250 mg/m2 compared with 175 mg/m2; see Table 34) indicated that OS was not statistically significantly different between the two paclitaxel regimens. The HR was adjusted for initial performance score, cell type, response to prior platinum, cooperative group and measurable disease. An unadjusted HR was not reported.
Paclitaxel weekly compared with paclitaxel every 3 weeks
Rosenberg et al.60 defined OS as time from date of randomisation to death or censored observation. In the full trial population, there was no statistically significant difference between treatment regimens in median OS (p = 0.98). Median OS was 13.6 months (95% CI 10.5 to 18.7 months) in the group receiving paclitaxel every 7 days compared with 14.7 months (95% CI 12.3 to 19.1 months) in the group receiving paclitaxel every 21 days. It is unclear how many events had occurred at the time of analysis.
Network meta-analysis (mixed PFIs)
The RCTs available for inclusion in the NMA evaluating OS in patients with mixed PFIs in recurrent ovarian cancer are summarised in Table 34. However, based on expert clinical opinion, the TAG decided not to evaluate this mixed patient population, as the results would not be considered clinically meaningful.
Progression-free survival
In oncology trials, progression of disease is typically assessed according to internationally recognised criteria, such as the RECIST criteria,69 which are based on clinical signs, ultrasound scans or X-rays. RECIST criteria encompass measurable and non-measurable disease. Increase in levels of CA125 biomarker is also used to determine disease progression, typically in patients with non-measurable lesions at baseline; according to criteria developed by Rustin et al.,80 increase in CA125 level has been shown to predate evidence of disease progression from clinical examinations or radiological scans in 70% of patients with ovarian cancer by a median of 4 months.76 There are two time-to-event measures of disease progression (definitions as reported in Food and Drug Administration guidance on conducting oncology trials):77
- PFS, which is defined as time from randomisation to disease progression or death (includes all deaths)
- TTP, which is defined as time from randomisation to disease progression (deaths before progression are censored).
The terms PFS and TTP are often used interchangeably. For example, a trial might refer to the outcome of PFS but the definition indicates that all-cause mortality has not been included in the analysis. For the purposes of the review, the TAG has considered PFS and TTP together and has reported the outcome as defined in the individual trials. As for OS, in some cases, PFS and TTP have been measured from the time of treatment initiation rather than randomisation.
Progressive events occur in a shorter timeframe and more frequently than OS events. Therefore, PFS data are available much sooner than OS data. Additionally, there is no confounding from postprogression therapy. However, because PFS is based on assessment of change in tumour size, there is a degree of subjective assessment, with associated potential for measurement errors. Assessment bias is more likely in an open-label trial. Differences in the timing of measurement between the groups may arise if the treatments under evaluation have different cycle lengths, which could lead to a difference in progression date. In clinical trials, it has been reported that an increase in CA125 level frequently triggers subsequent postprogression therapy before clinical or radiological confirmation of progression. The practice of using CA125 level alone also introduces disparity across trials in terms of the date of disease progression.
The criteria used to determine progression were initially developed for use in clinical trials using response rate as a primary end point (e.g. Phase II screening trials), with the goal of facilitating evaluation of changes in tumour burden during treatment rather than to associate the changes with a clinical benefit.78 However, changes in tumour size are recognised as signals of a drug’s anti-tumour activity.
Summary of results for progression-free survival/time to progression
Results are presented for PFS or TTP, as reported in the trial. PFS and TTP are often used interchangeably and, for the purposes of the results presented here, TTP has been assumed to approximate to PFS. Definitions as reported in the trials are provided in the main text. No trial was identified evaluating treatments in a population solely comprising patients who were allergic or intolerant to platinum-based chemotherapy. Here, results for patients with platinum-sensitive or PRR disease are summarised. For trials not limited to either platinum-sensitive or PRR patients (i.e. includes a mix of PFI), results for the full trial population are presented in the main text.
Results for progression-free survival/time to progression for the subgroup of patients with platinum-sensitive (relapse at ≥ 6 months after last platinum-based chemotherapy) ovarian cancer
Nine RCTs28–31,48,50,52,54,61 evaluating seven different head-to-head comparisons of interventions and comparators of interest reported on PFS/TTP (Table 35).
TABLE 35
Progression-free survival for patients with platinum-sensitive ovarian cancer
As for OS, based on trials identified, it was not possible to construct a complete network. Again, two discrete networks were generated, one evaluating platinum-based therapies and the second comparing non-platinum-based regimens. It should be stressed that results from the two discrete networks are not directly comparable.
In the network evaluating platinum-based chemotherapies, all combination chemotherapy regimens significantly improved PFS compared with platinum monotherapy (Table 36). In addition, PLDH plus carboplatin was found to be significantly more effective at prolonging PFS than paclitaxel plus carboplatin. No other statistically significant differences were identified between combination regimens.
TABLE 36
Results from NMA for PFS of platinum-based chemotherapies
Analysis of non-platinum-based regimens indicates that trabectedin plus PLDH significantly improves PFS compared with PLDH, paclitaxel and topotecan when given as monotherapy (Table 37). No statistically significant differences were identified among the monotherapies evaluated (PLDH, topotecan and paclitaxel).
TABLE 37
Results from NMA for PFS of non-platinum-based chemotherapies
Where available, PFS/TTP data were analysed for the subgroups of patients with FPS (relapse at > 12 months after last platinum-based treatment) and PPS (relapse at ≥ 6 to ≤ 12 months after last platinum-based treatment). As for OS, few trials involving platinum-sensitive patients evaluated treatment effect in these two subgroups: three trials afforded data on FPS and four trials on PPS. Two trials evaluated platinum-based regimens and two trials non-platinum-based regimens.
Results in patients with fully platinum-sensitive ovarian cancer
One65 of the three trials50,61,65 reported a HR as a measure of treatment effect (Table 38). The difference between trabectedin plus PLDH and PLDH monotherapy was not statistically significant. The two remaining trials50,61 did not report a HR for PFS but the proportion of people having an event was similar in each treatment group. The lack of HRs for two of the trials50,61 precluded carrying out a NMA.
TABLE 38
Progression-free survival for the subgroup of patients with FPS ovarian cancer
Results in patients with partially platinum-sensitive ovarian cancer
Two of the four trials56,65 evaluating treatments in the subgroup of patients with PPS ovarian cancer reported HR as a measure of effect. PLDH plus carboplatin was found to significantly prolong PFS compared with paclitaxel plus carboplatin (Table 39).56 In addition, trabectedin plus PLDH significantly improved PFS compared with PLDH alone.65 The two remaining trials50,61 did not report HRs. The proportion of patients experiencing an event was similar in the two treatment groups in each trial. The lack of HRs for two of the trials50,61 precluded carrying out a NMA.
TABLE 39
Progression-free survival for the subgroup of patients with PPS ovarian cancer
Results in progression-free survival for the subgroup of patients with platinum-resistant/-refractory ovarian cancer
Four RCTs23,52,54,62 reporting results for four different head-to-head comparisons involving PRR patients were identified. Two RCTs enrolled only patients with PRR23,62 with the remaining two RCTs52,54 reporting results from a subgroup of patients within the trial. None of the trials identified a significant difference in PFS/TTP between the two treatment groups evaluated (Table 40).
TABLE 40
Progression-free survival for the subgroup of patients with PRR ovarian cancer
Three23,52,54 of the four identified trials were included in the network; the treatment regimens evaluated in the trial reported by Lortholary et al.62 did not inform the network. Trabectedin plus PLDH is outside of the scope for this review for the population of PRR patients; data have been included within the network to capture all of the available evidence but are not included in the economic analysis. The results of the NMA are in alignment with the results of the individual trials, with no statistically significant differences in PFS among PLDH, paclitaxel and topotecan monotherapy (Table 41).
TABLE 41
Results from NMA for PFS of patients with PRR ovarian cancer
Platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
Bafaloukos et al.29 evaluated TTP, which was defined as the time from the initiation of treatment to the first disease progression. Deaths as a result of disease without previous documentation of progression were considered events in TTP. Median TTP was 11.8 months in the PLDH plus carboplatin group compared with 10.8 months in the paclitaxel plus carboplatin group (see Table 47), with no statistically significant difference between treatments for this outcome (p = 0.904). It is important to note that the study was not powered to detect differences in TTP. An accompanying HR was not reported.
TABLE 47
Summary of results for PFS for people with platinum-sensitive recurrent ovarian cancer
Progression-free survival was the primary outcome in the CALYPSO trial31 and primary analysis was based on the ITT population. Although a comprehensive description of criteria for categorisation of disease progression is provided, it is unclear when monitoring for progression began, that is, from randomisation or from first administration of study drug. Tumour assessment was carried out every 3 months while patients were receiving treatment.
After a median follow-up of 22 months, 832 PFS events had occurred. PLDH plus carboplatin significantly prolonged median PFS compared with carboplatin plus paclitaxel, with a median PFS gain of 1.9 months (median PFS 11.3 months with PLDH plus carboplatin vs. 9.4 months with paclitaxel plus carboplatin; HR 0.82, 95% CI 0.72 to 0.94; p = 0.005). The test for non-inferiority of PLDH plus carboplatin afforded a p-value of < 0.001. A similar proportion of patients in each group had disease progression based on RECIST criteria69 (Table 42).
TABLE 42
Breakdown of patients by measure used to evaluate disease progression
Exploratory analysis of the effects of several baseline characteristics on PFS was carried out using Cox proportional hazards regression. Factors evaluated were age; number of previous lines of chemotherapy; TFI; surgery at relapse; measurability status of tumour; size of tumour (< 5 cm or ≥ 5 cm); number of tumour sites (1 or > 1); tumour grade; histological classification of tumour cells; CA125 level; ECOG performance score; and treatment arm. Limited results are available in the full publication (summarised in Table 43). TFI, measurable disease, CA125 level of ≥ 100 and PLDH plus carboplatin were found to be associated with a significant effect on PFS. It is unclear whether the remainder of the putative prognostic factors had no effect on PFS.
TABLE 43
Multivariate regression analysis to evaluate the effect of baseline factors on PFS
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with carboplatin alone
Alberts et al.28 reported that PFS was measured as a secondary outcome but a definition of PFS was not provided. Based on 55 out of 61 women having progressed or died, Alberts et al.28 found a median PFS (unadjusted) of 12 months in the PLDH plus carboplatin group and 8 months in the carboplatin alone group (HR 0.54, 95% CI 0.32 to 0.93; p = 0.03; see Table 47). Longer-term data (all women had progressed or died) reported by Markman et al.55 found similar results, with median PFS of 12 months and 8 months in the PLDH plus carboplatin group and carboplatin alone group, respectively (HR not reported; p = 0.02).
Trabectedin plus PLDH compared with PLDH alone
PFS was the primary outcome of the OVA-301 trial, and was defined as time from random assignment to disease progression or death.30 Three analyses for PFS were performed, based on review by independent radiologists, independent oncologists and investigator. The primary analysis was based on review by independent radiologists who were masked to treatment allocation, with disease progression determined by radiological evaluation alone according to RECIST criteria.69 The primary analysis included only those patients who had measurable disease at baseline. A secondary analysis was based on review by independent oncologists who were also masked to treatment and who categorised disease progression based on radiological assessments together with clinical data. The secondary analysis included all randomised patients.
The sample size calculation estimates that 415 progressive events would be needed to test statistical difference at a two-sided 5% significance level with at least 90% power, based on assumed median PFS of 16 weeks and 22 weeks for PLDH alone and trabectedin plus PLDH, respectively. At the time of analysis of PFS, in the full trial population (includes platinum-resistant patients), 389 events had occurred according to independent radiology review and 432 events based on independent oncologist review. Based on event rate, the primary analysis of PFS could be underpowered. In the FAD for TA222, the Committee concluded that ‘despite the technical difficulties, the analysis based on the independent radiologists’ assessment was the most robust’.73 For this reason, the TAG has used results from the primary analysis of PFS in the NMA.
In the subgroup of patients with platinum-sensitive disease, all three analyses found that median PFS was significantly prolonged with trabectedin plus PLDH compared with PLDH alone (Table 44). Multivariate analysis of potential prognostic factors found that treatment with trabectedin plus PLDH remained significant after adjustment of prognostic factors; the multivariate analysis was based on the full trial population and is presented in the section outlining results in the full trial population.
TABLE 44
Summary of PFS in platinum-sensitive patients in OVA-301
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In Gordon et al.,49 PFS was defined as the time from the first day of study drug dosing to documented disease progression or death due to any cause while the patient was on the study drug or during the long-term follow-up period. In platinum-sensitive patients, Gordon et al.49 found that PLDH significantly prolonged PFS compared with topotecan (p = 0.037; HR not reported). Median PFS was reported to be 28.9 weeks and 23.3 weeks in the PLDH and topotecan groups, respectively. However, results presented in TA91,13 which are based on data provided by the manufacturer as part of the appraisal process, indicate that there is no statistically significant difference between PLDH and topotecan in PFS in platinum-sensitive patients, with a median PFS of 27.3 weeks with PLDH, and 22.7 weeks with the topotecan-treated group (HR 1.29, 95% CI 0.98 to 1.69; HR of > 1 favours PLDH). As data reported in TA9113 are more mature, the TAG has used the HR reported in TA91 in its NMA.
Topotecan compared with paclitaxel
ten Bokkel Huinink et al.21 evaluated TTP as a secondary outcome, defining TTP as time from first study drug to documented progression or administration of third-line therapy. Analysis of TTP for the subgroup of patients with platinum-sensitive (late relapse) disease is not reported in either publication by ten Bokkel Huinink et al.21,52 TA9113 found no statistically significant difference between topotecan and paclitaxel in TTP, reporting an unadjusted HR of 0.82 (95% CI 0.54 to 1.26; see Table 47) in platinum-sensitive patients, for which HR < 1 favours topotecan. There was no significant difference between topotecan and paclitaxel in TTP (p = 0.08), with a median TTP of 18.9 weeks in the topotecan group compared with 14.7 weeks in the paclitaxel group.
Gemcitabine plus carboplatin compared with carboplatin alone
PFS was the primary outcome in the trial reported by Pfisterer et al.50 and was defined as time from the date of randomisation to the date of disease progression or death from any cause. PD was based on clinical and/or radiological evaluation. CA125 elevation without accompanying clinical or radiological evidence was not sufficient to determine disease progression. Analysis occurred after observation of 325 events. Gemcitabine plus carboplatin was associated with a gain in median PFS of 2.8 months, with the difference between groups reaching statistical significance (HR 0.72, 95% CI 0.58 to 0.90; p = 0.0031). Median PFS was 8.6 months (95% CI 7.9 to 9.7 months) with gemcitabine plus carboplatin compared with 5.8 months (95% CI 5.2 to 7.1 months) with carboplatin alone.
Univariate analysis to investigate the effect of prespecified prognostic factors on PFS found PFI to be an important prognostic factor (p = 0.0015; Table 45).
TABLE 45
Results of univariate analysis of prespecified prognostic factors affecting PFS
Paclitaxel plus carboplatin compared with platinum-based therapy alone
ICON4/AGO-OVAR 2.261 defined PFS as the time from randomisation to first appearance of PD or death from any cause, which is the definition most commonly used across trials. Raised CA125 level without clinical or radiological evidence of PD was not considered to demonstrate disease progression. As for OS, patients known to be alive and without PD at the time of analysis were censored at their last follow-up. At analysis (median follow-up of 42 months), 717 (89%) of patients had developed PD or died. Paclitaxel plus platinum-based chemotherapy was associated with a significantly improved PFS compared with platinum-based therapy alone (HR 0.76, 95% CI 0.66 to 0.89; p = 0.0004). The improvement translates into an estimated absolute difference in 1-year PFS of 10% (40% vs. 50%) and an absolute difference in median PFS of 3 months in favour of combination treatment (median PFS 12 months with paclitaxel plus platinum-based chemotherapy vs. 9 months with platinum-based chemotherapy alone).
The authors carried out an exploratory analysis to investigate the effect of randomisation strata on PFS (summarised in Table 46).61 Again, as for OS, no statistically significant difference between treatment groups was identified for any of the subgroups analysed. A non-significant trend was observed within the subgroups of age (< 55 vs. 55–65 vs. > 65 years) and the number of previous lines of chemotherapy (1 vs. 2 vs. > 2).
Gonzalez-Martin et al.48 reported that paclitaxel plus carboplatin was associated with a significantly prolonged TTP compared with carboplatin alone (median TTP: 33.7 weeks with carboplatin alone vs. 49.1 weeks with paclitaxel plus carboplatin; HR 0.54, 95% CI 0.32 to 0.92; p = 0.021; see Table 47). TTP was defined as the time from date of randomisation to date of documentation of tumour progression. It should be noted that the study was not powered to identify a difference between groups in TTP and that the statistical comparative analysis was exploratory.
Network meta-analysis (platinum sensitive)
The RCTs available for inclusion in the NMA evaluating PFS in patients with platinum-sensitive recurrent ovarian cancer are summarised in Table 47. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Figure 7.

FIGURE 7
Networks for PFS for people with platinum-sensitive recurrent ovarian cancer. (a) Network 1; and (b) network 2.
Network 1 (see Figure 7a) consisted of the following comparators:
- paclitaxel plus carboplatin
- gemcitabine plus carboplatin
- PLDH plus carboplatin
- platinum as a monotherapy.
Paclitaxel plus carboplatin was chosen as the baseline treatment, as this would best help to inform the economic evaluation conducted by the TAG (see Chapter 4, Treatment effectiveness). However, results are reported in Table 48 sequentially covering all possible comparisons. Overall, only PLDH plus carboplatin had a significantly improved PFS (at the 5% level) compared with paclitaxel plus carboplatin. Platinum monotherapy was associated with a significant reduction in PFS compared with all doublet chemotherapies assessed.
TABLE 48
Results of the NMA for PFS for people with platinum-sensitive recurrent ovarian cancer
Network 2 (see Figure 7b) consisted of the following comparators:
- PLDH monotherapy
- trabectedin plus PLDH
- paclitaxel monotherapy
- topotecan monotherapy.
Pegylated liposomal doxorubicin hydrochloride monotherapy was chosen as the baseline treatment, as this would best help to inform the economic evaluation conducted by the TAG (see Chapter 4, Treatment effectiveness). However, results are reported in Table 48 sequentially covering all possible comparisons. Overall, only trabectedin plus PLDH demonstrated a significant difference increase in PFS (at the 5% level) compared with PLDH monotherapy. Trabectedin plus PLDH would also be considered to have a statistically significant prolonged PFS when compared directly with paclitaxel monotherapy or topotecan monotherapy. None of the other comparisons of chemotherapies would be considered significantly different from one another.
Fully platinum sensitive
Trabectedin plus PLDH compared with PLDH alone
In the subgroup of patients with FPS disease, in the primary analysis of PFS (independent radiologist), OVA-301 found no statistically significant difference between treatment groups in median PFS, with median PFS of 11.1 months in the trabectedin plus PLDH group compared with 8.9 months in the PLDH alone group (HR 0.70, 95% CI 0.47 to 1.03; see Table 49).65 Secondary analysis based on independent review by oncologists found the difference in PFS to be statistically significant and favouring trabectedin plus PLDH [median PFS: 11.1 months with trabectedin plus PLDH vs. 9.0 months with PLDH alone; HR 0.66, 95% CI 0.46 to 0.97; p = 0.0311 (log-rank)]. The FAD of the STA of trabectedin plus PLDH (TA22273) states that the primary analysis was thought to be the most robust analysis.
TABLE 49
Summary of results for PFS in people with FPS recurrent ovarian cancer
It is important to reiterate that, in the full trial population, fewer events had occurred than the planned event rate required to generate 90% power and, as a consequence, the analysis might have been underpowered. In a subgroup analysis, the power to detect a statistically significant difference is further reduced. In addition, analysis of results for FPS patients was not preplanned and is therefore hypothesis generating.
Gemcitabine plus carboplatin compared with carboplatin alone
In the trial reported by Pfisterer et al.,50 in the subgroup of patients with FPS ovarian cancer, at the time of analysis, a similar proportion in each treatment group had progressed or died [93/106 (87.7%) with gemcitabine plus carboplatin vs. 97/107 (90.7%) with carboplatin alone]. Median PFS in each treatment group for this population was not reported. An accompanying HR or p-value for the difference between treatment groups was not available in the full publication.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
As for OS, ICON4/AGO-OVAR 2.261 carried out a subgroup analysis to determine the effect of paclitaxel plus platinum chemotherapy on PFS in various subgroups, including time since completion of last chemotherapy regimen (≤ 12 months vs. > 12 months). In the subgroup of patients with FPS ovarian cancer (599 patients), a similar proportion of people in each treatment group had progressed or died at the time of analysis [256/300 (85.3%) with paclitaxel plus carboplatin vs. 262/299 (87.3%) with carboplatin alone]. Median PFS in each treatment group for this population was not reported. An accompanying HR or p-value for the difference between treatment groups was not available in the full publication.
Network meta-analysis (fully platinum sensitive)
The trials identified for potential inclusion in the NMA for PFS in patients with FPS recurrent ovarian cancer are detailed in Table 49. Of the three RCTs identified, only one trial30,65 reported the required data for analysis, and so it was not possible perform an indirect comparison.
Partially platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
A separate publication of CALYPSO31 reported an analysis of PFS in the subgroup of patients with PPS ovarian cancer.57 The PPS subgroup comprised 161 patients in the PLDH plus carboplatin group and 183 patients in the paclitaxel plus carboplatin group. Baseline characteristics were similar in the two treatment groups. Median follow-up was 23 months and 326 patients experienced an event (progression or death).
Pegylated liposomal doxorubicin hydrochloride plus carboplatin significantly prolonged median PFS compared with paclitaxel plus carboplatin in this subgroup of patients, with a gain of 0.6 months in PFS (median PFS: 9.4 months with PLDH plus carboplatin vs. 8.8 months with paclitaxel plus carboplatin; HR 0.73, 95% CI 0.58 to 0.90; p = 0.004 for superiority; see Table 50).
TABLE 50
Summary of results for PFS in people with PPS recurrent ovarian cancer
Trabectedin plus PLDH compared with PLDH alone
In the subgroup of patients with PPS ovarian cancer, in the primary analysis of PFS (independent radiologist), OVA-30164 found that trabectedin plus PLDH significantly prolonged median PFS compared with PLDH alone (HR 0.65, 95% CI 0.45 to 0.92; Table 50). Median PFS was 7.4 months in the trabectedin plus PLDH group compared with 5.5 months in the PLDH alone group. Results based on review by independent oncologist align with those of the primary analysis (HR 0.54, 95% CI 0.39 to 0.76). As noted above, analysis of results for PPS patients is potentially underpowered and was not preplanned.
Gemcitabine plus carboplatin compared with carboplatin alone
At the time of analysis of PFS in Pfisterer et al.,50 most patients categorised as having PPS ovarian cancer had progressed or died [69/71 (97.2%) with gemcitabine plus carboplatin vs. 65/71 (91.5%) with carboplatin alone]. Median PFS in each treatment group for this population was not reported. An accompanying HR or p-value for the difference between treatment groups was not available in the full publication.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
In the subgroup of patients with PPS disease in ICON4/AGO-OVAR 2.2,61 almost all patients in each treatment group had progressed or died at the time of analysis [90/92 (97.8%) with paclitaxel plus carboplatin vs. 109/111 (98.2%) with carboplatin alone]. Median PFS in each treatment group for this population was not reported. An accompanying HR or p-value for the difference between treatment groups was not available in the full publication.
Network meta-analysis (PPS)
The trials identified for potential inclusion in the NMA for PFS in patients with PPS recurrent ovarian cancer are detailed in Table 50. Of the four RCTs identified, only two trials30,31,50,63 reported the required data for analysis and as they did not contain a common comparator it was not possible to perform an indirect comparison.
Platinum resistant/refractory
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In the subgroup of patients with PRR ovarian cancer, Gordon et al.49 found no statistically significant difference in PFS between PLDH and topotecan (p = 0.733; HR not reported). Median PFS with PLDH was 9.1 weeks compared with 13.6 weeks with topotecan. Results presented in TA91 for this subgroup of patients are analogous to those reported in Gordon et al.,49 with a HR reported of 0.99 (95% CI 0.77 to 1.28).13
Topotecan compared with paclitaxel
Analysis of TTP for the subgroup of patients with PRR (refractory, early and interim relapse) disease is not reported in either publication by ten Bokkel Huinink et al.21,52 TA9113 found no statistically significant difference between topotecan and paclitaxel in TTP, reporting an unadjusted HR of 0.75 (95% CI 0.50 to 1.12; Table 51) in PRR patients, for which HR < 1 favours topotecan. There was no significant difference between topotecan and paclitaxel in TTP (p = 0.08), with a median TTP of 18.9 weeks in the topotecan group compared with 14.7 weeks in the paclitaxel group.
TABLE 51
Summary of results for PFS in people with PRR recurrent ovarian cancer
Paclitaxel plus carboplatin compared with paclitaxel alone
PFS was the primary outcome of the trial carried out by Lortholary et al.62 and was determined according to criteria set out by GCIG.70 Median PFS is based on 162 events occurring in a median follow-up of 15 months. No statistically significant differences in PFS were identified among the treatment arms, with a median PFS of 3.7, 4.8 and 5.4 months for weekly paclitaxel, weekly paclitaxel plus carboplatin, and weekly paclitaxel plus weekly topotecan, respectively. As discussed earlier, results from the weekly paclitaxel plus topotecan group are not of interest to this systematic review. The authors of the study were contacted with a request for the HR for the comparison of weekly paclitaxel compared with weekly paclitaxel plus carboplatin. The authors helpfully provided the requested information, which indicates that there is no significant difference between the two treatment groups in median PFS (HR 0.92, 95% CI 0.76 to 1.12; p = 0.42; see Table 51).
In addition, an exploratory analysis of PFS was carried out using a Cox model that adjusted for: age; number of metastatic sites; number of prior lines of chemotherapy (1 vs. ≥ 2); PFI (progression ≤ 1 month vs. > 1 month from last platinum dose); ECOG performance status (0 vs. 1 or 2); and tumour size (< 5 cm or ≥ 5 cm). The analysis found that (monotherapy vs. combination therapy) was not predictive of PFS. However, PFI and ECOG performance status were identified as independent predictors of PFS, with p-values of 0.03 and 0.01, respectively.
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
PFS was evaluated as a secondary outcome by Sehouli et al.23 A definition for PFS was not provided in the full publication. There was no statistically significant difference between treatments in PFS (HR 1.29, 95% CI 0.96 to 1.76; p = 0.088). Median PFS was 3.0 months with conventional topotecan compared with 4.4 months with weekly topotecan.
Network meta-analysis (PRR)
The RCTs available for inclusion in the NMA evaluating PFS in patients with PRR recurrent ovarian cancer are summarised in Table 51. The network of trials constructed for this outcome is depicted in Figure 8 and contains the following comparators:
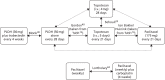
FIGURE 8
Networks for PFS for people with PRR recurrent ovarian cancer.
- PLDH monotherapy
- trabectedin plus PLDH
- paclitaxel monotherapy
- topotecan monotherapy; i.e. topotecan 1.25 or 1.5 mg/m2 daily for 5 days every 21 days
- topotecan monotherapy (weekly); i.e. topotecan 4.0 mg/m2 (weekly) on days 1, 8 and 15 of a 28-day cycle.
The results from this NMA are presented in Table 52. Overall, there was no significant difference in PFS (at the 5% level) for any of the chemotherapies assessed compared with PLDH monotherapy (or with each other).
TABLE 52
Results of the NMA for PFS for people with PRR recurrent ovarian cancer
A RCT that provided results for this population, but which did not share a common comparator within the network, compared low-dose paclitaxel (80 mg/m2) with low-dose paclitaxel (80 mg/m2) plus carboplatin.62 However, Lortholary et al.62 identified no significant difference in PFS between the two different treatment regimens (see Table 52). Trabectedin plus PLDH is outside of the scope for this review for the population of PRR patients; data have been included within the network to capture all of the available evidence but are not included in the economic analysis.
Full population (mixed platinum-free intervals)
Trabectedin plus PLDH compared with PLDH alone
In OVA-301 (all patients), after 389 events based on independent radiological review, trabectedin plus PLDH was found to significantly prolong PFS by 1.5 months compared with PLDH alone (median PFS: 7.3 months with trabectedin plus PLDH vs. 5.8 months with PLDH alone; HR 0.79, 95% CI 0.65 to 0.96; p = 0.019; see Table 54).30
TABLE 54
Summary of results for PFS in a population of mixed PFI
Multivariate analysis of baseline characteristics that are potential prognostic factors affecting PFS (based on independent radiology review) identified treatment with trabectedin plus PLDH and PFI (analysed as a continuum) as factors having a statistically significant effect on PFS (Table 53).30
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
For the full trial population, Gordon et al.49 observed a median PFS of 16.1 weeks with PLDH and of 17.0 weeks with topotecan, with no statistically significant difference between groups (HR 1.12, 95% CI 0.93 to 1.35; p = 0.095; see Table 54). The HR was not reported in the full publication and is as reported in TA91.13
Topotecan compared with paclitaxel
Data reported here are taken from the longer-term follow-up study reported by ten Bokkel Huinink et al.52 in which data had been collected for > 4 years. For analysis of TTP, 25% of patients in the topotecan group and 12.3% of patients in the paclitaxel group were censored. There was no statistically significant difference between topotecan and paclitaxel in TTP (p = 0.08), with a median TTP of 18.9 weeks in the topotecan group compared with 14.7 weeks in the paclitaxel group (Table 54).52 An accompanying HR was not reported in the full publication.52 However, TA9113 reported a HR of 0.81 (95% CI 0.60 to 1.09) for TTP, for which HR of < 1 favours topotecan.13 The HR was adjusted for stratification factors.
Paclitaxel compared with oxaliplatin
The methods section of Piccart et al.63 indicates that TTP was a secondary outcome measure, with TTP defined as the time from day 1 of treatment to first observation of disease progression as per WHO criteria. However, results are presented for both TTP and PFS. At the time of analysis, of the 86 patients randomised, 69 had progressed (80.2%). Of the remaining 17 patients who had not progressed, nine were in the paclitaxel group and eight were in the oxaliplatin group.
Median TTP (the number of patients reported includes only those who have progressed) and PFS were the same and were reported as 14 weeks in the paclitaxel group compared with 12 weeks in the oxaliplatin group. Statistical significance was not assessed in the full publication.
Topotecan oral compared with topotecan intravenous
Gore et al.24 evaluated TTP; TTP was not defined in the full publication. In the full trial population, median TTP was 13 weeks with oral topotecan compared with17 weeks with i.v. topotecan (difference reported to be non-significant; p-value not reported; see Table 54).24
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
In the trial carried out by Omura et al.,68 PFS did not differ appreciably between treatment regimens. Patients assigned to paclitaxel 175 mg/m2 had an estimated median PFS of 4.8 months compared 5.5 months for patients receiving paclitaxel 250 mg/m2 (see Table 54). The statistical significance between the groups was not assessed.
Paclitaxel weekly compared with paclitaxel every 3 weeks
Rosenberg et al.60 evaluated TTP as a secondary outcome, and defined TTP as time from first day of study treatment to the date of documented tumour progression (as per WHO criteria) or censored observation. In the full trial population, median TTP was 6.1 months (95% CI 5.0 to 8.0 months) in the group receiving paclitaxel weekly compared with 8.1 months (95% CI 6.4 to 9.7 months) in the group receiving paclitaxel every 21 days. The difference between groups in TTP did not reach statistical significance (p = 0.85). It is unclear how many events had occurred at the time of analysis.
Network meta-analysis (mixed PFIs)
The RCTs available for inclusion in the NMA evaluating PFS in patients with mixed PFIs in recurrent ovarian cancer are summarised in Table 54. However, based on expert clinical opinion, the TAG decided not to evaluate this mixed patient population, as the results would not be considered clinically meaningful.
Tumour response
As with PFS and TTP, for patients with measurable disease, assessment of tumour response is based on standard criteria, such as RECIST criteria.69 In patients without measurable disease, changes in CA125 level are used to evaluate tumour response as per the algorithm outlined by Rustin et al.76 There is some controversy over the use of CA125 level alone as an indicator for disease progression and for tumour response. However, an alternative opinion is that it is difficult to radiologically follow changes in measurable disease from baseline. ORR is typically reported as the combination of patients with a CR or those with a PR, as defined by the criteria implemented in the trial. ORR is considered to be a direct measure of the anti-tumour activity of a drug but not a direct measure of clinical benefit.76,77 As for PFS and TTP, evaluation of CR and PR is open to assessment bias, particularly in an open-label trial. When CR and PR have been reported separately, for the purposes of the NMA, the TAG has combined CR and PR results. Results for SD and PD are also reported for completeness.
Summary of results for tumour response
Results are presented for ORR, which has been defined as the number of patients achieving CR or PR as their best response. Definitions of CR and PR as reported in the trials are provided in the main text. No trial was identified evaluating treatments in a population solely comprising patients who were allergic or intolerant to platinum-based chemotherapy. Here, results for patients with platinum-sensitive or PRR disease are summarised. For trials not limited to either platinum-sensitive or PRR patients (i.e. includes a mix of PFI), results for the full trial population are presented in the main text.
Results for overall response rate for the subgroup of patients with platinum-sensitive (relapse at ≥ 6 months after last platinum-based chemotherapy) ovarian cancer
Twelve RCTs24,28–30,48,50,52,54,60,61,63,68 evaluating 11 different head-to-head comparisons of interventions and comparators of interest reported on ORR (Table 55). Of the 11 comparisons identified, only two trials30,50 reported a statistical significance in ORR. A larger proportion of patients treated with gemcitabine plus carboplatin achieved CR or PR than those treated with carboplatin alone. Trabectedin plus PLDH was also found to significantly improve the rate of CR or PR achieved compared with PLDH (50 mg/m2) alone.
TABLE 55
Overall response rate for patients with platinum-sensitive ovarian cancer
Based on the trials identified, it was not possible to construct a complete network. Again, two discrete networks were generated: one evaluating platinum-based therapies and the second comparing non-platinum-based regimens. It should be stressed that results from the two discrete networks are not directly comparable.
In the network evaluating platinum-based chemotherapies, paclitaxel plus carboplatin and gemcitabine plus carboplatin were found to have a significantly higher ORR than platinum monotherapy (Table 56). There was no significant difference between PLDH plus carboplatin and any of the chemotherapeutic treatments with which it was assessed.
TABLE 56
Results from NMA for ORR of platinum-based chemotherapies
Analysis of non-platinum-based regimens indicates that trabectedin plus PLDH significantly improves ORR compared with PLDH, and oral topotecan (Table 57). Compared with oral topotecan, i.v. topotecan was found to be associated with a significant increase in the proportion of patients achieving CR or PR. No other statistically significant differences were identified.
TABLE 57
Results from NMA for ORR of non-platinum-based chemotherapies
Most identified trials involving platinum-sensitive patients did not present data on tumour response separately for the subgroup of patients with FPS (relapse at > 12 months after last platinum-based treatment) and PPS (relapse at ≥ 6 to ≤ 12 months after last platinum-based treatment) ovarian cancer. No data were available for the subgroup of patients with FPS ovarian cancer.
Results in patients with partially platinum-sensitive ovarian cancer
Only the CALYPSO trial57 presented results (in an accompanying publication) for tumour response in patients with PPS ovarian cancer. There was no significant difference between PLDH plus carboplatin and paclitaxel plus carboplatin in the proportion of patients achieving CR or PR as their best response (OR 0.86, 95% CI 0.58 to 1.27).
Results in tumour response for the subgroup of patients with platinum-resistant/-refractory ovarian cancer
Eight RCTs23,24,49,52,60,62,63,68 reporting results for eight different head-to-head comparisons involving PRR patients were identified (Table 58). Two RCTs enrolled only patients with PRR, with the remaining six RCTs reporting results from a subgroup of patients within the trial. None of the trials identified a significant difference in ORR between the two treatment groups evaluated.
TABLE 58
Overall response rate for the subgroup of patients with PRR ovarian cancer
A NMA was carried out using five of the identified RCTs. Based on clinical expert advice, the decision was taken not to include the trial by Piccart et al.63 comparing paclitaxel vs. oxaliplatin as oxaliplatin is not licensed for the treatment of ovarian cancer and is rarely used in UK clinical practice. In addition, the treatment regimens evaluated in the trial reported by Lortholary et al.62 did not inform the network. In the NMA, PLDH was found to significantly increase ORR compared with paclitaxel (175 mg/m2) every 21 days and with an alternative regimen in which paclitaxel was given weekly at a dose of 67 mg/m2. PLDH monotherapy was also significantly more effective than an unconventional regimen of topotecan in which topotecan was administered weekly at a dose of 4 mg/m2.
No chemotherapeutic regimen was found to have a significantly higher ORR than PLDH monotherapy (Table 59). However, paclitaxel monotherapy, paclitaxel monotherapy (weekly) and topotecan monotherapy (i.v., weekly) were found to have significantly lower ORR than PLDH monotherapy. No other comparison of chemotherapies was found to have a statistically significant difference.
TABLE 59
Results from NMA for ORR in patients with PRR ovarian cancer
Platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
In Bafaloukos et al.,29 tumour response was evaluated using either WHO criteria for patients with measurable disease at baseline or repetitive CA125 level measurements using the algorithm proposed by Rustin et al.,80 and based on CA125 Rustin’s criteria for patients without measurable disease at baseline.78 Bafaloukos et al.29 included a small proportion of women with only CA125 level elevation at baseline as a marker of presence of disease [16/186 (8.6%)] for whom results were analysed both as part of the full trial population and as a subgroup. Tumour assessments for response were carried out every two cycles. A similar proportion of women achieved overall response (CR or PR) in the two treatment groups [47/93 (50.5%) with PLDH plus carboplatin vs. 56/96 (58.3%) with paclitaxel plus carboplatin; OR 0.886, 95% CI 0.535 to 1.402; see Table 60]. Bafaloukos et al.29 found no statistically significant difference between PLDH plus carboplatin and paclitaxel plus carboplatin for overall response in any of the populations assessed: the full trial population (p = 0.309); patients with measurable disease at baseline (p = 0.427); and patients with evaluable disease (elevated CA125 level and/or effusions) (p = 0.713). It is unclear whether the clinicians evaluating response had been masked to treatment, or whether tumour response was evaluated by a central review panel.
TABLE 60
Summary of results for response rate in people with platinum-sensitive recurrent ovarian cancer
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with carboplatin alone
Alberts et al.28 based the primary analysis of response rate on confirmed response rates for CR or PR, with CR and PR assigned according to RECIST criteria.69 Women with only CA125 level elevation at baseline as a marker of disease at study entry (six women) were excluded from the analysis of objective response. It is unclear whether the clinicians evaluating response had been masked to treatment, or whether tumour response was evaluated by a central review panel. Alberts et al.28 found no statistically significant difference between PLDH plus carboplatin and carboplatin alone in confirmed response rate [14/27 (52%) with PLDH plus carboplatin vs. 8/28 (29%) with carboplatin alone; p = 0.10]. However, a follow-up publication by Markman et al.55 reporting more mature data found the difference between groups to be statistically significant favouring PLDH plus carboplatin [16/27 (59%) with PLDH plus carboplatin vs. 8/29 (28%) with carboplatin alone; p = 0.10; OR 2.15, 95% CI 0.79 to 5.83; see Table 60]. As noted earlier, the duration of follow-up in the longer-term study is unclear. In addition, the follow-up publication does not discuss the inclusion of one additional patient in the analysis of the carboplatin group.
Trabectedin plus PLDH compared with PLDH alone
OVA-301.30 evaluated tumour response as the ORR (CR or PR) with response maintained at ≥ 4 weeks, based on RECIST criteria.69 The schedule for tumour assessment is unclear. The primary analysis was based on assessments by independent radiology review. In the subgroup of patients with platinum-sensitive ovarian cancer (218 patients in the trabectedin plus PLDH group vs. 213 patients in the PLDH alone group), trabectedin plus PLDH significantly improved ORR compared with PLDH alone [77/218 (35.3%) with trabectedin plus PLDH vs. 48/213 (22.5%) with PLDH alone; p = 0.042; OR 1.567, 95% CI 1.043 to 2.354; see Table 60]. It should be noted that most patients achieved PR. In the full trial population, only six patients achieved a CR: two in the trabectedin plus PLDH group and four in the PLDH alone group.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In Gordon et al.,49 tumour response was determined by ORR, which comprised CR and PR. Patients achieving either a CR or PR underwent repeat radiological assessment at least 4 weeks later to confirm the response. CR was defined as complete disappearance of all measurable and assessable disease, no new lesions and no disease-related symptoms. PR was defined as a ≥ 50% reduction in the sum of products of the perpendicular diameters of all measurable lesions for at least 4 weeks. Although open-label in design, scans for assessment of disease response and progression underwent independent radiological review.
In the subgroup of patients with platinum-sensitive disease, a similar proportion in the PLDH and topotecan groups achieved either CR or PR as their best response [31/109 (28.4%) with PLDH vs. 32/111 (28.8%) with topotecan; p = 0.964; OR 0.987, 95% CI 0.563 to 1.727; see Table 60]. The difference between groups did not reach statistical significance. In addition, a similar proportion of patients in each group achieved SD as their best response [41/109 (37.6%) with PLDH vs. 42/111 (37.8%) with topotecan; see Table 60].
Topotecan compared with paclitaxel
Response rate was a primary outcome evaluated by ten Bokkel Huinink et al.21 Response included patients achieving either CR or PR as their best response, with CR or PR assigned as per WHO criteria. All claimed responses were independently reviewed and scans confirmed by a radiologist masked to treatment allocation. The timing of tumour assessment is unclear. Patients who were not fully assessed for efficacy or who were not evaluated for response were considered to be non-responders. Data for the subgroup of patients with platinum-sensitive disease (late relapse; relapse at > 6 months after cessation of chemotherapy) were reported separately. In this subgroup of patients, a larger proportion of patients in the topotecan group achieved either CR or PR compared with paclitaxel [15/52 (28.8%) with topotecan vs. 11/55 (20.0%) with paclitaxel; see Table 60] but the statistical significance of this result was not evaluated in the full publication.21 The OR calculated by the TAG indicates the difference to be non-significant (OR 1.442, 95% CI 0.607 to 3.427; see Table 60).
Gemcitabine plus carboplatin compared with carboplatin alone
Pfisterer et al.50 implemented SWOG criteria to determine degree of tumour response. The outcome evaluated was overall response, which included patients achieving a CR or PR as their best response. SWOG defines a CR as complete disappearance of all measurable and evaluable disease and no evidence of non-evaluable disease and PR as sum of products of all lesions decreased by > 50% for at least 3–6 weeks, with no new lesions and no progression of evaluable lesions. Patients were assessed before random assignment, before every cycle during treatment, and every 2–3 months after treatment for at least 2 years. It is unclear from the full publication whether there was an independent review of claimed CR or PR.
Gemcitabine plus carboplatin significantly improved ORR compared with carboplatin alone, with 47.2% (84/178) of patients treated with gemcitabine plus carboplatin achieving CR or PR compared with 30.9% (55/178) of patients treated with carboplatin (p = 0.0016; OR 1.527, 95% CI 1.025 to 2.275; see Table 60).
Paclitaxel plus carboplatin compared with platinum-based therapy alone
The ICON4/AGO-OVAR 2.2 investigators defined response rate as patients achieving CR or PR.61 It is unclear from the full publication which criteria (e.g. WHO, SWOG, or RECIST) were used to assign CR or PR. Timing of response assessment varied with protocol, with those in the AGO protocol assessed after the second and fourth cycles of treatment and those in the Italian ICON4 protocol assessed after three cycles. No further details are reported.
The authors reported that there was no statistically significant difference between treatment regimens in response rate, with 66% (78/119) patients in the paclitaxel plus platinum-based treatment achieving CR or PR compared with 54% (69/128) patients in the platinum chemotherapy alone group, which translates to a difference of 12% (95% CI –0.1% to 24%; p = 0.06). Although the methods state that all efficacy analyses are based on the ITT principle, it should be noted that the number of patients included in the analysis of response is not equal to the number of patients randomised to each group. One potential explanation of this potential discrepancy could be that the patients included in the analysis were those with measurable disease at baseline; number of patients with measurable disease was not reported in the table of baseline characteristics presented in the full publication.
Gonzalez-Martin et al.48 used the WHO criteria to evaluate response in those with measurable disease at baseline, with tumour response assessed every three cycles. For patients without measurable disease at baseline, response was determined according to Rustin’s criteria. The RCT found that paclitaxel plus carboplatin significantly improved ORR (CR plus PR) compared with carboplatin alone [75.6% with paclitaxel plus carboplatin vs. 50.0% with carboplatin alone (p = 0.017; see Table 60)]. The authors commented that based on study design, paclitaxel plus carboplatin was the ‘winner’. Although analysis was based on the ITT population, it should be noted that the comparative statistical analysis was carried out as an exploratory exercise and the reported p-value should be interpreted with caution. In addition, overall response combines data for women with and without measurable disease at baseline.
Paclitaxel compared with oxaliplatin
Objective confirmed response rate was the primary efficacy end point in the trial carried out by Piccart et al.63 Confirmed response was defined as CR or PR as per WHO criteria, and that was observed on at least two consecutive evaluations at least 4 weeks apart. Confirmed response was verified by two independent radiologists. ORR was defined by the total number of patients in each treatment group. Only patients receiving at least two treatment cycles were considered assessable for response. Of the 86 patients randomised, only five were not assessable: two in the paclitaxel group and three in the oxaliplatin group; four patients were deemed ineligible and one patient died 6 days after the first dose of oxaliplatin owing to causes unrelated to treatment.
In the subgroup of patients with platinum-sensitive disease (23 patients), 20% (2/10) of patients in the paclitaxel group achieved PR compared with 38% (5/13) of patients in the oxaliplatin group. The statistical significance of the difference was not assessed in the full publication. The TAG calculated the OR to be 0.520 (paclitaxel vs. oxaliplatin), with a 95% CI of 0.083 to 3.259 (non-significant difference). No patient achieved a CR. The authors caution that, because of the low number of patients in the analysis, conclusions cannot be drawn on the comparative effectiveness of treatments in this subgroup.
Topotecan oral compared with topotecan intravenous
In Gore et al.,24 tumour response was assessed based on WHO criteria such that a CR was the complete disappearance of all known measurable and evaluable disease determined by two measurements not < 4 weeks apart. A PR was defined as a > 50% decrease in measurable lesion size for at least 4 weeks, with no simultaneous increase in a known lesion or appearance of new lesions or increase in evaluable disease. Timing of assessment was determined by radiological method used to measure disease at baseline. Patients evaluated by CT or MRI at baseline were assessed for response at the end of alternate cycles, whereas those evaluated by chest radiograph or photography were assessed at the end of every cycle.
In the platinum-sensitive subgroup (relapse at > 6 months after initial response), although a larger proportion of patients in the i.v. topotecan group achieved a CR or PR as their best response, the difference between treatment groups did not reach statistical significance [11/58 (19%) with oral topotecan vs. 20/56 (36%) with i.v. topotecan; reported as not significant; p-value not reported; see Table 60].
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
Omura et al.68 analysed ORR based on platinum sensitivity. A statistically significant treatment subgroup interaction was identified (p = 0.041). In the subgroup of patients with platinum-sensitive disease, there was no statistically significant difference between paclitaxel 250 mg/m2 and paclitaxel 175 mg/m2 in the proportion of patients achieving a CR or PR (OR 0.63, 95% CI 0.191 to 2.07). The OR was adjusted for histological cell type (papillary serous compared with clear cell or mucinous cell vs. other cell types), cooperative group, performance status and prior platinum sensitivity. The proportion of patients achieving either CR or PR in each group was 36.0% (9/25) and 48.1% (13/27) in the 250 mg/m2 and 175 mg/m2 groups, respectively. Unadjusted OR as calculated by the TAG was 0.748 (95% CI 0.273 to 2.051; see Table 60). For the purposes of the NMA, based on clinical expert advice, it has been assumed that doses of paclitaxel of 175 mg/m2 up to 250 mg/m2 are of equivalent clinical effectiveness and thus this trial has not been included in the NMA.
Paclitaxel weekly compared with paclitaxel every 3 weeks
In the trial carried out by Rosenberg et al.,60 patients were stratified at randomisation based on platinum resistance (relapse ≤ 6 months vs. > 6 months after primary platinum-based treatment). Results for the primary outcome of tumour response were reported separately for the subgroups categorised by platinum resistance. Evaluations of tumour size were carried out at baseline and subsequently every 6 weeks using the same imaging technique for all assessments. Tumour response was categorised as per WHO criteria, with overall response including CR or PR as a best response.
In the subgroup of patients with platinum-sensitive disease, a similar proportion of patients achieved either CR or PR in each treatment group [26/48 (54.2%) with paclitaxel every 7 days vs. 25/52 (48.1%) with paclitaxel every 21 days; see Table 60]. The statistical significance of the result in this subgroup of patients was not reported in the full publication. The OR calculated by the TAG indicates that the difference between groups did not reach statistical significance (OR 1.127, 95% CI 0.574 to 2.212; see Table 60). It should be noted that the results include patients with unconfirmed CR and PR. In the full trial population, 3 patients in each group had unconfirmed CR, and 7 and 6 patients in the paclitaxel every 7 days and paclitaxel every 21 days, respectively, had unconfirmed PR. The corresponding number of patients in the platinum-sensitive subgroup is not reported.
Network meta-analysis (platinum sensitive)
The RCTs available for inclusion in the NMA evaluating ORR in patients with platinum-sensitive recurrent ovarian cancer are summarised in Table 60. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Figure 9.

FIGURE 9
Networks for ORR for people with platinum-sensitive recurrent ovarian cancer. (a) Network 1; and (b) network 2.
Network 1 (see Figure 9a) consisted of the following comparators:
- paclitaxel plus carboplatin
- PLDH plus carboplatin
- platinum as a monotherapy
- gemcitabine plus carboplatin.
Although ORR does not inform the economic evaluation conducted by the TAG (see Chapter 4, Independent economic assessment), for consistency with OS and PFS, paclitaxel plus carboplatin was chosen as the baseline treatment. However, results are reported in Table 61 sequentially covering all possible comparisons. Overall, there was no significant difference (at the 5% level) for any of the doublet chemotherapies assessed compared with paclitaxel plus carboplatin (or with each other). Platinum monotherapy was associated with a significant reduction in ORR compared with all doublet chemotherapies, with the exception of PLDH plus carboplatin, where no significant difference was found.
TABLE 61
Results of the NMA for ORR for people with platinum-sensitive recurrent ovarian cancer
Network 2 (see Figure 9b) consisted of the following comparators:
- PLDH monotherapy
- trabectedin plus PLDH
- topotecan monotherapy (i.v.)
- paclitaxel monotherapy, i.e. 175 mg/m2 or 200 mg/m2 every 21 days
- topotecan (oral)
- paclitaxel monotherapy (weekly); i.e. paclitaxel 67 mg/m2 every week for 21 days.
Pegylated liposomal doxorubicin hydrochloride monotherapy was chosen as the baseline treatment in order to maintain consistency with the results reported for the NMAs for OS and PFS. However, results are reported in Table 61 sequentially covering all possible comparisons. Overall, only trabectedin plus PLDH demonstrated a significant increase in ORR (at the 5% level) compared with PLDH monotherapy. Trabectedin plus PLDH would also be considered to have a statistically significant increased ORR when compared directly with topotecan monotherapy (oral) but to have no significant difference from any other treatment assessed. None of the other comparisons of chemotherapies would be considered significantly different from one another, with the exception of topotecan monotherapy (oral), which was found to have a significantly lower ORR than topotecan monotherapy (i.v.).
Partially platinum sensitive
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
An accompanying publication to CALYPSO31 presents results for PFS and response rate (secondary outcome) for a subgroup of patients with PPS (TFI 6–12 months).57 The principal publication provided a comprehensive description of the criteria for progression and indicated that tumour assessments occurred every 3 months and states that ORR was ‘response maintained ≥ 4 weeks by RECIST’.31 Table 2 in the accompanying publication indicates that confirmed best responses are based on RECIST criteria,69 and ORR is the total of confirmed CR and PR.57
There was no statistically significant difference between PLDH plus carboplatin and paclitaxel plus carboplatin in ORR [63/161 (39%) with PLDH plus carboplatin vs. 83/183 (45%) with paclitaxel plus carboplatin; p = 0.691]. The proportion of patients achieving CR, PR and SD, together with PD, are presented in Table 62.
TABLE 62
Summary of results for response rate in people with PPS recurrent ovarian cancer
Network meta-analysis (PPS)
As only a single trial was identified with data to inform ORR in patients with PPS recurrent ovarian cancer (see Table 62) no NMA was possible for this subgroup.
Platinum resistant/refractory
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
Gordon et al.49 found no statistically significant difference between PLDH and topotecan in the proportion of patients with PRR ovarian cancer achieving either CR or PR as their best response [16/130 (12.3%) with PLDH vs. 8/124 (6.5%) with topotecan; p = 0.118; see Table 64]. However, a larger proportion of patients in the topotecan group achieved SD as their best response [36/130 (27.7%) with PLDH vs. 53/124 (42.7%) with topotecan; significance not assessed; see Table 64].
TABLE 64
Summary of results for response rate in population with PRR recurrent ovarian cancer
Topotecan compared with paclitaxel
In the subgroup of patients with PRR disease (resistant, early relapse and interim relapse; 119 patients), ten Bokkel Huinink et al.21 that 13.3% (8/60) of patients treated with topotecan and 6.8% (4/59) of patients treated with paclitaxel achieved either CR or PR as their best response (significance not assessed; see Table 64). Results for the individual categories that make up PRR are presented in Table 63.
TABLE 63
Response rate for resistant, early relapse and interim relapse
Paclitaxel plus carboplatin compared with paclitaxel alone
Lortholary et al.62 based response rate on the proportion of patients who achieved either a CR or PR as their best response. Patient response was determined according to RECIST criteria69 for patients with measurable disease and Rustin’s criteria for CA125 levels for patients with non-measurable disease. Chest CT and abdominopelvic or MRI were obtained every two cycles or as needed for assessment of duration of response. Objective response was to be confirmed radiologically at least 4 weeks after initial response. Lortholary et al.62 found that a similar response rate was achieved in the weekly paclitaxel and weekly paclitaxel plus carboplatin groups [20/57 (35.1%) with weekly paclitaxel vs. 19/51 (37.3%) with weekly paclitaxel plus carboplatin; see Table 64]. The statistical significance of the difference between groups was not assessed by Lortholary et al.62 The TAG calculated an OR of 1.06 (95% CI 0.510 to 2.209), which indicates that the difference between groups is not statistically significant.
Paclitaxel compared with oxaliplatin
In the subgroup of patients with PRR ovarian cancer (63 patients), Piccart et al.63 found that 16% (5/31) of patients in the paclitaxel group achieved PR compared with 6% (2/32) of patients in the oxaliplatin group (see Table 64). No patient achieved a CR. The statistical significance of the difference was not assessed in the full publication.63 The authors caution that, because of the low number of patients in the analysis, conclusions cannot be drawn on the comparative effectiveness of treatments in this subgroup.
Topotecan oral compared with topotecan intravenous
In the subgroup of patients with PRR ovarian cancer (progression or SD during treatment or relapse at < 6 months after initial response), Gore et al.24 found that a small proportion of patients in each group achieved a CR or PR as their best response, with no statistically significant difference between groups [6/77 (7.8%) with oral topotecan vs. 6/75 (8.0%) with i.v. topotecan; reported as not significant; p-value not reported; see Table 64].
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
Clinical benefit rate was the primary outcome in the trial carried out by Sehouli et al.23 Clinical benefit rate comprised CR, PR and SD as best response. By contrast, most trials identified have evaluated ORR of CR or PR. In the trial, tumour response could be determined radiologically and categorised as per RECIST criteria69 or by change in CA125 level as per GCIG criteria,70 with choice of method of assessment at the discretion of the investigator. Schedule of assessment of response was not reported. It should be noted that, despite most patients having measurable disease at baseline, only a small proportion of women were evaluated radiologically for response (19.8%).
For the primary outcome of clinical benefit, 58% (46/80) of patients treated with the conventional dose of topotecan achieved CR, PR or SD compared with 47% (36/76) of patients receiving topotecan weekly. The statistical significance of the difference between groups was not reported. Considering ORR (CR or PR), the proportion of patients achieving CR or PR as best response was 18.8% (15/80) and 9.2% (7/76) in the conventional topotecan compared with weekly topotecan groups, respectively.
Of the 80 patients in the conventional topotecan group, response was evaluated by CA125 level alone in 62 patients (CR or PR = 13 patients). By comparison, 63 out of 76 patients were evaluated by CA125 level alone (CR or PR = five patients).
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
In the subgroup of patients with PRR disease, Omura et al.68 found that paclitaxel 250 mg/m2 significantly increased the proportion of patients achieving a CR or PR compared with paclitaxel 175 mg/m2 (OR 2.59, 95% CI 1.36 to 4.95). The OR was adjusted for histological cell type (papillary serous compared with clear cell or mucinous cell vs. other cell types), cooperative group, performance status and prior platinum sensitivity. The proportion of patients achieving either CR or PR in each group was 36.7% (40/109) and 22.1% (23/104) in the 250 mg/m2 and 175 mg/m2 groups, respectively. Unadjusted OR as calculated by the TAG was 1.659 (95% CI 0.930 to 2.961; see Table 64), which is a non-statistically significant difference.
Paclitaxel weekly compared with paclitaxel every 3 weeks
In the subgroup of patients with PRR, Rosenberg et al.60 found that a similar proportion of patients achieved either CR or PR in each treatment group [11/57 (19.3%) with paclitaxel every 7 days vs. 13/51 (25.5%) with paclitaxel every 21 days; Table 64]. The statistical significance of the result in this subgroup of patients was not reported in the full publication. As noted earlier, unconfirmed CR and PR is not broken down by subgroup and it is unclear how many patients in the PRR analysis had unconfirmed CR or PR.
Network meta-analysis (PRR)
The RCTs available for inclusion in the NMA evaluating ORR in patients with PRR recurrent ovarian cancer are summarised in Table 64. The network of trials constructed for this outcome is depicted in Figure 10 and contains the following comparators:

FIGURE 10
Networks for ORR in people with PRR recurrent ovarian cancer.
- PLDH monotherapy
- topotecan monotherapy (i.v.); i.e. topotecan 1.25 or 1.5 mg/m2 daily for 5 days every 21 days
- paclitaxel monotherapy; i.e. 175 mg/m2 or 200 mg/m2 every 21 days
- topotecan monotherapy (oral)
- paclitaxel monotherapy (weekly); i.e. paclitaxel 67 mg/m2 every week for 21 days
- topotecan monotherapy (i.v., weekly); i.e. topotecan 4.0 mg/m2 (weekly) on days 1, 8 and 15 of a 28-day cycle.
The results from this NMA are presented in Table 65. Overall, no chemotherapy was found to have a significantly higher ORR (at the 5% level) than PLDH monotherapy. However, paclitaxel monotherapy, paclitaxel monotherapy (weekly) and topotecan monotherapy (i.v., weekly) were found to have significantly lower ORR than PLDH monotherapy. No other comparison of chemotherapies was found to have a statistically significant difference.
TABLE 65
Results from NMA for ORR in people with PRR recurrent ovarian cancer
A RCT that provided results for this population but which did not share a common comparator within the network compared low-dose paclitaxel (80 mg/m2) with low-dose paclitaxel (80 mg/m2) plus carboplatin.62 However, Lortholary et al.62 identified no significant difference in OS between the two different treatment regimens (OR 1.062, 95% CI 0.510 to 2.209).
Full population (mixed platinum-free intervals)
Trabectedin plus PLDH compared with PLDH alone
OVA-30130 evaluated tumour response as the ORR (CR or PR) with response maintained ≥ 4 weeks based on RECIST criteria.69 In the full trial population, trabectedin plus PLDH significantly improved ORR compared with PLDH alone [93/337 (27.6%) with trabectedin plus PLDH vs. 63/335 (18.8%) with PLDH alone; p = 0.080; see Table 67). It should be noted that most patients achieved PR, with only six patients being assessed as CR: two in the trabectedin plus PLDH group and four in the PLDH alone group.
TABLE 67
Summary of results for response rate in population with mixed PFIs
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
In the full trial population, Gordon et al.49 found no statistically significant difference between PLDH and topotecan in the proportion of patients achieving either CR or PR as their best response [47/239 (19.7%) with PLDH vs. 40/235 (17.0%) with topotecan; p = 0.390; see Table 67]. In addition, a similar proportion of patients in each group achieved SD as their best response [77/239 (32.2%) with PLDH vs. 95/235 (40.4%) with topotecan; see Table 67].
Topotecan compared with paclitaxel
In patients who received at least one dose of study drug (226 patients), ten Bokkel Huinink et al.21 found no statistically significant difference between topotecan and paclitaxel in ORR [CR or PR; 23/112 (20.5%) with topotecan vs. 15/114 (13.2%) with paclitaxel; p = 0.138; see Table 67]. It should be noted that, of the 226 patients included in the analysis, only 202 were evaluated for response, with the remaining 24 patients considered to be non-responders.
The authors carried out an analysis of response rate relative to baseline disease characteristics. Higher response rates in both groups were observed in patients without ascites at baseline, with better performance status scores (lower score is better), with smaller tumour burden (< 5 cm), and in those who responded to first-line chemotherapy (summarised in Table 66).
TABLE 66
Response rate relative to baseline characteristics for topotecan relative to paclitaxel
Paclitaxel compared with oxaliplatin
Piccart et al.63 found that a similar proportion of patients achieved PR in the paclitaxel and oxaliplatin groups [7/41 (17.1%) with paclitaxel vs. 7/45 (15.6%) with oxaliplatin]. No patient achieved a CR. The statistical significance of the difference was not assessed in the full publication.
Topotecan oral compared with topotecan intravenous
In the full trial population, Gore et al.24 found no statistically significant difference between oral and i.v. topotecan in the proportion of patients achieving a CR or PR as their best response [17/135 (13%) with oral topotecan vs. 26/131 (20%) with i.v. topotecan; reported as not significant; p-value not reported; see Table 67].
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
Omura et al.68 evaluated only patients with measurable disease for tumour response (131 patients treated with paclitaxel 175 mg/m2 vs. 134 patients treated with paclitaxel 250 mg/m2). ORR comprised patients with CR (disappearance of all gross evidence of disease for at least 4 weeks) or PR (≥ 50% reduction in the product of perpendicular measurements of each lesion for at least 4 weeks). Response was assessed before every other cycle of therapy. It is unclear from the methods whether the assessor was masked to treatment allocation.
In the full trial population, a significantly larger proportion of patients in the paclitaxel 250 mg/m2 group than in the 175 mg/m2 group achieved either CR or PR as their best response [49/134 (36%) with paclitaxel 250 mg/m2 vs. 36/131 (27%) with paclitaxel 175 mg/m2; see Table 67]. The accompanying OR was 1.89 (95% CI 1.07 to 3.31; p = 0.027). The OR had been adjusted for histological cell type (papillary serous compared with clear cell or mucinous cell vs. other cell types), cooperative group, performance status and prior platinum sensitivity.
In patients randomised to paclitaxel 250 mg/m2 and who were subsequently randomised to filgrastim 5 or 10 µg/kg, there was no statistically significant difference among the filgrastim groups in the proportion of patients achieving CR or PR [24/68 (35%) with 5 µg/kg filgrastim vs. 25/66 (37.9%) with 10 µg/kg filgrastim].
Paclitaxel weekly compared with paclitaxel every 3 weeks
Rosenberg et al.60 found no statistically significant difference between paclitaxel every 7 days and paclitaxel every 21 days in the proportion of patients achieving either CR or PR [37/105 (35.2%) with paclitaxel every 7 days vs. 38/103 (36.9%) with paclitaxel every 21 days; reported as not significant; p-value not reported]. As noted, patients with unconfirmed CR (six patients) and PR (13 patients) are included in this analysis and this should be borne in mind when interpreting the results.
Network meta-analysis (mixed PFIs)
The RCTs available for inclusion in the NMA evaluating ORR in patients with mixed PFIs in recurrent ovarian cancer are summarised in Table 67. However, based on expert clinical opinion, the TAG decided not to evaluate this mixed-patient population, as the results would not be considered clinically meaningful.
Quality of life
Of the 16 RCTs identified, 10 reported some level of data on QoL.21,23,30,31,48–50,61–63 A systematic review of health-related QoL reporting in ovarian cancer trials identified considerable disparity in the level of reporting of QoL results, the questionnaires used to evaluate QoL and the time points for evaluation.8 Given the often palliative nature of second- and subsequent-line therapeutic treatments for ovarian cancer, there has been a move to place greater emphasis on assessment of QoL in this condition.
The most commonly used scale in the identified trials is the EORTC QLQ-C30 questionnaire,81 which was developed to assess the QoL of cancer patients and can be supplemented with disease-specific modules for individual cancers, including ovarian cancer. The QLQ-C30 questionnaire81 comprises six questions that address dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea and financial impact, in addition to one global QoL scale, five functional scales (physical, role, emotional, cognitive, and social) and three symptom scales (fatigue, pain and nausea/vomiting).
Here, a narrative description of QoL is presented for those trials providing data on this outcome.
Summary of results for quality of life
Owing to a paucity of data, results for individual trials assessing QoL are summarised here. It should be noted that, generally, reporting of results was limited, with few trials reporting scores generated from responses to the questionnaires.
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
Baseline QoL scores showed impaired global health scores and considerable symptom burden. At 3 months, PLDH plus carboplatin was associated with a significant improvement in global health compared with paclitaxel plus carboplatin. However, this benefit was not maintained at 6 months.
The QLQ-OV28 questionnaire indicated that paclitaxel plus carboplatin was associated with significantly worse peripheral neuropathy and other chemotherapy side effects at 3 months and 6 months compared with PLDH plus carboplatin.71
Trabectedin plus pegylated liposomal doxorubicin hydrochloride compared with pegylated liposomal doxorubicin hydrochloride alone
Mean change in scores from baseline to end of treatment were similar between trabectedin plus PLDH and PLDH monotherapy, with no differences reaching statistical significance on any questionnaire. The difference between groups in mean scores for the QLQ-C30 global health status scale81 did not reach ≥ 5 at any time point, which indicated non-significance. Additional information on QoL in the subgroup of patients with PPS ovarian cancer provided in the MS indicates a difference in global health status score among responding patients beyond cycle 5, with patients in the trabectedin plus PLDH group having a higher score than those receiving PLDH monotherapy (higher score is favourable).
Topotecan compared with paclitaxel
The EORTC QLQ-C3081 scores were similar between the groups and neither paclitaxel nor topotecan was associated with any compromise of QoL.
Paclitaxel plus carboplatin compared with platinum-based monotherapy
The ICON4/AGO-OVAR 2.261 investigators evaluated QoL using the EORTC QLQ-C3081 questionnaire. It was reported that, in the first 6 months after randomisation, patients receiving platinum monotherapy scored significantly worse on the nausea and vomiting symptom scale than did the paclitaxel plus platinum-based chemotherapy group. However, this difference seemed to be transient and was observed for only the first 15 weeks after randomisation. All other worst scores or AUCs were reported to be similar between treatment groups for the remaining eight symptom scales, the five functional scales, and global health status of the QLQ-C30.81
Gonzalez-Martin et al.48 also evaluated QoL using the QLQ-C3081 questionnaire. No differences between treatments in the five functional components of the QLQ-C30 were reported.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
Quality of life was assessed using the EORTC QLQ-C3081 questionnaire. At week 12, no significant differences between the groups in any of the measured scores were noted. The proportion of patients who had a worsened global QoL score was also reported to be similar in the two treatment groups. Topotecan was associated with a significantly more favourable rating on the pain subscale of the EORTC QLQ-C30.
Gemcitabine plus carboplatin compared with carboplatin alone
Based on responses to the EORTC QLQ-C3081 and QLQ-C28,71 no statistically significant differences between treatment groups for all scales/items at baseline or in changes in score from baseline to treatment discontinuation were noted.
Paclitaxel plus carboplatin compared with paclitaxel alone
Response to EORTC QLQ-C3081 indicated that global health scores were stable over time and similar across treatment arms. Among symptom and functional scales, patients receiving weekly paclitaxel plus carboplatin experienced improvements in constipation, abdominal/gastrointestinal symptoms, appetite loss, pain and emotional functioning. Patients treated with weekly paclitaxel alone experienced improvements in attitude to disease and insomnia, but worsening of dyspnoea and peripheral neuropathy.
Paclitaxel compared with oxaliplatin
Mean QoL score on the EORTC QLQ-C3081 increased by > 10 points between baseline and cycle 4 for patients in the paclitaxel group, irrespective of study withdrawal. By contrast, in the oxaliplatin group, the mean QoL score decreased through cycle 2, but by < 10 points, after which most patients’ mean scores returned to baseline levels.
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
It was reported that there were no differences between treatment groups in EORTC QLQ-OV28 scores.71
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
Quality-of-life data were collected during CALYPSO31 using the EORTC QLQ-C3081 questionnaire and supplemented by the ovarian cancer-specific OV28 module. QoL was assessed at baseline and subsequently at the 3-, 6-, 9- and 12-month assessments. QoL was not assessed after progression of disease. Results for QoL are presented in full in an accompanying publication.59
Analyses of QoL were restricted to those patients with both a completed baseline questionnaire and at least one QoL form completed during follow-up. At baseline, 90% of patients completed the questionnaires [421/467 (90.1%) with PLDH plus carboplatin vs. 458/509 (90.0%) with paclitaxel plus carboplatin]. Compliance remained high at 3 months’ follow-up (79.3% with PLDH plus carboplatin vs. 73.5% with paclitaxel plus carboplatin) but steadily declined over the remaining 9 months (completed questionnaires: 6 months – 68.3% with PLDH plus carboplatin vs. 60.3% with paclitaxel plus carboplatin; 12 months – 50.6% with PLDH plus carboplatin vs. 49.7% with paclitaxel plus carboplatin). Given that only 50% of patients were compliant at 12 months, the authors restricted reporting of results to data collected up to 9 months’ follow-up.
Baseline QoL scores showed impaired global health scores and considerable symptom burden (see Table 68).
TABLE 68
The QLQ-C30 and QLQ-OV28 scores at baseline and at 3 and 6 months’ follow-up
At 3 months, PLDH plus carboplatin was associated with a significant improvement in global health compared with paclitaxel plus carboplatin (mean score at 3 months [standard deviation (sd) –2.2 (22.7) with paclitaxel plus carboplatin vs. 2.6 (26.0) with PLDH plus carboplatin; p = 0.01]. However, this benefit was not maintained at 6 months, at which time the difference between groups for this measure was not statistically significant [4.8 (24.4) with paclitaxel plus carboplatin vs. 2.4 (26.4) with PLDH plus carboplatin; p = 0.31]. It should be noted that the difference between groups is modest. Results from QoL analyses are presented in Table 68.
Other symptom scores for which there was a significant difference at 3 months, but which was not maintained at 6 months, are physical functioning; nausea and vomiting; pain; dyspnoea; and sexual functioning.
Assessment of QLQ-OV28 indicated that paclitaxel plus carboplatin was associated with significantly worse peripheral neuropathy and other chemotherapy side effects at 3 months and 6 months compared with PLDH plus carboplatin.
Trabectedin plus pegylated liposomal doxorubicin hydrochloride compared withpegylated liposomal doxorubicin hydrochloride alone
OVA-30130 evaluated patient-reported outcomes as an exploratory end point using the cancer-specific EORTC QLQ-C3081 and QLQ-OV28 questionnaires, together with the generic European Quality of Life-5 Dimensions (EQ-5D) questionnaire, which is the utility measure preferred by NICE. Results from the analyses were reported in a follow-up publication by Krasner et al.67
Patients completed questionnaires at baseline, on day 1 of each treatment cycle before administration of the allocated treatment, and at the end of treatment. Statistical analyses of QoL were based on all randomised patients. Non-random withdrawal from treatment across groups, most frequently as a result of disease progression or poor tolerability, is well recognised in trials evaluating treatments in cancer. To account for the potential imbalance in patients lost to follow-up between the groups, the authors implemented a pattern mixture model.
Compliance was high, with an overall rate of missing questionnaires of 15%, which was balanced across the groups (14.4% with trabectedin plus PLDH vs. 15.2% with PLDH alone). At most time points, the rate of missing questionnaires was < 10%, but at the end of treatment the rate rose to 34%.
Mean change in scores from baseline to end of treatment were similar between trabectedin plus PLDH and PLDH alone, with no differences reaching statistical significance on any questionnaire. The authors report that the difference between groups in mean scores for the QLQ-C3081 global health status scale did not reach ≥ 5 at any time point, which indicated non-significance. Mean change in QLQ-C30 global health status scale over time is presented in Figure 11. Minor, sporadic differences in the fatigue symptom scale were observed in cycles 3 and 9, with some worsening of fatigue for subjects with trabectedin plus PLDH.
FIGURE 11
Mean QLQ-C30 global health status score over time (reproduced with permission from PharmaMar’s submission).
In the submission received from PharmaMar, additional information on QoL in the subgroup of patients with PPS ovarian cancer is provided. The manufacturer notes that a difference in global health status score was observed among responding patients beyond cycle 5 in the PPS subgroup, with patients in the trabectedin plus PLDH group having a higher score than those receiving PLDH alone (higher score is favourable) (Figure 12). The manufacturer comments that the benefit associated with trabectedin plus PLDH is clinically meaningful. It should be noted that the analysis seems to be based on patients with PPS ovarian cancer who responded to treatment (n is reported to be 51) rather than the full PPS subgroup. In addition, all QoL analyses are exploratory.

FIGURE 12
Mean QLQ-C30 global health status score over time for the PPS subgroup (reproduced with permission from PharmaMar’s submission). SEM, standard error of the mean.
Topotecan compared with paclitaxel
ten Bokkel Huinink et al.21 evaluated QoL using the EORTC QLQ-C3081 questionnaire. It is reported that between 75% and 85% of patients enrolled in the study had evaluable QoL data. However, no results are reported in either of the two identified publications.21,52 The authors comment that scores were similar between the groups and that neither paclitaxel nor topotecan was associated with any compromise of QoL.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
Gordon et al.49 report that QoL was assessed using the EORTC QLQ-C3081 questionnaire. All patients completed a QLQ-C30 questionnaire before study entry, during every cycle and 4 weeks after the last treatment dose. The full publication reports that about 82% of patients completed the questionnaire at baseline and that at study entry function and symptom scale scores were similar between the groups. At week 12, it is reported that there were no significant differences between the groups in any of the measured scores. No further details are reported in the full publication. Additional detail is reported in TA91,13 which is summarised here.
Technology appraisal no. 91 reports that only 50% of patients completed the questionnaire at 12 weeks.13 At week 12, similar proportions of patients in the PLDH and topotecan groups had improved or stable global QoL scores, with no statistically significant difference identified between groups [68/239 (28.5%) with PLDH vs. 55/235 (23.4%) with topotecan; relative risk (RR) 0.82, 95% CI 0.61 to 1.12]. The proportion of patients who had a worsened global QoL score was also reported to be similar in the two treatment groups [49/239 (20.5%) with PLDH vs. 48/235 (20.4%) with topotecan; RR 0.97, 95% CI 0.70 to 1.42]. Considering the subscales of the QLQ-C30,79 a statistically significant difference between PLDH and topotecan was identified for only the pain subscale score (RR 1.26, 95% CI 1.08 to 1.50), which favoured topotecan (results from TA9113 summarised in Table 69).
TABLE 69
Percentage of patients with a maintained or improved QoL score at 12 weeks’ follow-up for PLDH vs. topotecan (collated from table 10 and figure 7 in TA91)
Gemcitabine plus carboplatin compared with carboplatin alone
Pfisterer et al.50 evaluated QoL using the EORTC QLQ-C3081 and QLQ-C28 (version 2) questionnaires. QoL was assessed 2 weeks before enrolment and before commencement of each treatment cycle. Questionnaire completion rate was high, with 85.4% (152/178) and 82.6% (147/178) of patients in the gemcitabine plus carboplatin and carboplatin alone groups, respectively, having completed a questionnaire at baseline and at least one postbaseline questionnaire. The authors report that there were no statistically significant differences between treatment groups for all scales/items at baseline or in changes in score from baseline to treatment discontinuation. No further details reported.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
The ICON4/AGO-OVAR 2.2 investigators evaluated QoL using the EORTC QLQ-C3081 questionnaire. In total, 90% (482/536) of patients enrolled in centres following the MRC CTU ICON4 protocol completed the questionnaire at baseline, before receiving any study drug. The authors report that all scales were balanced across the two treatment groups at baseline and that most patients had little or no functional difficulties and few had moderate or severe symptoms at baseline (no further details reported). In the first 6 months after randomisation, patients receiving platinum monotherapy scored significantly worse on the nausea and vomiting symptom scale than did the paclitaxel plus platinum-based chemotherapy group (p = 0.0014 for worst score and p = 0.005 for AUC). However, this difference seemed to be transient and was observed for only the first 15 weeks after randomisation. All other worst scores or AUCs were reported to be similar between treatment groups for the remaining eight symptom scales, the five functional scales, and global health status of the QLQ-C3081 (no further details reported).
Gonzalez-Martin et al.48 also evaluated QoL using the QLQ-C3081 questionnaire. The authors reported that there were no differences between treatments in the five functional components of the QLQ-C30. No other details were reported.
Paclitaxel plus carboplatin compared with paclitaxel alone
Lortholary et al.62 explored QoL using the EORTC QLQ-C3081 and QLQ-OV28 questionnaires. Completion rate of questionnaires ranged between 40% and 70%, with questionnaires collected at baseline, and after two, four and six cycles of treatment. Global health scores were stable over time and similar across treatment arms. Among symptom and functional scales, patients receiving weekly paclitaxel plus carboplatin experienced improvements in constipation, abdominal/gastrointestinal symptoms, appetite loss, pain and emotional functioning. Patients treated with weekly paclitaxel experienced improvements in attitude to disease and insomnia, but worsening of dyspnoea and peripheral neuropathy. No further details reported.
Paclitaxel compared with oxaliplatin
Piccart et al.63 used the EORTC QLQ-C3081 questionnaire and a specific checklist to evaluate QoL. Patients were to complete the questionnaires at least 8 days before their first treatment and, subsequent to start of treatment, every 6 weeks or every two visits. At baseline, completed questionnaires were available for 66 patients. However, at the end of the second treatment cycle (week 6) only 47 patients had completed their questionnaires, with a further drop to 31 completed questionnaires by the end of the fourth treatment cycle (12 weeks). The authors report that the mean QoL score increased by > 10 points between baseline and cycle 4 for patients in the paclitaxel group, irrespective of study withdrawal. By contrast, in the oxaliplatin group, the mean QoL score decreased through cycle 2, but by <10 points, after which most patients’ mean scores returned to baseline levels. The authors propose that the initial decrease in score in the oxaliplatin group is associated with peripheral neurotoxicity. No further details on scores are reported.
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
Sehouli et al.23 explored disease-specific QoL using the EORTC QLQ-OV28 questionnaire. Details on schedule of completion of questionnaires were not reported. Baseline data were available for 120 patients (65 treated with conventional topotecan vs. 55 treated with weekly topotecan). A second assessment was available for considerably fewer patients (39 treated with conventional topotecan vs. 20 treated with weekly topotecan), but it is unclear at what point in the trial the second questionnaire was completed. Patients with at least a completed baseline and at least one follow-up assessment reported an improvement in neuropathy scales but a worsening in body image. The authors reported that there was no difference in scores between treatment groups. No further details were reported.
Adverse events
Summary of results for adverse effects
Data for adverse effects for individual trials are reported in the main text. Within each trial, the most frequently reported adverse effects were as expected for the individual treatments based on the SmPC. Commonly occurring adverse effects were alopecia, nausea and vomiting, haematological toxicities (neutropenia, anaemia, thrombocytopenia and leucopenia).
Based on expert clinical advice, the TAG restricted its comparison of AEs to those considered most problematic for patients or most likely to consume substantial health-care resource. The potential for a NMA was, therefore, investigated for the following severe (grades 3 and 4) AEs: allergic reaction; alopecia; anaemia; fatigue; febrile neutropenia; nausea and vomiting; and neuropathy. The results of each investigation are presented in the main text. The results were mixed, with most found to be non-significant or with chemotherapies having significant lower risk of one or more AEs but then being found to have significantly higher risks of others (e.g. PLDH plus carboplatin has significantly less risk of allergic reaction and alopecia but significantly higher risk of anaemia and nausea and vomiting when compared with paclitaxel plus carboplatin). In many cases, a NMA was not possible owing to the lack of available data in the trials assessed. In these instances, the individual trial results are reported with the ORs and 95% CIs calculated. Overall, no chemotherapy was consistently associated with either a lower risk or a higher risk of the severe AEs assessed.
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with paclitaxel plus carboplatin
Bafaloukos et al.29 based the safety analysis on the 177 patients who received at least one cycle of allocated treatment (84 in the PLDH plus carboplatin group vs. 89 in the carboplatin plus paclitaxel group). A significantly larger proportion of patients in the paclitaxel plus carboplatin group discontinued treatment because of associated toxicity (13.5% with paclitaxel plus carboplatin vs. 3% with PLDH plus carboplatin; p = 0.016).
Neutropenia (grades 3 and 4) was the most commonly observed severe toxicity, with a similar proportion of people between groups experiencing this adverse effect (30% with paclitaxel plus carboplatin vs. 35% with PLDH plus carboplatin); the difference between groups did not reach statistical significance (p-value not reported).
Pegylated liposomal doxorubicin hydrochloride plus carboplatin was associated with a significantly higher rate of severe thrombocytopenia (grades 3 and 4: 11% with PLDH plus carboplatin vs. 2% with paclitaxel plus carboplatin; p = 0.016; Table 70) and PPE and skin toxicity (grades 1 and 2; 38% with PLDH plus carboplatin vs. 9% with paclitaxel plus carboplatin; p = 0.003). By contrast, paclitaxel plus carboplatin was associated with a significantly higher rate of severe neurotoxicity (7% with paclitaxel plus carboplatin vs. 0% with PLDH plus carboplatin; p = 0.029) and alopecia (20% with paclitaxel plus carboplatin vs. 5% with PLDH plus carboplatin; p = 0.003).
TABLE 70
Adverse effects as reported by Bafaloukos et al.
In the CALYPSO trial,31 significantly fewer patients treated with PLDH plus carboplatin discontinued treatment early as a result of adverse effects compared with patients treated with paclitaxel plus carboplatin (6% with PLDH plus carboplatin vs. 15% with paclitaxel plus carboplatin; p < 0.001). There were two treatment-related deaths in the PLDH plus carboplatin group: one attributed to cerebral haemorrhage and one to acute myeloid leukaemia.
Overall, severe (grades 3 and 4) non-haematological toxicity occurred significantly more frequently in the paclitaxel plus carboplatin group (36.8% with paclitaxel plus carboplatin vs. 28.4% with PLDH plus carboplatin; p = 0.001). Incidence of anaemia and febrile neutropenia were similar between treatment groups. However, grade 3 and grade 4 neutropenia and thrombocytopenia were significantly more frequent in the paclitaxel-plus-carboplatin group (neutropenia: p < 0.01; thrombocytopenia: p < 0.001; Table 71).
Adverse events that occurred significantly more frequently in the paclitaxel plus carboplatin group than in the PLDH plus carboplatin group were grade 2 alopecia (complete or total hair loss) (p < 0.001), hypersensitivity reactions (p < 0.001), and sensory and motor neuropathy (sensory, p < 0.001; motor, p = 0.002; see Table 71). By contrast, PLDH plus carboplatin was associated with a significantly higher incidence of hand–foot syndrome (grades 2 and 3; p < 0.001), nausea (p < 0.001), vomiting (p < 0.001) and mucositis (p < 0.001; Table 71).
Pegylated liposomal doxorubicin hydrochloride plus carboplatin compared with carboplatin alone
Alberts et al.28 reported that the most common grade 3 and grade 4 AEs in the PLDH plus carboplatin group were haematological, with eight patients (26%) experiencing a grade 4 haematological AE (thrombocytopenia and neutropenia; Table 72). No patient in the PLDH plus carboplatin group had an allergic reaction compared with nine patients treated with carboplatin alone.
Trabectedin plus pegylated liposomal doxorubicin hydrochloride compared with pegylated liposomal doxorubicin hydrochloride alone
In OVA-301,30 safety was evaluated using NCI-CTC for AEs and the safety analysis population included all randomly assigned patients who received one or more doses of trabectedin or PLDH. Deaths were summarised by treatment and primary cause. Nineteen patients died during treatment (8 in the PLDH group vs. 11 in the trabectedin plus PLDH group). Twelve patients died (six in each group) as a result of disease progression. One patient in the PLDH group and five patients in the trabectedin plus PLDH group died as a result of an adverse effect. The full publication presented the most common grade 3 and grade 4 AEs, together with other AEs of interest that were potentially related to treatment, which are presented in Table 73. Grade 3 and grade 4 haematological adverse effects were more common in the trabectedin plus PLDH group than in the PLDH alone group. The incidence of known toxicities associated with PLDH, such as hand–foot syndrome, stomatitis and mucosal inflammation, was lower in the trabectedin plus PLDH arm than the PLDH monotherapy arm, although the number of events was low in the combination group.
Pegylated liposomal doxorubicin hydrochloride compared with topotecan
Gordon et al.49 reported withdrawal rates due to adverse effects of 18% and 16% from the PLDH and topotecan groups, respectively. Almost all patients reported an adverse effect. The incidence of grade 1, 2 or 3 events was reported to be similar across the groups but grade 4 events occurred more frequently in the topotecan group. Gordon et al.49 note that the toxicity profiles of topotecan and PLDH were different, with PLDH associated with adverse effects of mild to moderate severity. The most common adverse effect in the PLDH group was severe PPE, with the difference between PLDH and topotecan reaching statistical significance (p < 0.001; Table 74). By contrast, incidence of severe (grades 3 and 4) haematological toxicity was significantly higher with topotecan [neutropenia (p < 0.001) and leucopenia (p < 0.001); see Table 74].
Technology appraisal no. 91 presents additional data on adverse effects, reporting treatment-emergent AEs that occurred in at least 10% of patients (Table 75).13 TA91 identified statistically significant differences between PLDH and topotecan for various grade 3 events. Adverse effects that were significantly higher in the PLDH compared with the topotecan group were:
TABLE 75
Treatment-emergent AEs that occurred in at least 10% of patients as reported in TA91 (reproduced with permission)
- mucous membrane disorder (RR 0.05, 95% CI 0.006 to 0.56)
- stomatitis (RR 0.056, 95% CI 0.01 to 0.31)
- PPE (RR 0.009, 95% CI 0.001 to 0.087)
- rash (RR 0.11, 95% CI 0.017 to 0.61).
By contrast, adverse effects that were significantly higher in the topotecan group than the PLDH group were as follows:
- fever (RR 4.07, 95% CI 1.00 to 16.82)
- anaemia (RR 4.62, 95% CI 2.64 to 8.16)
- leucopenia (RR 4.02, 95% CI 2.6 to 6.27)
- neutropenia (RR 1.7, 95% CI 1.04 to 3.00)
- thrombocytopenia (RR 13.56, 95% CI 4.54 to 40.99)
- alopecia (RR 5.09, 95% CI 1.60 to 16.27).
Although a larger proportion of patients treated with PLDH experienced grade 4 pain, stomatitis and PPE, the difference between PLDH and topotecan did not reach statistical significance for these outcomes.13 By contrast, incidence of grade 4 fever, anaemia, leucopenia, neutropenia and thrombocytopenia remained statistically significantly higher in the topotecan group than in the PLDH group.
Pegylated liposomal doxorubicin hydrochloride compared with paclitaxel
Technology appraisal no. 91 reports that 16.7% (18/108) of patients in the PLDH group and 6.5% (7/108) of patients in the paclitaxel group discontinued treatment because of adverse effects.13 The five most commonly reported treatment emergent AEs associated with PLDH were nausea (51.9%), PPE (50.9%), stomatitis (48.1%), alopecia (43.5%), and asthenia (38.9%). In the paclitaxel group, the five most commonly reported AEs were alopecia (87.0%), nausea (43.5%), paraesthesia (43.5%), constipation (38.0%) and asthenia (33.3%).
The treatment-emergent AEs that occurred in at least 10% of participants in either treatment group for all grades, grades 3 and 4 are presented in Table 76. The incidence of grade 4 events was low in each group, with neutropenia being the only grade 4 event occurring in both the PLDH and paclitaxel groups (0.9% with PLDH vs. 2.8% with paclitaxel).
TABLE 76
Treatment-emergent AEs in a least 10% of participants by preferred term for PLDH vs. paclitaxel as reported in TA91
Technology appraisal no. 9113 presented forest plots to illustrate the significance of the difference between groups. Grade 3 events occurred in a significantly smaller proportion of people in the paclitaxel group compared with the PLDH group (RR of < 1 indicates paclitaxel was associated with a lower rate of AE):
- PPE (0% with paclitaxel vs. 14.8% with PLDH); RR 0.031, 95% CI 0.003 to 0.297
- stomatitis (0.9% with paclitaxel vs. 10.2% with PLDH); RR 0.091, 95% CI 0.02 to 0.53.
Alopecia was the only grade 3 adverse effect occurring significantly more frequently with paclitaxel than with PLDH (18.5% with paclitaxel vs. 2.8% PLDH; RR 6.67, 95% CI 2.20 to 20.66; see Table 76).
Topotecan compared with paclitaxel
ten Bokkel Huinink et al.21 evaluated adverse effects according to the NCI-CTC. There were two treatment-related deaths in the topotecan group, which were attributed to topotecan-induced sepsis. There were no treatment-related deaths in the paclitaxel group. Ten patients (seven in the topotecan group vs. four in the paclitaxel group) discontinued treatment as a result of an adverse effect. Febrile neutropenia, infection and sepsis were the causes of withdrawal from the topotecan group, whereas discontinuations from the paclitaxel group were as a result of neurotoxicity. Severe (grades 3 and 4) haematological adverse effects predominantly occurred more frequently in the topotecan group than in the paclitaxel group, with differences between groups in grade 4 leucopenia, neutropenia, and thrombocytopenia reaching statistical significance (Table 77). The only haematological adverse effect that occurred more frequently in paclitaxel-treated patients was grade 3 neutropenia (see Table 77).
Most non-haematological adverse effects were mild to moderate in severity (grades 1 and 2). The most frequently reported adverse effects considered related or possibly related to treatment in both groups were alopecia and gastrointestinal disturbances, including nausea, vomiting, diarrhoea and constipation (see Table 77). A larger proportion of patients in the paclitaxel group experienced alopecia than in the topotecan group. Mild to moderate nausea, vomiting and constipation occurred more frequently in the topotecan group. By contrast, more patients in the paclitaxel group experienced mild to moderate diarrhoea.
Gemcitabine plus carboplatin compared with carboplatin alone
In the trial reported by Pfisterer et al.,50 grade 3 and grade 4 haematological toxicities were significantly more frequent in the gemcitabine plus carboplatin group than in the carboplatin alone group, with neutropenia the predominant haematological toxicity (Table 78). The proportion of patients discontinuing treatment as a result of a haematological AE was small in each group (5.1% with gemcitabine plus carboplatin vs. 4.0% with carboplatin alone). Grade 3 and grade 4 non-haematological AEs were infrequent in each group, with < 5% of patients in each group experiencing a non-haematological toxicity reported in the full publication (see Table 78). Grade 2 alopecia occurred in 14.3% of patients treated with gemcitabine plus carboplatin compared with 2.3% of patients treated with of carboplatin alone (statistical significance of result not reported). AEs were graded according to the NCI-CTC guidance.
Paclitaxel plus carboplatin compared with platinum-based therapy alone
In ICON4/AGO-OVAR 2.2,61 paclitaxel plus platinum-based therapy was associated with higher rates of alopecia compared with conventional platinum-based therapy alone [322/392 (86%) with paclitaxel plus platinum-based chemotherapy vs. 95/410 (25%) with conventional platinum-based therapy; Table 79).61 Additionally, the proportion of patients experiencing a grade 2–4 neurological toxicity was higher in the paclitaxel plus platinum chemotherapy group [76/392 (20%)] than in the conventional platinum-based therapy group [4/410 (1%)]. By contrast, incidence of moderate or severe (grades 2–4) haematological adverse effects was higher in the conventional platinum-based therapy group.
Gonzalez-Martin et al.48 based the safety analysis on 78 patients who received at least one cycle of treatment. Adverse effects were graded according to NCI-CTC criteria. Grades 3 and 4 haematological toxicity was similar between the groups. Although severe neutropenia (grades 3 and 4) was more common in the paclitaxel plus carboplatin group, the difference between groups was not statistically significant (p = 0.24; see Table 80). Treatment with paclitaxel plus carboplatin was associated with a higher incidence of grade 2–4 non-haematological adverse effects and with significantly higher incidences of alopecia, mucositis, myalgia/arthralgia and peripheral neuropathy than treatment with carboplatin alone (Table 80).
Paclitaxel plus carboplatin compared with paclitaxel alone
In the trial reported by Lortholary et al.,62 one patient randomised to treatment with weekly paclitaxel did not receive a dose of study drug and was therefore not included in the safety analysis. No deaths were categorised as treatment related. Non-haematological toxicity was similar between treatment groups, with the exception of hypersensitivity reactions, which occurred more frequently with combination treatment than with weekly paclitaxel alone (Table 81). A larger proportion of patients treated with weekly paclitaxel plus carboplatin experienced grade 3 and grade 4 leucopenia and neutropenia. Discontinuation rate because of adverse effects was also higher in the group receiving combination therapy (see Table 81). No patient in the weekly paclitaxel group discontinued treatment because of haematological toxicity, whereas 14% in the weekly paclitaxel plus carboplatin group discontinued treatment for this reason.
Paclitaxel compared with oxaliplatin
Piccart et al.63 reported safety analysis based on all 86 patients randomised: all patients had received at least one treatment cycle and were assessable for the safety analysis. Only grade 3 and grade 4 AEs were reported (presented in Table 82), with grade assigned according to NCI-CTC. Considering haematological toxicities, severe neutropenia (grades 3 and 4) occurred only in the paclitaxel group [9/41 (22%)], whereas grade 3 thrombocytopenia was reported only in the oxaliplatin group [2/45 (4%)]. Severe anaemia was rare, and no episodes of febrile neutropenia were observed. Of the non-haematological AEs reported, the number of patients experiencing an AE was low in each group. No episodes of grade 4 nausea and vomiting were reported. The most frequently reported non-haematological adverse effect was pain, with 12% (5/41) and 4% (2/45) of patients in the paclitaxel and oxaliplatin groups, respectively, experiencing a grade 3 pain event (see Table 82). The proportion of patients experiencing a grade 3 neurosensory AE was similar between the two treatment groups (7% with paclitaxel vs. 9% with oxaliplatin; see Table 82).
Topotecan oral compared with topotecan intravenous
Gore et al.24 reported that neutropenia and leucopenia were the most common haematological toxicities occurring in both treatment groups, although the rate of both AEs was higher in the group receiving topotecan intravenously rather than orally (Table 83). Seven deaths were attributed to haematological toxicity, two in the oral treatment group and five in the i.v. treatment group. A similar proportion of patients in each group developed grade 3 and grade 4 thrombocytopenia or anaemia. Gastrointestinal disturbances were the most common non-haematological toxicity, with most events reported as mild to moderate in severity. Incidence of gastrointestinal adverse effects was higher in the oral topotecan group (see Table 83). Grades 3 and 4 non-haematological toxicities generally occurred in <10% of patients. Incidence of Grades 3 and 4 nausea, diarrhoea, vomiting, and fever was marginally higher in patients treated with oral topotecan compared with i.v. topotecan (see Table 83).
Topotecan administered on five consecutive days (conventional regimen) compared with topotecan administered weekly
Sehouli et al.23 report that, of the 194 patients randomised, five patients did not receive any dose of study drug, which differs slightly from the number reported in the CONSORT diagram (two patients in each group). The methods state that all analyses are based on the ITT principle. However, it is unclear from the reporting of the adverse effects whether all patients have been analysed. It should be noted that, although the comparator is referred to as conventional topotecan, the dose administered in this group is 1.25 mg/m2 for five consecutive days compared with the licensed dose of 1.5 mg/m2.
Compared with the conventional dosing schedule, weekly topotecan was associated with significantly fewer episodes of severe (grades 3 and 4) haematological events (anaemia, leucopenia, neutropenia and thrombocytopenia; see Table 84). Incidence of severe non-haematological events was low in each group, with no difference between groups reaching statistical significance (Table 84).
Paclitaxel high dose (250 mg/m2) compared with paclitaxel standard dose (175 mg/m2)
Omura et al.68 reported that febrile neutropenia was the most commonly observed severe toxicity. After the first cycle of therapy, the incidence of neutropenic fever did not differ significantly between:
- patients receiving paclitaxel 175 mg/m2 (without filgrastim) and those assigned to paclitaxel 250 mg/m2 with filgrastim (22% paclitaxel 175 mg/m2 and no filgrastim vs. 19% with paclitaxel 250 mg/m2 and filgrastim; p-value not reported)
- filgrastim 10 µg/kg and filgrastim 5 µg/kg among women receiving paclitaxel 250 mg/m2 (19% with 5 µg/kg filgrastim vs. 18% with 10 µg/kg filgrastim; 95% CI –11% to 13%, no point estimate reported).
Patients receiving the higher paclitaxel dose (250 mg/m2) reported a numerically greater incidence of anaemia, thrombocytopenia, nausea and vomiting, neuropathy and myalgia/arthralgia than those receiving paclitaxel 175 mg/m2. The difference between groups was statistically significant for thrombocytopenia (15% with 250 mg/m2 vs. 7% with 175 mg/m2; p = 0.009), neuropathy (16% with 250 mg/m2 vs. 7% with 175 mg/m2; p = 0.024) and myalgia/arthralgia (10% with 250 mg/m2 vs. 3% with 175 mg/m2; p = 0.022). Adverse effects as reported in Omura et al.68 are summarised in Table 85.
Paclitaxel weekly compared with paclitaxel every 3 weeks
Of the 208 patients randomised in the trial reported by Rosenberg et al.,60 205 received at least one dose of paclitaxel and were included in the safety analysis. No treatment-related deaths occurred in the trial. Considering haematological adverse effects, paclitaxel given every 3 weeks was associated with a significantly higher incidence of severe neutropenia (grades 3 and 4) compared with the once weekly regimen [19/104 (18%) with paclitaxel weekly vs. 45/101 (45%) with paclitaxel every 3 weeks; p < 0.001; Table 86]. Of the other haematological adverse effects assessed, number of episodes of severe anaemia, leucopenia and thrombocytopenia were similar between the two treatment groups, with none of the differences between groups reaching statistical significance. However, assessment of haematological toxicities of grades 1–4 identified a statistically significantly higher incidence of anaemia in patients treated with paclitaxel weekly compared with every 3 weeks [81/104 (78%) with paclitaxel weekly vs. 65/101 (64%) with paclitaxel every 3 weeks; p = 0.04; see Table 86]. The difference between groups in neutropenia remained significant and favoured paclitaxel weekly (i.e. smaller proportion of patients experienced an event; see Table 86).
TABLE 86
Adverse effects as reported by Rosenberg et al. (reproduced with permission)
No grade 4 non-haematological adverse effects were reported. Grade 1–3 non-haematological adverse effects were common, with high incidences of neuropathy, alopecia and arthralgia/myalgia (see Table 86). The difference between the two paclitaxel regimens in neuropathy and in alopecia was not statistically significant. However, paclitaxel every 3 weeks was associated with a significantly higher incidence of arthralgia/myalgia compared with the weekly regimen [61/104 (59%) with paclitaxel weekly vs. 85/101 (84%) with paclitaxel every 3 weeks; p = 0.04; see Table 86]. A larger proportion of patients treated with weekly paclitaxel experienced problems with their nails (discolouration and/or loosening from the nail bed) compared with patients treated every 3 weeks [37/104 (36%) with paclitaxel weekly vs. 2/101 (2%) with paclitaxel every 3 weeks; p < 0.001; see Table 86]. Considering only grade 3 non-haematological events, episodes of grade 3 neuropathy and grade 3 alopecia were significantly higher in the paclitaxel every 3 weeks regimen compared with the weekly regimen (see Table 86). Problems with nail changes remained significantly more common in the paclitaxel weekly group. Incidence of nausea/vomiting and of arthralgia/myalgia was similar in each group, with no statistically significant difference between the two treatment groups (see Table 86).
Network meta-analysis
For the NMA, studies that reported combined grades of AEs (e.g. grades 2–4, including grades 3 and 4) were excluded from the analysis. When data were reported separately for vomiting and nausea in the same study, this was combined for the purposes of the analysis, as were data on neurosensory events. It is acknowledged that this might have led to double-counting. For trials that specified they would record all AEs, events rates of zero were not imputed; only data reported in the papers were used to inform the analysis. Network diagrams for the AEs analysed in the NMA are presented in Appendix 4.
To give focus to the evaluation of AEs, the TAG consulted with its expert clinical advisors and identified the following severe AEs (grades 3 and 4) as those most problematic for patients or most likely to consume substantial health-care resource:
- allergic reaction
- alopecia
- anaemia
- fatigue
- febrile neutropenia
- nausea and vomiting
- neuropathy.
The treatments evaluated for these serious AEs are as follows:
- gemcitabine plus carboplatin
- platinum monotherapy
- PLDH monotherapy
- PLDH plus carboplatin
- paclitaxel monotherapy, i.e. 175 mg/m2 or 200 mg/m2 every 21 days
- paclitaxel monotherapy (weekly), i.e. paclitaxel 67 mg/m2 every week for 21 days
- topotecan monotherapy (i.v.), i.e. topotecan 1.25 mg/m2 or 1.5 mg/m2 daily for 5 days every 21 days
- topotecan monotherapy (oral)
- topotecan monotherapy (i.v., weekly), i.e. topotecan 4.0 mg/m2 (weekly) on days 1, 8 and 15 of a 28-day cycle.
Unlike the efficacy outcomes reported earlier, the evaluation of severe AEs is based on the total population regardless of PFI, i.e. it is not broken down by the various subgroups based on platinum sensitivity (or insensitivity). However, for consistency the baseline treatment for each network assessed are consistent with the efficacy analyses.
Allergic reaction
The absolute numbers for the RCTs included in the NMA evaluating allergic reaction in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Appendix 4.
The results from this NMA are presented in Table 87. Overall, only PLDH plus carboplatin was found to have significantly less risk of an allergic reaction (at the 5% level) than paclitaxel plus carboplatin. PLDH plus carboplatin is also associated with significantly less risk of allergic reaction than platinum as monotherapy. No other comparison of chemotherapies was found to have a statistically significant difference.
TABLE 87
Results of the NMA for allergic reaction for people with recurrent ovarian cancer
As only one trial62 provided data on this AE for network 2, it was not possible to conduct a NMA. Lortholary et al.62 compared low-dose paclitaxel (80 mg/m2) with low-dose paclitaxel (80 mg/m2) plus carboplatin. Low-dose paclitaxel was found to have significantly less risk of causing an allergic reaction than paclitaxel plus carboplatin (OR 0.114, 95% CI 0.014 to 0.942).
Alopecia
The absolute numbers for the RCTs included in the NMA evaluating alopecia in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Appendix 4.
As only one trial29 provided data on this AE for network 1 it was not possible to conduct a NMA. Bafaloukos et al.29 compared PLDH plus carboplatin to paclitaxel plus carboplatin. PLDH plus carboplatin was found to have significantly less risk of causing alopecia than paclitaxel plus carboplatin (OR 0.235, 95% CI 0.077 to 0.724).
The results for the NMA of network 2 are presented in Table 88. Overall, all chemotherapies assessed were found to have a significantly higher risk of alopecia (at the 5% level) than PLDH monotherapy. Paclitaxel monotherapy was also found to have a significantly higher risk of alopecia than paclitaxel monotherapy (weekly). No other comparison of chemotherapies was found to have a statistically significant difference.
TABLE 88
Results of the NMA for alopecia for people with recurrent ovarian cancer
Anaemia
The absolute numbers for the RCTs included in the NMA evaluating anaemia in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Appendix 4.
The results of the NMA from network 1 are presented in Table 89. Overall, PLDH plus carboplatin and gemcitabine plus carboplatin were found to have significantly higher risk of anaemia (at the 5% level) than paclitaxel plus carboplatin. Gemcitabine plus carboplatin was also found to have a significantly higher risk of anaemia than platinum monotherapy. No other comparison of chemotherapies was found to have a statistically significant difference.
TABLE 89
Results of the NMA for anaemia for people with recurrent ovarian cancer
The results of the NMA from network 2 are also presented in Table 89. Overall, topotecan monotherapy (i.v.), topotecan monotherapy (oral) and PLDH plus trabectedin were found to have significantly higher risk of anaemia (at the 5% level) than PLDH monotherapy. PLDH plus trabectedin, paclitaxel monotherapy and topotecan monotherapy (i.v., weekly) were also found to have significantly higher risk of anaemia than topotecan monotherapy (i.v.). Paclitaxel monotherapy was found to have significantly less risk than topotecan monotherapy (oral) and topotecan monotherapy (i.v.). No other comparison of chemotherapies was found to have a statistically significant difference.
One additional trial62 provided data on this AE but it was not possible to include this in either network owing to the atypical doses of paclitaxel compared. Lortholary et al.62 compared low-dose paclitaxel (80 mg/m2) with low-dose paclitaxel (80 mg/m2) plus carboplatin. No significant difference in risk of anaemia was identified (OR 0.273, 95% CI 0.071 to 1.048).
Fatigue
The absolute numbers for the RCTs included in the NMA evaluating fatigue in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Appendix 4.
A NMA of network 1 could not be performed owing to zero events in a link in the network29 and non-comparable doses and/or treatment regimen in the remaining available trials. Individual trial results are presented in Table 90.
TABLE 90
Results of the individual trials for network 1 for fatigue for people with recurrent ovarian cancer
The results of the NMA from network 2 are presented in Table 91. No comparison of chemotherapies was found to have a statistically significant difference (at the 5% level).
TABLE 91
Results of the NMA for fatigue for network 2 for people with recurrent ovarian cancer
Febrile neutropenia
The absolute numbers for the RCTs included in the NMA evaluating febrile neutropenia in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, no NMA could be performed due to zero events in three of the available trials.28,50,62 Individual trial results are presented in Table 92.
TABLE 92
Results of the individual trials for febrile neutropenia for people with recurrent ovarian cancer
Nausea and vomiting
The absolute numbers for the RCTs included in the NMA evaluating nausea and vomiting in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, as described earlier, a single network could not be constructed out of the available trials. The two networks constructed for this outcome are depicted in Appendix 4.
The results of the NMA from network 1 are presented in Table 93. Overall, PLDH plus carboplatin was found to have significantly higher risk of nausea and vomiting (at the 5% level) than paclitaxel plus carboplatin. No other comparison of chemotherapies was found to have a statistically significant difference.
TABLE 93
Results of the NMA for nausea and vomiting for people with recurrent ovarian cancer
The results of the NMA from network 2 are also presented in Table 93. Overall, paclitaxel monotherapy was found to have significantly lower risk of nausea and vomiting (at the 5% level) than PLDH monotherapy. Topotecan monotherapy (oral) and PLDH plus trabectedin were found to have significantly higher risk of nausea and vomiting than PLDH monotherapy (and any of the other chemotherapies assessed). However, when compared with each other no significant difference was found. No other comparison of chemotherapies was found to have a statistically significant difference.
Neuropathy
The absolute numbers for the RCTs included in the NMA evaluating neuropathy in patients with recurrent ovarian cancer are reported in Adverse effects, above. Unfortunately, no NMA could be performed due to zero events in four of the available trials.21,23,29,48 Individual trial results are presented in Table 94.
TABLE 94
Results of the individual trials for neuropathy for people with recurrent ovarian cancer
Discussion
The population of ovarian cancer patients that is the focus of this MTA is those who have relapsed following first-line treatment with platinum-based therapy or have disease that is refractory to platinum-based chemotherapy. Diagnosis of recurrent disease varies in UK clinical practice, with diagnosis based on clinical examination, biochemical markers (CA125) or radiological confirmation – or any combination of these three. Clinical expert advice is that, typically, a patient is diagnosed as relapsed if they have a serial rise in CA125 level or have developed clinical signs, such as ascites. Diagnosis is typically confirmed with radiological scans. If a patient has no clinical symptoms but does have a rise in CA125 level, although possibly classified as relapse, the patient might not start a new chemotherapeutic regimen until they go on to develop symptoms. Date of relapse by CA125 level is likely to be about 4 months earlier than date of relapse based on radiological scans.
A patient’s response to first-line platinum-based therapy is indicative of their response to second and subsequent lines of platinum-based treatment, with the length of the PFI and the extent of relapse (site and number of tumours) particularly prognostic of response. However, most patients will develop resistance to platinum-based therapy over time, with decreasing length of PFI with increasing rounds of treatment. Platinum-resistant ovarian cancer has a particularly poor prognosis, with a reported median OS of < 12 months.
The systematic review of clinical effectiveness evidence carried out to address the decision problem that is the focus of this MTA identified 16 RCTs, evaluating 14 pairwise comparisons. Of the 16 RCTs identified, five evaluated the intervention and comparator within their licensed indication, and dose and route of administration. The remaining 11 RCTs evaluated the intervention or comparator outside the parameters specified in the licence. However, the scope of the evidence identified was insufficient to fully address the decision problem; therefore, where possible the TAG has carried out synthesis of the evidence within NMAs.
Based on clinical expert advice, the TAG has focused on the clinical effectiveness of interventions in populations defined by degree of platinum sensitivity [i.e. platinum sensitive (i.e. recurrence ≥ 6 months after last platinum-based treatment) and platinum resistant (i.e. recurrence < 6 months after last platinum-based treatment) or refractory (progression during platinum-based treatment)].
The identified RCTs facilitated the construction of three distinct networks for the outcomes of OS and PFS, two of which considered patients with platinum-sensitive disease; the remaining network considered patients with disease that is PRR. As the systematic review was conducted in such a way as to identify all trials with at least one intervention of interest, a wider selection of treatments were assessed, but, unfortunately, this did not uncover one or more trials that could link the disconnected networks in patients with platinum-sensitive disease. Furthermore, owing to time constraints, the decision was taken not to search for non-randomised trials.
The two networks, for OS and PFS, constructed in patients with platinum-sensitive disease were:
- platinum sensitive network 1, which compared regimens containing platinum, in particular: platinum plus paclitaxel, PLDH plus platinum, gemcitabine plus carboplatin, and platinum alone
- platinum sensitive network 2, which compared non-platinum-based therapies, in particular: PLDH, trabectedin plus PLDH, paclitaxel and topotecan.
Platinum-sensitive patients
Overall survival and PFS data were identified for eight and seven different head-to-head comparisons of interventions and comparators of interest, respectively. Of these, three reported a statistically significant difference in OS between the treatments considered. In particular, Parmar et al.61 reported a statistically significant difference in OS between paclitaxel plus platinum vs. conventional platinum treatment (HR 0.82, 95% CI 0.69 to 0.97) observed in the ICON4/AGO-OVAR 2.261 trial. Gonzalez-Martin et al.48 reported a statistically significant difference between paclitaxel plus carboplatin vs. carboplatin alone (HR 0.31, 95% CI 0.14 to 0.68) and Gordon et al.54 present a statistically significant difference between PLDH and topotecan (HR 1.43, 95% CI 1.07 to 1.92). Six of the identified head-to-head comparisons identified a statistically significant difference in PFS. These were:
- CALYPSO31 PLDH plus carboplatin vs. paclitaxel plus carboplatin (HR 0.82, 95% CI 0.72 to 0.94)
- ICON4/AGO-OVAR 2.261 Paclitaxel plus platinum vs. conventional platinum treatment (HR 0.76, 95% CI 0.66 to 0.89)
- Gonzalez-Martin et al.48 Paclitaxel plus carboplatin vs. carboplatin alone (HR 0.54, 95% CI 0.32 to 0.92)
- Alberts et al.28 PLDH plus carboplatin vs. carboplatin alone (HR 0.54, 95% CI 0.32 to 0.93)
- OVA-30130 Trabectedin plus PLDH vs. PLDH (HR 0.73, 95% CI 0.56 to 0.95)
- Pfisterer et al.50 Gemcitabine plus carboplatin vs. carboplatin alone (HR 0.72, 95% CI 0.58 to 0.90).
In the NMA evaluating platinum-based chemotherapies, PLDH plus carboplatin and paclitaxel plus carboplatin were found to significantly improve OS compared with platinum monotherapy. However, no statistically significant differences in OS were identified between the remaining treatments considered in the network. When compared with platinum monotherapy, PFS was estimated to significantly improve in patients treated with paclitaxel plus carboplatin, gemcitabine plus carboplatin or PLDH plus carboplatin. In addition, a statistically significant difference in PFS was estimated for paclitaxel plus carboplatin compared with PLDH plus carboplatin.
However, the TAG consider it important to note that examination of the baseline characteristics of trials included in NMAs of platinum-based therapies, revealed an imbalance in baseline performance score (ECOG) within one of the included trials. In particular, the trial carried out by Gonzalez-Martin et al.,48 in which paclitaxel plus carboplatin is compared with platinum monotherapy; the proportion of patients with a baseline ECOG score of 2 that were randomised to treatment with platinum monotherapy was 17.9% vs. 5.6% of patients randomised to treatment with paclitaxel plus carboplatin. The TAG notes that this imbalance is likely to result in an overestimation of the relative treatment effect of paclitaxel plus carboplatin vs. platinum monotherapy.
Furthermore, the TAG notes the presence of clinical heterogeneity in the duration of PFI between trials. In particular, patients enrolled in the ICON-4/AGO-OVAR 2.261 trial had a comparably longer PFI than patients enrolled in the other trials included in NMA of OS and PFS data. Similarly, a comparatively high proportion of patients enrolled in the trial carried out by Gonzalez-Martin et al.48 were diagnosed as recurrent based on assessment of CA125 levels; therefore these patients are likely to be more susceptible to platinum therapy than patients enrolled in the other included trials. However, the TAG notes that although patients in ICON-4/AGO-OVAR 2.261 and Gonzalez-Martin et al.48 may be expected to experience greater benefit than patients enrolled in the other trials, the magnitude of this difference is unlikely to affect estimates of the relative effect of treatment.
Network meta-analysis of non-platinum-based therapies indicated that PLDH monotherapy and trabectedin plus PLDH are both significantly more effective at prolonging OS than topotecan monotherapy. No other significant OS differences were identified. Analysis of non-platinum-based regimens indicates that trabectedin plus PLDH statistically significantly improves PFS compared with PLDH, paclitaxel and topotecan when given as monotherapies. No statistically significant differences in PFS were identified among the monotherapies evaluated (PLDH, topotecan, and paclitaxel). However, as a result of the use of subgroup data to inform these analyses, assessment of the presence of clinical heterogeneity was not possible. In addition, the TAG considers it import to highlight that subgroup data from the included trials were not sufficiently powered to detect a difference in OS or PFS.
Overall response rate was reported for 11 different head-to-head comparisons of interventions and comparators of interest. Of these, only two were statistically significant: trabectedin plus PLDH vs. PLDH from OVA-30130 (OR 1.57, 95% CI 1.04 to 2.35); gemcitabine plus carboplatin vs. carboplatin alone from Pfisterer et al.50 (OR 1.527, 95% CI 1.025 to 2.275).
Based on the trials identified, it was not possible to construct a complete network informing ORR. Akin to analyses of OS and PFS, two discrete networks were generated: one evaluating platinum-based therapies (paclitaxel plus carboplatin, gemcitabine plus carboplatin, PLDH plus carboplatin and platinum monotherapy) and the second comparing non-platinum-based regimens [PLDH, trabectedin plus PLDH, topotecan (i.v.), paclitaxel (every 3 weeks), topotecan (oral) and paclitaxel (weekly)].
In the NMA evaluating platinum-based chemotherapies, paclitaxel plus carboplatin and gemcitabine plus carboplatin were found to have a significantly higher ORR than platinum monotherapy. There was no significant difference between PLDH plus carboplatin and any of the chemotherapeutic treatments assessed. Analysis of non-platinum-based regimens indicates that trabectedin plus PLDH significantly improves ORR compared with PLDH, and oral topotecan. Compared with oral topotecan, i.v. topotecan was found to be associated with a significant increase in the proportion of patients achieving CR or PR. No other statistically significant differences were identified.
Platinum-resistant/-refractory patients
The OS and PFS data were reported for five and four different head-to-head comparisons in PRR patients, respectively. Two RCTs enrolled only patients with PRR, with the remaining RCTs reporting results from a subgroup of patients within the trial. None of the trials identified a significant difference in OS or PFS between the two treatment groups evaluated. Furthermore, no statistically significant differences in ORR were reported in the eight different head-to-head comparisons involving PRR patients. Similarly, no statistically significant differences in OS or PFS were identified in the NMA of treatment with paclitaxel, PLDH and topotecan. However, NMA of ORR estimated that PLDH significantly increased ORR compared with paclitaxel (175 mg/m2) every 21 days and with an alternative regimen in which paclitaxel was given weekly at a dose of 67 mg/m2. PLDH monotherapy was also significantly more effective than an unconventional regimen of topotecan in which topotecan was administered weekly at a dose of 4 mg/m2. As a result of the use of subgroup data to inform these analyses, the TAG notes that the individual trial data may have been underpowered to detect a difference in OS, PFS or ORR. Furthermore, as baseline characteristics were not reported for the subgroups, an assessment of the presence of clinical heterogeneity was not possible.
Health-related quality of life
Treatments for newly diagnosed ovarian cancer are given with curative intent; however, for women with advanced, recurrent disease, second- and subsequent-line therapies are typically given with palliative rather than curative intent, with the aim of alleviating symptoms and prolonging survival. Thus, key considerations in the choice of treatment at these stages in the pathway are maintaining the patient’s QoL. Of the 16 RCTs identified, 10 reported some level of data on QoL. However, reporting of results was generally limited, with few trials reporting scores generated from responses to the questionnaires. The most commonly used scale in the identified trials is the EORTC QLQ-C30 questionnaire,81 which was developed to assess the QoL of cancer patients and can be supplemented with disease-specific modules for individual cancers, including ovarian cancer. For many comparisons, scores on QoL scales were similar between treatments. Differences in QoL include:
- For PLDH plus platinum versus paclitaxel plus platinum, at 3 months, PLDH plus platinum was associated with a significant improvement in global health compared with paclitaxel plus platinum. However, this benefit was not maintained at 6 months.
- For paclitaxel plus platinum versus platinum-based chemotherapy patients receiving platinum monotherapy scored significantly worse on the nausea and vomiting symptom scale than did the paclitaxel plus platinum-based chemotherapy group. However, this difference seemed to be transient and was observed for only the first 15 weeks after randomisation.
- For trabectedin plus PLDH versus PLDH in the subgroup of patients with PPS ovarian cancer, it is indicated that there is difference in global health status score among responding patients beyond cycle 5, with patients in the trabectedin plus PLDH group having a higher score than those receiving PLDH alone (higher score is favourable).
- In comparison with PLDH, topotecan was associated with a significantly more favourable rating on the pain subscale of the EORTC QLQ-C30.
- For paclitaxel plus platinum versus paclitaxel, patients receiving weekly paclitaxel plus platinum experienced improvements in constipation, abdominal/gastrointestinal symptoms, appetite loss, pain and emotional functioning. Patients treated with weekly paclitaxel alone experienced improvements in attitude to disease and insomnia, but worsening of dyspnoea and peripheral neuropathy.
- For paclitaxel versus oxaliplatin, mean QoL score on the EORTC QLQ-C3081 increased by > 10 points between baseline and cycle 4 for patients in the paclitaxel group, irrespective of study withdrawal. By contrast, in the oxaliplatin group, the mean QoL score decreased through cycle 2, but by < 10 points, after which most patients’ mean scores returned to baseline levels.
Adverse events
An important consideration in the choice of second-line treatment is the adverse effect of neurotoxicity, which is commonly associated with paclitaxel and also with carboplatin. Neurotoxicity can persist for up to 2 years after the end of treatment. Patients who relapse after first-line treatment with paclitaxel–platinum combination therapy and are subsequently rechallenged with the same regimen within 12 months (i.e. those who are PPS) are at an increased risk of developing neurotoxicity. However, despite the associated increased risk of neurotoxicity, paclitaxel plus carboplatin is generally the preferred second-line treatment in UK practice in recurrent platinum-sensitive cancer, particularly for patients who relapse > 12 months after completion of first-line chemotherapy. Carboplatin is chosen over cisplatin because of its more favourable adverse effect profile.
Within each of the identified trials, the most frequently reported adverse effects were as expected for the individual treatments based on the SmPC. Commonly occurring adverse effects were alopecia, nausea and vomiting, haematological toxicities (neutropenia, anaemia, thrombocytopenia and leucopenia). Based on expert clinical advice the TAG restricted its comparison of AEs to those considered most problematic for patients or most likely to consume substantial health-care resource.
The potential for a NMA was, therefore, investigated for the following severe (grades 3 and 4) AEs: allergic reaction, alopecia, anaemia, fatigue, febrile neutropenia, nausea and vomiting, and neuropathy. In many cases a NMA was not possible owing to the lack of available data in the trials assessed. In these instances, the individual trial results are reported with the ORs and 95% CIs that were calculated. The majority of NMA results, supplemented by the individual trial results where a NMA was not possible, indicated that the likelihood of AEs were not statistically significantly different across treatment regimens. However, in some instances, chemotherapies were estimated as having significantly lower risks of one or more AEs but significantly higher risks of other AEs. For example, when compared with paclitaxel plus platinum, PLDH plus platinum is associated with significantly lower risks of allergic reaction and alopecia but significantly higher risks of anaemia and nausea and vomiting. Overall, no chemotherapy was consistently associated with either a lower risk or a higher risk of the severe AEs assessed.
- Assessment of clinical effectiveness - Topotecan, pegylated liposomal doxorubici...Assessment of clinical effectiveness - Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: a systematic review and economic evaluation
- Results - A clinical and economic evaluation of Control of Hyperglycaemia in Pae...Results - A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial
- Methods - Helping pregnant smokers quit: a multi-centre randomised controlled tr...Methods - Helping pregnant smokers quit: a multi-centre randomised controlled trial of electronic cigarettes versus nicotine replacement therapy
- List of abbreviations - Optimal pharmacotherapy pathway in adults with diabetic ...List of abbreviations - Optimal pharmacotherapy pathway in adults with diabetic peripheral neuropathic pain: the OPTION-DM
- List of abbreviations - Progesterone to prevent miscarriage in women with early ...List of abbreviations - Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...