Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Westwood M, van Asselt T, Ramaekers B, et al. High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis. Southampton (UK): NIHR Journals Library; 2015 Jun. (Health Technology Assessment, No. 19.44.)

High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis.
Show detailsThis chapter explores the cost-effectiveness of hs-cTn assays (used singly or in series, up to 4 hours from the onset of chest pain/presentation) compared with the current standard of serial Tn T and/or I testing on admission and at 10–12 hours after the onset of symptoms for the early rule-out of AMI in people with acute chest pain.
Review of economic analyses of high-sensitivity cardiac troponin assays
Search strategy
Searches were undertaken to identify cost-effectiveness studies of high-sensitivity TnT/I. As with the clinical effectiveness searching, the main EMBASE strategy for each set of searches was independently peer reviewed by a second Information Specialist, using the Canadian Agency for Drugs and Technologies in Health (CADTH) Peer Review Checklist.28 Search strategies were developed specifically for each database and keywords associated with high-sensitivity TnT/I were adapted according to the configuration of each database. Full search strategies are reported in Appendix 1.
The following databases were searched for relevant studies from 2005 to October 2013:
- MEDLINE (OvidSP): 2005–2013/10/wk1.
- MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP): up to 2013/10/1.
- EMBASE (OvidSP): 2005–2013/10/17.
- NHS Economic Evaluation Database (NHS EED) (Wiley): Cochrane Library Issue 3 2005 to July 2013.
- Health Economic Evaluation Database (HEED) (Wiley): 2005–2013/10/18.
- EconLit (EBSCO): 2005–2013/09/01.
- Science Citation Index (SCI) (Web of Science): 2005–2013/10/21.
- Conference Proceedings Citation Index – Science (CPCI) (Web of Science): 2005–2013/10/21.
- Research Papers in Economics (REPEC) (Internet): up to 2013/10/21 http://repec.org/.
Identified references were downloaded in EndNote X4 software for further assessment and handling.
References in retrieved articles were checked for additional studies.
Inclusion criteria
Studies reporting a full economic analysis, which related explicitly to the cost-effectiveness of hs-cTn or standard cTn (with cTn implying either cTnI or cTnT) testing, with survival and/or quality-adjusted life-years (QALYs) as an outcome measure, were eligible for inclusion. Specifically, one of the strategies had to include cTn testing. Studies that reported only a cost-analysis of cTn testing were not included in the review.
Quality assessment
Full cost-effectiveness studies were appraised using the Drummond checklist.77
Results
The literature search identified 152 reports. After initial screening of titles and abstracts, five reports7,19,78–80 were considered to be potentially relevant: two full papers79,80 and three HTA reports.7,19,78 Two additional reports81,82 were provided by a clinical expert: a Canadian optimal use report82 (similar to an HTA report) and an abstract81 that was referred to in this report. All seven identified reports7,19,78–82 fulfilled inclusion criteria based on full-text assessment. The seven publications related to five studies. Figure 13 shows the flow of studies through the review process, Table 9 lists the study details and the results of the quality assessment are shown in Table 10.

FIGURE 13
Flow of studies through the review process. CA, conference abstract; HTA, Health Technology Assessment; JA, journal article.
TABLE 9
Summary of included full papers
TABLE 10
Checklist of study quality for full papers included
Goodacre (2011)78 and Fitzgerald (2011)79
This study was based on the multicentre, pragmatic controlled trial ‘Randomised Assessment of Treatment using Panel Assay of Cardiac Markers’ (RATPAC). An economic evaluation was undertaken to assess the cost-effectiveness of management based on testing with a panel of point-of-care cardiac markers compared with management without point-of-care panel assessment. The included population consisted of patients presenting to hospital with chest pain attributable to suspected, but not proven, AMI and no other potentially serious alternative pathology or comorbidity. The analysis was performed from an NHS perspective, using trial data to estimate the mean costs per patient of chest pain-related care and the mean number of QALYs accrued by patients in each arm of the trial, with a time horizon of 3 months. In addition, a decision-analytic model was constructed to duplicate (validate) trial results and extrapolate results to a longer time horizon.
Resource-use data were collected for all patients. Cost and outcome data were collected using patient notes and self-completed questionnaires. Unit prices were based partly on a microcosting study on a sample of patients, partly on a study previously undertaken by the investigators, and partly on purchase price and national unit costs. QALYs were calculated based on European Quality of Life-5 Dimensions (EQ-5D) measurements. In a sensitivity analysis, productivity costs were included as reported by the patients.
As it was anticipated that the trial would have limited power to detect a difference in major adverse events, the decision-analytic model was intended to explore whether uncertainty around the effect of the intervention upon the major adverse event rate could influence the potential cost-effectiveness of the intervention. The model used trial data to estimate costs and QALYs up to 3 months. Beyond this, lifetime cost and QALYs were estimated from a previous study.86 It was assumed that patients who had died at 3 months would accrue no further costs or QALYs. Those who had survived non-fatal myocardial infarction (MI) would accrue costs and QALYs associated with CHD (estimated at £10,079 and 6.829, respectively). Those without CHD were assigned zero costs and 20 QALYs.
Empirical results showed that the point-of-care test strategy was dominated by standard care, which delivered slightly more QALYs at a lower cost. The probability that point-of-care testing would be more cost-effective than standard care at a willingness-to-pay threshold of £20,000 per QALY was < 1%. The decision-analytic model again resulted in higher costs and less effect for the point-of-care panel assay compared with standard care, also when extrapolated to lifetime survival. The probability of the point-of-care panel assay being cost-effective for the 3-month and lifetime model was 22.3% and 33.6%, respectively.
The main conclusion was that point-of-care panel assay testing is unlikely to be considered cost-effective in the NHS, with an 89% probability that standard care was dominant. Cost-effectiveness was mainly driven by differences in mean cost, with point estimates suggesting that, per patient, point-of-care panel assessment was £211 more expensive than standard care.
Vaidya (2012)81
This study aimed to assess the cost-effectiveness of a hs-TnT assay, alone or in combination with the H-FABP assay in comparison with the conventional cTnT assay for the diagnosis of AMI in patients presenting to hospital with chest pain. A decision-analytic model was developed to perform both a cost–utility analysis (cost per QALY gained) and a cost-effectiveness analysis [cost per life-year (LY) gained and cost per AMI averted], using a health-care perspective and a lifetime time horizon. One-way and probabilistic sensitivity analyses (PSAs) were conducted.
The incremental cost-effectiveness ratio (ICER) for hs-TnT compared with conventional cTnT was €3748 per QALY gained. For hs-cTnT in combination with H-FABP compared with conventional cTnT the ICER was €5717 per QALY gained. For LY and AMI averted, no ICERs were reported in the abstract. The PSA showed the hs-TnT assay to be the preferable strategy, with a probability of > 90%, at a ceiling ratio of €4800 per QALY. This led to the conclusion that the hs-TnT assay is very cost-effective relative to the conventional cTnT assay. Combining hs-TnT with H-FABP did not seem to offer any additional economic or health benefit over the hs-TnT test alone.
Goodacre (2013)7 and Thokala (2012)80
This study aimed to estimate the cost-effectiveness of using alternative biomarker strategies to diagnose MI, and using biomarkers, computed tomography coronary angiography (CTCA) and exercise ECG to risk-stratify Tn-negative patients. As the second aim was outside the scope of this review, we have summarised only the analysis that compares the biomarker strategies for diagnosing MI, referred to in the HTA report as ‘the diagnostic phase model’. The different diagnostic strategies were applied to a hypothetical cohort of patients attending the ED with suspected, but not proven, ACS. Patient characteristics were defined using data from the RATPAC trial,87 as well as patients’ arrival times during the day at the ED. The model assigned each patient a probability of re-infarction or death depending on their characteristics and whether or not they had treatment. The model took a lifetime time horizon. The economic perspective was that of the NHS in England and Wales.
The following strategies were applied to each patient:
- no testing – discharge all patients without treatment (hypothetical)
- standard Tn assay measured at presentation using the 10% CV as the threshold for positivity
- standard Tn assay measured at presentation using the 99th percentile threshold
- high-sensitivity Tn assay measured at presentation using the 99th percentile threshold
- standard Tn assay measured at presentation and 10 hours after symptom onset using the 99th percentile threshold.
Blood tests at presentation were assumed to be taken in the ED, and so a decision could be made within 1 hour of the test results becoming available. For the 10–12 hours’ Tn measurement, three different scenarios were tested:
- ‘doctor-on-demand’ scenario, with medical staff available 24 hours a day to make a disposition decision within 1 hour of the results being available
- twice-daily ward round scenario, with medical staff only available at twice daily ward rounds to make disposition decisions
- once-daily ward round scenario, with medical staff only available at a once daily ward round to make disposition decisions.
Sensitivity and specificity estimates for the presentation Tn tests were obtained by performing meta-analysis of estimates from individual primary studies included in the accompanying review. The 10-hour Tn test was assumed to have perfect sensitivity and specificity as it was the reference standard for the review. This implies that FPs of the hs-Tn testing at presentation will still be discharged home after the 10- to 12-hour Tn test but FNs will be discharged home without treatment. The ‘discharge without testing or treatment’ by definition has perfect specificity, but a sensitivity of 0%.
The risk of re-infarction and death for patients with MI was based on a study by Mills et al.83 Life expectancy of patients with MI, and MI with re-infarction, was estimated from Polanczyk et al.,88 whereas the utility of patients with MI was based on Ward et al.85 The utility of patients with re-infarction was estimated by using a multiplicative factor of 0.8 for patients with MI (expert opinion). Patients without MI were assigned the life expectancy and utility scores of the general population. Lifetime costs for patients with MI were based on Ward et al.85 One-way sensitivity analyses were performed, as well as a PSA. In a secondary analysis, a strategy was added that involved alternative biomarkers in combination with the presentation Tn testing.
The results showed that measuring a 10-hour Tn level in all patients was the most effective strategy (ICER £27,546–103,560). However, at a threshold of £30,000 per QALY, the optimal strategy in all but one scenario was measurement of high-sensitivity Tn at presentation, with a 10-hour Tn test if positive and discharge home if negative (ICER £7487–17,191 per QALY). The exception was a scenario involving patients without known CAD and a doctor available on demand to discharge the patient, where, using the £30,000 per QALY threshold, the strategy of measuring a 10-hour Tn level in all patients was optimal (ICER of £27,546 per QALY). Sensitivity analyses showed the optimal strategy to vary with different levels of sensitivity and timing of the tests.
The report concluded that the additional costs that are likely to be incurred by measuring a 10-hour Tn level, compared with a presentation high-sensitivity Tn level, are unlikely to represent a cost-effective use of NHS resources in most of the scenarios tested.
Canadian Agency for Drugs and Technologies in Health optimal use report
This report82 aimed to determine the cost-effectiveness of hs-cTnT and hs-cTnI assays compared with each other, as well as with cTnI assays in patients with suspected ACS symptoms in the ED. For this purpose, three comparators were considered: hs-cTnT, hs-cTnI and cTnI. As cTnT is no longer available in Canada, it was not taken into account in the analysis. The target population consisted of 65-year-old patients presenting to the ED, without ST segment elevation, who required cTn testing for diagnosis of NSTEMI. For the economic evaluation, a decision tree was constructed, which calculated lifetime cost per QALY from the perspective of a publicly funded health-care system.
The model consisted of a short-term part, which had a time horizon of 1 year, and a long-term part. The short-term part incorporated the testing and treatment procedures and short-term outcomes. Patients were tested at presentation at the ED and, if they were not admitted to hospital after the first test, they were tested again after 6 hours. When the patient was admitted after the first test, treatment was said to be initiated early, and when a patient was admitted after the second test, treatment was late. One-year mortality depended on whether a patient had NSTEMI and whether they were treated early, treated late, or untreated (in the case of FN test results). Those not suffering from NSTEMI were further stratified into UA or not having ACS (non-ACS). The annual probability of death in the long-term part of the model was dependent on patient age, sex, and whether or not they had suffered a NSTEMI, UA or did not have any type of ACS in the short-term part of the model.
The sensitivity and specificity for each cTn test at presentation to the ED was derived from the systematic review which was also part of this study. In the model, patients with a negative cTn test at presentation were assumed to be observed and have a second cTn test 6 hours later. After the second cTn test, 90% of these FNs were assumed to become TPs.
Short-term mortality rates and relative risks (RRs) for treated/non-treated were taken from published clinical studies and one non-referenced study. The RR for late treatment compared with early treatment was derived from expert opinion. Long-term mortality rates were taken from published clinical studies, and one non-referenced study. QALYs were calculated by incorporating an age-specific utility decrement for patients with NSTEMI. A number of one-way sensitivity analyses were performed, as well as a PSA.
The base-case results indicated that hs-cTnI was dominated by hs-cTnT, when compared with cTnI, at an ICER of US$119,377 per QALY. The PSA showed that, for willingness-to-pay thresholds of up to US$124,000, cTnI had the highest probability of being cost-effective. For thresholds > US$124,000, hs-cTnT had the highest probability of being cost-effective. The hs-cTnI test was not likely to be cost-effective for any value of the threshold.
The authors concluded that hs-cTnT would be considered the most cost-effective testing strategy if willingness to pay for a QALY is US$119,377 or more, otherwise cTnI would be the most cost-effective test. However, there was a lot of uncertainty in results when model assumptions were changed.
Collinson (2013)19
This study used the decision tree developed in the related HTA by Goodacre et al.7 to compare the cost-effectiveness of five diagnostic strategies to a hypothetical cohort of patients presenting to hospital with symptoms suggestive of MI but with no diagnostic ECG changes, no known history of CHD and no major comorbidities requiring inpatient treatment. Essentially, this was a substudy of the point-of-care arm of the RATPAC trial. All methods and model inputs were identical to the study by Thokala et al.80 and the HTA report by Goodacre et al.,7 but with slightly different strategies applied to the cohort of patients:
- No testing – discharge all patients without treatment (theoretical ‘zero’ option)
- high-sensitivity cTnT at presentation – discharge home if test is negative or admit to hospital for Tn-testing at 10–12 hours if positive
- high-sensitivity cTnT and H-FABP at presentation – discharge home if both tests are negative or admit to hospital for Tn testing at 10–12 hours if either test is positive
- high-sensitivity cTnT at presentation and at 90 minutes as in the RATPAC protocol – discharge home if both tests are negative or admit to hospital testing at 10–12 hours if either test is positive
- standard Tn testing at 10–12 hours (current standard as per NICE guidelines).
The difference with the other studies is in the addition of H-FABP in the third strategy and in the second high-sensitive Tn test at 90 minutes in the fourth strategy. In a secondary analysis, cTnT was replaced by cTnI. Sensitivity and specificity of presentation biochemical testing were estimated using data from within the study (RATPAC). Standard Tn testing at 10–12 hours was assumed to have perfect sensitivity and specificity as this was again the reference standard.
At the £20,000 per QALY threshold, 10-hour Tn testing was cost-effective (£12,090 per QALY) in the doctor-on-demand scenario, but not in the other scenarios (once-daily ward round and twice-daily ward rounds), when high-sensitivity cTnT and H-FABP measurement at presentation was cost-effective. At the £30,000 per QALY threshold, 10-hour Tn testing was cost-effective in the doctor-on-demand scenario and twice-daily ward rounds scenario (£24,600 per QALY), whereas the TnT and H-FABP measurement at presentation strategy was cost-effective (£14,806 per QALY) in the once-daily ward round scenario. Secondary analysis using cTnI instead of cTnT showed that cTnI testing at presentation and at 90 minutes was cost-effective in all three scenarios at the £20,000 per QALY threshold, and in two of the scenarios at the £30,000 per QALY threshold, with 10-hour Tn being cost-effective only in the doctor-on-demand scenario (£24,327 per QALY). The overall conclusion was that 10-hour Tn testing is likely to be cost-effective compared with rapid rule-out strategies only if patients can be discharged as soon as a negative result is available and a £30,000 per QALY threshold is used.
Summary of studies included in the cost-effectiveness review
Most of the studies identified in this review have found that the question of whether hs-Tn testing is cost-effective cannot be answered unequivocally. In favour of hs-Tn testing, the abstract by Vaidya et al.81 concluded that hsTnT testing is ‘very cost-effective’ and the study by Goodacre et al.7 concluded that ‘the optimal strategy in all but one scenario was high-sensitivity Tn at presentation, with a 10 hour Tn test if positive and discharge home if negative’ (p. xv). The other papers reported ICERs that were considerably higher and with substantial uncertainty. The accuracy of high-sensitivity tests and the efficiency of decision-making based on test results were important drivers of cost-effectiveness.
Model structure and methodology
Troponin tests considered in the model
The health-economic analysis will estimate the cost-effectiveness of different Tn testing methods for diagnosing or ruling out NSTEMI, in patients presenting at the ED with suspected NSTE-ACS, who have no major comorbidities requiring hospitalisation (e.g. as HF or arrhythmia) and in whom STEMI has been ruled out. Those diagnosed with NSTEMI will then be admitted to the hospital for AMI treatment and those diagnosed as without NSTEMI can be discharged without AMI treatment and further hospital stay. AMI treatment might include aspirin, statins and ACE inhibitors and consideration of coronary revascularisation for high-risk cases.7 Initiating AMI treatment for NSTEMI will reduce the probability of MACEs, particularly cardiac death and re-infarction.
Standard serial Tn testing, for patients with acute chest pain attributable to possible ACS, does not achieve optimal sensitivity in detecting AMI until 10–12 hours after onset of symptoms. Waiting for 10–12 hours after symptom onset is burdensome for patients and induces additional health-care costs. Therefore, various alternatives have been proposed, using more sensitive Tn tests, for the early rule-out of NSTEMI (within the 4-hour NHS ED target).89
Two hs-cTn assays (Roche Elecsys hs-cTnT and Abbott ARCHITECT hs-cTnI) are currently used in NHS laboratories in England and Wales. One additional assay (Beckman Coulter hs-cTnI) was listed in the scope for this assessment, pending CE marking. However, each of these tests can be used at different time points and with different diagnostic thresholds, resulting in multiple possible strategies for each test. Whether or not a test strategy was included in the economic model was decided based on optimal diagnostic performance, given the available evidence on accuracy for a population with STEMI ruled out, and on applicability in clinical practice (see Results of the assessment of clinical effectiveness assessment, above). The test strategies evaluated in the model are:
- Standard Tn at presentation and at 10–12 hours (reference standard).
- Roche Elecsys hs-cTnT at presentation: 99th centile threshold.
- Roche Elecsys hs-cTnT (optimal strategy): LoB threshold at presentation followed by 99th centile threshold peak within 3 hours and/or Δ20% (compared with presentation test) at 1–3 hours (see Figure 9).
- Abbott ARCHITECT hs-cTnI at presentation: 99th centile threshold.
- Abbott ARCHITECT hs-cTnI (optimal strategy): LoD threshold at presentation, followed by 99th centile threshold at 3 hours (see Figure 11).
- Beckman Coulter hs-cTnI at presentation: 99th centile threshold.
- No testing, discharge all patients without testing or treatment (only in sensitivity analyses). A Tn test may not be indicated when clinical judgement assesses the probability that a patient is experiencing an AMI as low. Therefore, consistent with the protocol, this hypothetical strategy is included in sensitivity analyses wherein the AMI prevalence is varied.
In the base case, it was assumed that standard Tn had perfect sensitivity and specificity (reference case) for diagnosing AMI. Using this assumption, all patients testing positive on a hs-cTn test but negative on the standard Tn would be classified as FPs. This implies that their risk for adverse events would be the same as for those patients testing negative on both the hs-cTn test and the standard Tn, and that they ought to be discharged home without further immediate treatment. However, recent evidence has shown that patients with a negative standard Tn, but a positive hs-cTn, may be at higher risk for adverse events than patients who test negative on both the standard and the high-sensitive Tn (Goodacre S, Medstar Washington Hospital Center, Washington, DC, USA; Lipinski M, University of Sheffield, Sheffield, UK: 2014, personal communication). A secondary analysis was therefore performed, which attributed a higher risk of adverse events to a proportion of patients testing FP with the hs-cTn test.
Based on the available evidence, two analyses were performed:
- Base-case analysis.
- Secondary analysis, assuming that FPs in the hs-cTn testing strategies do not have the same risk for adverse events as TNs. Instead, these patients were assigned a higher risk for (re-)infarction and death, to reflect the idea that when the hs-cTn test gives a positive result, in some cases this must be caused by a disease process, whether or not the strict definition of AMI is met. The risk of adverse events in patients with positive hs-cTn but a negative standard Tn is higher than the patients testing negative on both the hs-cTn test and the standard Tn, but lower than risk of adverse events in patients diagnosed with NSTEMI (i.e. both positive hs-cTn and standard Tn).
Model structure
This assessment uses the HTA report by Goodacre et al.7 as a starting point for cost-effectiveness modelling. The Goodacre report compared the cost-effectiveness of several diagnostic strategies for ACS. The assessment group received the health-economic model (in SIMUL8 2011, Simul8 Corporation, Boston, MA, USA) that this HTA was based on, and this model was used as a starting point to develop a de novo model (in Microsoft Excel 2003: Microsoft Corporation, Redmond, WA, USA) adapted to better fit the scope of the current assessment. In the health-economic model the mean expected costs and QALYs were calculated for each alternative strategy. These long-term consequences were estimated based on the accuracy of the different testing strategies followed by AMI treatment or discharge from the hospital without AMI treatment for patients presenting at the ED with suspected NSTE-ACS, including patients with NSTEMI and patients without NSTEMI, who are further subdivided into ‘No ACS, no UA’ and ‘Unstable angina’. For this purpose a decision tree and a Markov model were developed. The decision tree was used to model the 30-day outcomes after presentation, based on test results and the accompanying treatment decision. These outcomes consisted of ‘No ACS, no UA’, ‘Unstable angina’, ‘Non-fatal AMI (untreated)’, ‘Non-fatal AMI (treated)’ and ‘Death’. The decision tree is shown in Figure 14.

FIGURE 14
Decision tree structure.
The long-term consequences in terms of costs and QALYs were estimated using a Markov cohort model (Figure 15) with a lifetime time horizon (60 years). The cycle time was 1 year, except for the first cycle, which was adjusted to 335.25 days (365.25–30) to ensure that the decision tree period (30 days) and the first cycle combined summed to 1 year. The following health states were included:

FIGURE 15
Markov model structure. a, During the first year post AMI, a distinction is made between treated and untreated AMI.
- ‘No acute coronary syndrome and no unstable angina (no ACS, no UA)’
- ‘Unstable angina’
- ‘Post AMI (treated and untreated)’
- ‘Post AMI with re-infarction’
- ‘Death’.
Model parameters
Estimates for the model input parameters were retrieved from the literature and by consulting experts for unpublished data. Accuracy estimates were derived from the systematic review component of this assessment (see Results of the assessment of clinical effectiveness assessment, above).
Transition probabilities
An overview of transition probabilities is provided in Table 11.
TABLE 11
Transition probabilities
Decision tree
The proportions of patients testing positive or negative (and thus commencing AMI treatment or being discharged from the hospital) were based on the estimated accuracy of the testing strategies considered (Table 12) and the estimated prevalence of NSTEMI in the UK [17.0% with standard error (SE) 2.8%; see Table 11].39,40,46,64 This prevalence was higher than that derived from the RATPAC trial78 and used in the Goodacre model,7 because the RATPAC study population was a low-risk population.79,87 The proportion of TPs, FPs, FNs and TNs were calculated as follows:
TABLE 12
Test accuracy
- TP = NSTEMI prevalence × sensitivity
- FP = (1 – NSTEMI prevalence) × (1 – specificity)
- FN = NSTEMI prevalence × (1 – sensitivity)
- TN = (1 – NSTEMI prevalence) × specificity.
Subsequently, the proportions of patients who receive AMI treatment (TP + FP), and who are discharged without AMI treatment (TN + FN) were calculated. These results are listed in Table 13.
TABLE 13
Test outcomes
After treatment, TP patients in the decision tree were allocated to ‘Non-fatal AMI (treated)’ and FP patients were further subdivided between ‘No ACS, no UA’ and ‘Unstable angina’ (based on the proportion of UA among non-NSTEMI patients; see Table 11). After being discharged, TN patients were also subdivided between ‘No ACS, no UA’ and ‘Unstable angina’, whereas FN patients were allocated to ‘Non-fatal AMI (untreated)’. The proportions of FNs, reported in Table 13, can be considered as the proportions of AMIs that would have been missed when assuming that standard Tn testing has perfect accuracy. Finally, to calculate the total number of deaths in the decision tree, the probability of 30-day mortality was assigned based on abovementioned subdivision (see Table 11). It was assumed that UA is always correctly diagnosed, hence the mortality probability for treated UA was used.
Markov model
The age-dependent AMI incidence in the UK92 was used to model the occurrence of AMI for patients in the health states ‘No ACS’ and ‘Unstable angina’. It was assumed that all AMIs in the Markov trace are diagnosed correctly and thus receive treatment. For patients in the ‘Post-MI’ health state, the probability of re-infarction after treated AMI was retrieved from a UK record linkage study (n = 387,452), which assessed long-term survival and recurrence after AMI.93 For the current assessment the probabilities for females and males were weighted according to the estimated proportion of females and males in the population (males = 58.1%7). The re-infarction probability for the ‘Post-MI with re-infarction’ health state is equal to the re-infarction probability for the ‘Post-MI’ health state. The re-infarction RR for people with untreated AMI compared with treated AMI was calculated from a recent study by Mills et al.83 based on patients with a Tn concentration of 5–19 ng/l. This RR was assumed only for the first year after presentation at ED, after which no increased risk was assumed (i.e. RR = 1.0 for untreated vs. treated AMI after year 1).
Age-dependent mortality from the general population was used for patients in the ‘No ACS, no UA’ health state.91 For the ‘Post-MI’ and ‘Post-MI with re-infarction’ health states, mortality was extracted from the record linkage study.93 Again, the study by Mills et al.83 was used to calculate the mortality RR for untreated AMI compared with treated AMI for the first year, after which an RR of 1.0 was used. Finally, a multivariate adjusted mortality hazard ratio (HR) for UA compared with NSTEMI was retrieved from a study by Allen et al.94 to calculate mortality after UA.
All input parameters for the Markov model are reported in Table 11.
Health-state utilities
Age-dependent utility scores, from the UK general population, were calculated for patients in the ‘No ACS, no UA’ health state based on a linear regression model.85 These age-dependent utility scores from the general population were combined with age-dependent disutilities for AMI82 to calculate utilities for the ‘Post-MI’ health states (with or without re-infarction). Utility scores for the ‘Unstable angina’ health state were calculated based on Post-MI utility scores and a utility increment of 0.010 (Table 14).85
TABLE 14
Utility scores
Resource use and costs
Test-specific resource use consisted of the number of tests performed and the duration of hospital stay (hours) before discharge/AMI treatment (Table 15).
TABLE 15
Resource use (test specific)
Health-state costs (Table 16) were mainly retrieved from previous economic evaluations conducted in the UK.85,97 Health-state costs for the ‘Unstable angina’, ‘Post-MI’ and ‘Post-MI with re-infarction’ consisted of costs for three 15-minute general practitioner consultations and medication costs.85 For the first year in the ‘Unstable angina’ health state, costs for clopidogrel (for 60%) and hospitalisation (for 50%) were added to this. The first year costs for both ‘Post-MI’ health states were based on resource data from the Nottingham Heart Attack Register.97
TABLE 16
Health-state costs, event costs and unit prices
Additionally, costs of fatal events, retrieved from a UK economic evaluation,85 were accumulated for all fatal AMIs. For this purpose, it was assumed that all 30-day deaths after ‘true’ NSTEMI were due to a fatal AMI event. In addition, AMI treatment costs were calculated based on the national tariff for non-elective AMI without complications [Healthcare Resource Group (HRG) code: EB10Z].98 To calculate the hospital stay costs for patients, based on the number of hours before the test results become available, non-elective NHS reference costs for the general medical ward were used (HRG code: EB01Z).98 For this purpose, it was assumed that doctors were available on demand, and the time to discharge was delayed because of time between arrival at the ED and start of first sampling (1 hour) and the time between sampling and the results being available (2 hours). In the case of multiple testing, the 1-hour delay between arrival at the ED and start of sampling was applied to only the first test; however, this also affected the timing of the second test if applicable. The 2-hour delay before test results become available applies to all tests performed. Incorporating these time delays effectively implies that only tests at presentation and tests performed 1 hour after presentation could inform decisions within the NHS 4-hour ED target. All other multiple testing strategies, as well as standard Tn testing at 10–12 hours, would require a transfer from the ED to the general ward (patients are transferred to the general ward 4 hours after presentation at the ED). Finally, the test costs include panel (including reagent, machine and maintenance), calibration and quality control costs. Depending on the annual number of panels, the test costs varied between £16.18 and £21.33, for annual rates of testing of 1500 and 3000, respectively.80 Based on clinical expert input, the average test costs were estimated to be £20 (2011 price level).7,80
Overview of main model assumptions
The main assumptions in the health-economic analyses were:
- Serial Tn testing (comparator) has perfect accuracy (sensitivity = 1.0 and specificity = 1.0).
- For the Roche Elecsys hs-cTnT and Abbott ARCHITECT hs-cTnI optimal strategies it was assumed that the sensitivity and specificity for the subpopulation not discharged after the presentation test is equal to the sensitivity and specificity for the initial group (presenting at the ED).
- The life expectancy, quality of life and costs for FP patients is, in the base-case analysis, equal to the life expectancy, quality of life and costs of TN patients. This assumption was amended in the secondary and sensitivity analyses.
- In contrast with AMIs occurring during the decision tree period, all AMIs (either first or re-infarction) occurring in the Markov trace are diagnosed correctly and thus treated.
- UA is always correctly diagnosed and thus treated.
- The re-infarction probability for the ‘Post-MI with re-infarction’ health state is equal to the re-infarction probability for the ‘Post-MI’ health state.
- The increased ‘Post-MI’ re-infarction and mortality probabilities for untreated AMI were assumed to last 1 year: afterwards a RR of 1.0 was applied (for untreated vs. treated AMI).
- There is no additional benefit of starting treatment early, so treatment effect for high-sensitive strategies is equal to treatment effect for standard Tn strategy.
- All 30-day deaths (after presentation at the ED) are due to fatal AMI events and will receive the associated costs.
Model analyses
Expected costs, LYs and QALYs were estimated for all Tn testing methods. Discount rates of 3.5% and a half-cycle correction were applied for both costs and effects. Incremental cost and QALYs for each strategy compared with standard Tn, and compared with the next best alternative, were calculated. The ICER was then calculated by dividing the incremental costs by the incremental QALYs. PSAs (10,000 simulations) were performed, and cost-effectiveness acceptability curves (CEACs) and cost-effectiveness acceptability frontiers (CEAFs) were constructed. Although CEACs can be used to illustrate decision uncertainty, the option with the highest probability of being cost-effective may not necessarily be the most cost-effective option according to the expected values. Moreover, CEAFs can be used to illustrate the decision uncertainty surrounding the most cost-effective option.100
Secondary analysis
For the base case, it was assumed that patients who tested negative on standard Tn and positive on hs-cTn tests would experience life expectancy and quality of life equal to TN patients. This assumption is, however, debatable, as unpublished data (Goodacre S, Lipinski M, personal communication) show that patients with a negative standard Tn test and positive hs-cTn test have an increased risk of (re-)infarction and mortality compared with those who test negative on both standard Tn and hs-cTn tests. Although this risk was not as high as in patients with both positive standard Tn and positive hs-cTn tests, it could still be considered prognostically important. Therefore, in this secondary analysis the risk of re-infarction and mortality was adjusted for patients who tested FP (see Table 11). It was assumed that for this proportion of patients, the relative treatment benefit would be equal to that for TP patients. As the prevalence of this ‘higher risk subgroup’ is likely to be the same for all comparators, it was assumed that this proportion was equal to the lowest proportion of FP patients for all hs-cTn tests (see Table 13). This ‘higher risk subgroup’ was assumed to be treated for all hs-cTn tests (as they tested positive with these tests) and untreated for the standard Tn test (as they tested negative with this test), thus affecting the probability of adverse outcomes and treatment costs. In addition, the post-MI utility and health-state costs were used for this ‘higher-risk subgroup’.
Sensitivity analysis
For both the base case and the secondary analysis, the following one-way sensitivity analyses were performed to assess the impact of model assumptions and input parameters on the estimated outcomes:
Model assumptions:
- The assumption that the increased post AMI re-infarction and mortality probabilities for untreated AMI lasts for only 1 year was replaced by the assumption that these probabilities would remain elevated for a lifetime.
- The assumption that a doctor will be available on demand and thus that a decision could be made immediately (as in the base case) was replaced with an assumed delay (1, 2 or 3 hours) before a doctor is available and a decision could be made.
- As for the previous sensitivity analysis, except that the delay (1, 2 or 3 hours) applies only once patients are transferred to the general ward 4 hours after presentation (no delay in the ED).
- A total delay of 1.5 hours is assumed (includes delay from the time at which sampling could be performed to the time at which results became available and delay between arrival at hospital and Tn assessment commencing) rather than assuming a total delay of 3 hours (base case).
- AMI treatment costs are applied for patients who tested FP rather than using no treatment costs, as assumed in the base-case analysis.
- In addition to the health-state costs of UA during the first year, the AMI treatment costs are also applied for patients with UA (during the first year), rather than assuming no additional treatment costs.
Model input parameters (varied to lower and upper boundary of the 95% CI unless stated otherwise):
- test costs [test costs was varied over a wider range (£5–40) than the 95% CI]
- AMI treatment costs (±25%)
- post-MI first-year health-state costs
- utility increment for UA compared with AMI
- post-MI disutility compared with no ACS
- mortality (30 day) treated AMI (decision tree)
- mortality (30 day) untreated AMI (decision tree)
- annual re-infarction (after initial AMI)
- RR re-infarction (untreated vs. treated AMI)
- annual post-MI mortality
- annual post-MI mortality after re-infarction
- HR mortality (UA vs. NSTEMI)
- RR mortality (untreated vs. treated AMI).
Subgroup analysis
For both the base case and the secondary analysis, a number of subgroup analyses were performed. The main subgroup analyses were based on age- and sex-dependent re-infarction probabilities, mortality probabilities (for all health states), AMI incidence and quality of life, and could be applied to all test strategies. Accuracy was thus assumed to be subgroup independent (equal to the base case values). The following subgroups were identified:
- Sex.
- Age (45, 55, 65, 75 and 85 years).
- People with a history of previous NSTEMI. For this purpose, a proportion of 0% UA was assumed and the probabilities for the initial ‘Post-MI’ health state were used for the ‘No ACS, no UA’ health state and the probabilities for ‘Post-MI with re-infarction’ were used for the ‘Post-MI’ and ‘Post-MI with re-infarction’ health states. This subgroup analysis was performed for only the base case, as for the secondary analysis this would lead to lower mortality probabilities for FP patients than TN patients (which seems implausible).
- Subgroups with varying AMI prevalence (1%, 5%, 10%, 20%, 30%). In these analyses the no-testing strategy was included as a comparator, as a Tn test may not be indicated when clinical judgement assesses that the probability that a patient is experiencing an AMI is low. For the no-testing strategy it is assumed that patients will be discharged immediately.
It should be noted that the main subgroup analyses (described above) differ from the subgroups described in the systematic review component of this assessment (see Chapter 3, Presentation samples), for which specific accuracy and prevalence data were available. Additional subgroup analyses were performed based on these subgroup-specific accuracy data. However, these analyses could be performed for only the Roche Elecsys hs-cTnT assay at presentation sample, using the 99th centile diagnostic threshold, compared with standard Tn testing; no subgroup-specific accuracy data were available for the other two hs-cTn assays. The following subgroups were considered:
- age ≤ 70 years and age > 70 years
- patients with pre-existing CAD and patients without pre-existing CAD
- symptom onset at < 3 hours before presentation and symptom onset at ≥ 3 hours before presentation.
The subgroups with high pre-test probability and low-to-moderate pre-test probability were not considered, as the prevalence data for these subgroups were unknown.
Results of cost-effectiveness analyses
This section describes the results using probabilistic analyses for the base-case analysis and the secondary analysis. In addition, the sensitivity analyses (deterministic) and subgroup analyses are described (these deterministic analyses are also presented in tabulated form in Appendices 5–9.
Base-case analysis
The base-case analysis includes six test strategies. Tables 17 and 18 show the probabilistic results of this analysis. Standard Tn testing was both most effective (15.101 LYs, 11.730 QALYs) and most expensive (£2697). The Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was least effective (15.076 LYs, 11.712 QALYs) and least expensive (£2253). Compared with standard Tn testing, hs-cTn testing resulted in ICERs ranging between £90,725 and £24,019 savings per QALY lost.
TABLE 17
Probabilistic results for base-case analysis: LYs
TABLE 18
Probabilistic results for base-case analysis: costs and QALYs
Comparisons based on the next best alternative showed that for willingness-to-pay values of < £6600 per QALY, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, would be cost-effective. For thresholds between £6600 and £30,631 per QALY, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective; above £30,631 per QALY the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective. Standard Tn becomes cost-effective at a threshold of £90,725 or higher (see Table 18).
At willingness-to-pay thresholds of £20,000 and £30,000 per QALY, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, had probabilities of being cost-effective of 47% and 35%, respectively. Although the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective at a willingness-to-pay threshold of £30,000 per QALY, the Abbott ARCHITECT hs-cTnI optimal strategy had the highest probability of being cost-effective (35%) at this threshold (Figures 16 and 17).
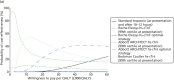
FIGURE 16
Cost-effectiveness acceptability curve and incremental cost-effectiveness plane (incremental costs and QALYs compared with standard Tn) for base-case analysis.

FIGURE 17
Cost-effectiveness acceptability frontier for base-case analysis.
Secondary analysis
The secondary analysis includes the same six test strategies. This analysis assumed that in a proportion of patients with a FP hs-cTn test (i.e. positive hs-cTn test and a negative standard Tn test), there is prognostic significance [i.e. it is associated with an increased risk of adverse events (mortality and re-infarction)].
Standard Tn testing was least effective (14.785 LYs, 11.464 QALYs) and most expensive (£3058). The Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was the least effective hs-cTn test strategy (14.833 LYs, 11.501 QALYs) and, overall, the least expensive strategy (£2781). The Abbott ARCHITECT hs-cTnI optimal strategy was most effective (14.855 LYs, 11.518 QALYs). Standard Tn testing was dominated by all hs-cTn testing strategies.
Comparisons based on the next best alternative showed that for willingness-to-pay values of < £13,623 per QALY, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective. For thresholds between £13,623 and £14,562 per QALY, the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was cost-effective; above £14,562 per QALY the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective (Tables 19 and 20).
TABLE 19
Probabilistic results for secondary analysis: LYs
TABLE 20
Probabilistic results for secondary analysis: costs and QALYs
At willingness-to-pay thresholds of £20,000 and £30,000 per QALY, the Abbott ARCHITECT hs-cTnI optimal strategy had the highest probability of being cost-effective (53% and 67%, respectively; Figures 18 and 19).
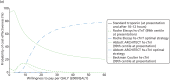
FIGURE 18
Cost-effectiveness acceptability curve and incremental cost-effectiveness plane (incremental costs and QALYs compared with standard Tn) for secondary analysis.
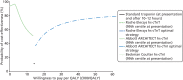
FIGURE 19
Cost-effectiveness acceptability frontier for secondary analysis.
Sensitivity analysis
The deterministic analysis for the base-case analysis is presented in Appendix 5. When it was assumed that the post-MI re-infarction and mortality probabilities would remain elevated for untreated AMI for a life-time period, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £1642 per QALY, at which point the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to a threshold of £7602 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £7602 and £26,532 per QALY. Standard Tn testing was cost-effective for thresholds of > £26,532 per QALY. Consistent with the base-case analysis, all ‘no doctor on demand’ sensitivity analyses (1, 2 or 3 hours) showed that the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between approximately £8000 and £40,000 per QALY. Similarly, where the total delay decreased to 1.5 hours (and assuming availability of a doctor on demand), the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £7778 and £29,653 per QALY, at which point the ARCHITECT hs-cTnI optimal strategy became cost-effective. Adding AMI treatment costs for the patients with a FP test substantially impacted upon the results: standard Tn testing was cost-effective for all threshold values of > £16,050 per QALY. Adding AMI treatment costs to the UA health state for the first year had a negligible impact on the incremental outcomes.
The following input parameters had a noticeable impact on the estimated cost-effectiveness: 30-day mortality for treated and untreated AMI (decision tree) and the mortality RR for treated AMI compared with untreated AMI (Markov trace). Varying the remaining parameters did not have a substantial impact on the results (i.e. the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between approximately £10,000 and £35,000 per QALY).
The deterministic analysis for the secondary analysis is presented in Appendix 6. When assuming that the post-AMI re-infarction and mortality probabilities would remain elevated for untreated AMI for a life-time period, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £1853 per QALY, at which point the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to a threshold of £2017 per QALY. The Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £2017 and £5889 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds of > £5889 per QALY. For all ‘no doctor-on-demand’ sensitivity analyses, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £18,000 per QALY for 1, 2 and 3 hours’ delay. The Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £18,000 and £19,000, £20,000 and £22,000 per QALY in case of 1, 2 and 3 hours’ delay, respectively. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for higher thresholds. Similarly to the deterministic base case, for which the total delay decreased to 1.5 hours (assuming availability of a doctor on demand), the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of below £14,956, at which point the ARCHITECT hs-cTnI optimal strategy became cost-effective. Adding AMI treatment costs for all patients with a FP test gave similar results to the deterministic analysis: the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for all threshold values of < £15,508 per QALY, at which point the Abbott hs-cTnI optimal strategy became the preferred option. Adding AMI treatment costs to the ‘Unstable angina’ health state for the first year had a negligible impact on the incremental outcomes.
The following input parameters had a noticeable impact on the estimated cost-effectiveness of the secondary analysis: increased test cost (of £40 per test), 30-day mortality for treated and untreated AMI (decision tree), and the re-infarction and mortality RR for treated AMI compared with untreated AMI (Markov trace). Varying the remaining parameters did not have a substantial impact on the results.
Subgroup analysis
Additional analyses were performed for subgroups based on age, sex, people with a history of previous NSTEMI, and AMI prevalence. These deterministic subgroup analyses (for the base case) analysis are presented in Appendix 7. Consistent with the base-case analyses, analyses based on age and sex subgroups indicated that, up to an age of 75 years, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between approximately £10,000 and £35,000 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for higher thresholds up to £115,000–170,000, at which point standard Tn testing became cost-effective. For females aged > 85 years, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £15,793 and £74,597 per QALY; the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £74,597 and £259,592 per QALY, and standard Tn testing was cost-effective for thresholds of £259,592 per QALY and higher. For males aged > 85 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £28,711 per QALY; the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £28,711 and £143,225 per QALY and the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £143,225 and £503,476 per QALY, at which point standard Tn testing became cost-effective. The results for the subgroup with a history of previous NSTEMI were almost identical to the base-case analysis.
For subgroup analyses considering AMI prevalence, no testing was included as additional comparator. For an AMI prevalence of 1%, the no-testing strategy was cost-effective up to thresholds of £27,409 per QALY, at which point the Beckman Coulter hs-cTnI (99th centile) test became cost-effective up to a threshold of £447,934 per QALY. For an AMI prevalence of 5–20%, the no-testing strategy was cost-effective up to thresholds of £8759–11,703 per QALY, at which point the Beckman Coulter hs-cTnI (99th centile) test became cost-effective up to thresholds of £32,042–97,709 per QALY. For an AMI prevalence of 30%, the no-testing strategy was cost-effective up to a threshold of £8431 per QALY, at which point the Beckman Coulter hs-cTnI (99th centile) test became cost-effective up to a threshold of £24,745 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £24,745 and £70,942 per QALY.
In addition, cost-effectiveness estimates for the subgroups, described in Chapter 3 (see Presentation samples), based on subgroup-specific accuracy and prevalence, are reported in Appendix 9 (only comparing the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, and standard Tn testing). The results of these analyses indicated that differences in accuracy and AMI prevalence between subgroups had a substantial impact on the cost-effectiveness of the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, compared with standard Tn testing (ICER range: £22,111–355,571; deterministic base case: £41,233).
The deterministic subgroup analyses for the secondary analysis are presented in Appendix 8. For females aged 45 years and males aged 45 or 55 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £16,023–17,836 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective for higher thresholds. For females aged 55 or 65 years and males aged 65 or 75 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £13,064–16,994 per QALY. From this threshold up to £18,999–25,149 per QALY, the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was most cost-effective. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for higher thresholds. For females aged 75 or 85 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective up to thresholds of £12,392£21,140 per QALY, at which point the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to thresholds of £16,407–26,911 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective for thresholds of > £24,020–45,709 per QALY. For males aged 85 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £66,418 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective for higher thresholds.
For subgroup analyses considering AMI prevalence, no testing was included as additional comparator. For an AMI prevalence of 1%, the no-testing strategy was cost-effective up to a threshold of £4563 per QALY, at which point the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to a threshold of £109,991 per QALY, where the Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective. Similarly, for AMI prevalences of 5% and 10% the thresholds were £5209 and £35,574, and £5820 and £22,684, respectively. For a AMI prevalences of 20% and 30%, the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds of > £16,319 and £15,410, respectively.
In contrast with the base-case analysis (described above), the subgroup-specific accuracy and prevalence (only comparing the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, and standard Tn testing) did not have an important impact on the cost-effectiveness (see Appendix 9). The Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was dominant for all subgroups.
- Assessment of cost-effectiveness - High-sensitivity troponin assays for the earl...Assessment of cost-effectiveness - High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis
- Cost-effectiveness review - The use of fenestrated and branched endovascular ane...Cost-effectiveness review - The use of fenestrated and branched endovascular aneurysm repair for juxtarenal and thoracoabdominal aneurysms: a systematic review and cost-effectiveness analysis
- Health economics methods and results - Accurate diagnosis of latent tuberculosis...Health economics methods and results - Accurate diagnosis of latent tuberculosis in children, people who are immunocompromised or at risk from immunosuppression and recent arrivals from countries with a high incidence of tuberculosis: systematic review and economic evaluation
- List of abbreviations - Folate Augmentation of Treatment – Evaluation for Depres...List of abbreviations - Folate Augmentation of Treatment – Evaluation for Depression (FolATED): randomised trial and economic evaluation
- Drosophila arizonae strain AZTUS-31 antares (antr) gene, complete cdsDrosophila arizonae strain AZTUS-31 antares (antr) gene, complete cdsgi|744515636|gb|KP236491.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...