Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Sharma P, Scotland G, Cruickshank M, et al. The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2015 Jun. (Health Technology Assessment, No. 19.48.)

The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation.
Show detailsWe assessed the cost-effectiveness of self-monitoring (self-testing and self-management) using CoaguChek system and alternative point-of-care testing devices compared with standard monitoring care in people receiving long-term vitamin K antagonist therapy.
Systematic review of existing cost-effectiveness evidence
Initial scoping searches revealed a number of previous systematic reviews of economic studies evaluating point-of-care testing devices for people receiving long-term vitamin K antagonist therapy.21,32 Further systematic searches of MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Science Citation Index, Health Management Information Consortium, NHS Economic Evaluation Database and the HTA databases were undertaken to identify any further relevant studies. The search strategies are detailed in Appendix 1.
The searches identified 12 economic evaluations of potential relevance to the scope of this assessment. All of these evaluations comparing INR self-monitoring strategies with standard care were appraised against the NICE reference case, and the methods and findings of each study are summarised briefly below in a narrative fashion and tabulated for comparison in Table 12. The studies were assessed against the NICE reference case and their relevance to the scope is shown in Table 13.
TABLE 12
Summary of identified economic evaluations included in the review
TABLE 13
Assessment of published economic evaluations against the NICE reference case
Critique of the included studies
Taborski 199982
This German study assessed the cost-effectiveness of patient self-management versus anticoagulation clinic-based management by a family physician or specialist. The study included costs relevant to the primary cost carrier: in this case, the government-controlled health insurance fund. Information regarding the costs of self-management and clinic management, and the costs of acute treatment and rehabilitation for complications were acquired from patients and published literature. Quality of life was not considered in the analysis. When costs of complications were included in the analysis, self-management was estimated to be less costly and more effective than clinic-managed care – owing to its estimated impact on the incidence of both thromboembolic and bleeding events. However, the estimated effects of self-management on these adverse events were selected from a small number of studies reporting high baseline rates and large beneficial effects of self-management.
Lafata 200083
This study, carried out in the USA, constructed a Markov model with a 5-year time horizon to examine the cost-effectiveness of three anticoagulation management strategies: usual care with a family physician (without a point-of-care monitoring device), anticoagulation clinic testing with a point-of-care monitor, and patient self-testing with a point-of-care monitor. The self-testing strategy required participants to telephone their anticoagulation clinic for dosing instructions. For each strategy it was assumed that the time within, above and below the therapeutic range differed and that time out of the target range influenced the risk of complications. Time in range was modelled to be highest for self-testing, followed by point-of-care anticoagulation clinic testing, followed by usual care. The actual estimates were based on a number of cohort studies and clinical trials, but these did not appear to be systematically identified.
The Markov model parameter values were estimated from available literature, routine health service data, and expert opinion where necessary. The analysis was conducted for a hypothetical cohort of participants, aged 57 years, initiating long-term warfarin therapy. Both a health service provider (direct medical care costs only) and a wider societal perspective (including costs incurred by participants and their caregivers) were adopted. The patient self-testing strategy assumed the highest number of annual tests (n = 52), compared with anticoagulation clinic testing (n = 23) and usual care (n = 14). The 5-year direct health service costs (per 100 participants) were higher for the self-testing strategy ($526,014) than for usual care ($419,514) or anticoagulation clinic testing ($405,560). However, when patient and caregiver costs were included, self-testing accumulated lower 5-year costs than anticoagulation clinic testing ($622,727 vs. $645,671). From the health service provider perspective, anticoagulation clinic testing with a point-of-care monitor was considered the most favourable strategy. When patient and caregiver costs were included, self-testing dominated point-of-care anticogulation clinic testing, but remained more costly and more effective than usual care.
A number of one-way sensitivity analyses were conducted to test key parameter and structural assumptions in the model. Model findings were found to be most sensitive to assumptions regarding the frequency of yearly tests and time spent in the therapeutic range with the different strategies. Given that the setting of this study was the USA, the results cannot be generalised to the UK.
Muller 200184
This economic analysis was conducted to assess the cost-effectiveness of patient self-management compared with standard family physician-managed anticoagulation monitoring in people following a mechanical heart valve replacement. The focus was on preventing coagulation-related complications. The incidence of stroke was estimated for a hypothetical cohort of 10,000 patients from the German Experience with Low Intensity Anticoagulation (GELIA) study.89 Data from the USA, adapted to German standards, were used to inform lifetime costs of stroke. The study assumed that self-management would reduce the incidence of severe complications by 30%, compared with family physician-managed care. The incremental cost-effectiveness ratio (ICER) was estimated to be Deutsche Mark (DM) 105,000 per life-year gained for self-management versus physician-managed care. The authors concluded that PSM may reduce the incidence of fatal strokes at an acceptable ICER.
Sola-Morales 200385
This evaluation was published in Catalan by the Catalan Agency for HTA. It was assessed partially based on a summary in a previous review21 and using a web-based translation interface to translate key passages.
The study compared several strategies including standard laboratory testing, patient self-management, patient self-testing, point-of-care monitoring by a GP and point-of-care monitoring in a hospital setting. A Markov model was constructed with a 5-year time horizon. Data to populate the model were acquired from a systematic literature review. The study assumed a higher incidence of adverse clinical outcomes for usual care than for those strategies utilising a point-of-care monitoring device. It was assumed that all strategies involving the use of a point-of-care monitor had equivalent effects. Based on these assumptions, the results indicated that, from a health insurer perspective, the use of point-of-care monitors in a hospital setting was the preferred option on grounds of cost-effectiveness. However, it was not clear what the relative cost-differences were between the monitoring strategies.
Jowett 200687
This cost–utility analysis was conducted alongside the largest UK-based RCT of patient self-management versus standard primary or secondary care INR monitoring. The follow-up period was 12 months. The analysis relied on individual patient-level cost and utility data (derived from responses to the EQ-5D), collected alongside the RCT.
The cost-effectiveness of patient self-management (average 30 tests per year with CoaguChek S) versus usual clinic management (average 10 tests per year at a combination of hospital and primary care clinics) was estimated from the perspective of the NHS and also from a wider perspective incorporating patient costs. The trial recruited 617 participants receiving long-term anticoagulation. Quality-adjusted life-years (QALYs) were derived from participant responses to the EQ-5D at baseline, 6 weeks and 6 months. Multiple imputations were used to replace missing EQ-5D data and a regression-based approach was used to estimate incremental QALYs associated with self-management.
Costs for patient self-management included training and assessment costs, device and testing strip costs, costs of any telephone calls relating to INR or device queries, and costs associated with any adverse events. Costs of standard care clinic monitoring visits were estimated for the various types of standard care on offer (from a sample of participating centres) and applied on a per-visit basis. Costs associated adverse events were taken from the NHS reference cost. Wider patient costs included out-of pocket travel costs and the value of time lost from work to attend appointments.
Based on intention to treat, the results indicated that from both the health service and the wider perspective, mean costs in the patient self-management arm were significantly higher than those in the usual care arm (+£294 and +£282.93). There was a very small, non-significant increase in QALYs in the self-management arm at 12 months (0.009, 95% CI –0.012 to 0.030).
From the health service provider perspective, the ICER for patient self-management was £32,716 per QALY gained, and an ICER of £31,437 per QALY gained was reported from a wider societal perspective. At a ceiling ratio of £20,000 per QALY gained, patient self-management had a 30% probability of being cost-effective; this probability increased to 46% when the ceiling ratio rose to £30,000 per QALY gained.
The authors concluded that, based on the general decision rules for interpreting cost-effectiveness findings in the UK, it was unlikely that self-management would be considered cost-effective compared with usual care. However, it was noted that although patient self-management incurred a higher initial cost, it could reduce the number of people attending outpatient clinics and, therefore, free up clinician time for other patients. Furthermore, the results were based on only 12 months’ follow-up of a single trial that was not powered to detect a difference in adverse events.
Regier 200686
This Canadian study assessed the cost-effectiveness of patient self-managed and family physician-managed (with laboratory testing) long-term anticoagulation therapy. A Bayesian Markov model was constructed from the perspective of a Canadian health-care payer, and analysed over a 5-year time horizon. The adopted model structure accounted for the time spent by patients within, above or below the specified INR therapeutic range, and determined patients’ risks of thromboembolic and haemorrhagic events based on this.
Model input parameter estimates were derived from a number of sources. TTR was obtained from a Canadian trial of self-management versus physician-managed warfarin therapy. Event risks for time spent in, above and below therapeutic range were derived from a prospective cohort of 2745 people with atrial fibrillation, AHVs and venous thromboembolism. Cost and utility parameters were taken from a number of different sources.
It was assumed that under the self-management strategy, people would perform 52 tests per year, while under physician-managed care, only 14 tests would be performed each year, with dosing information from the laboratory test being communicated to the patient by telephone.
The mean per-patient cost over the 5-year period was higher for the self-management strategy (C$6116) than for the physician-managed strategy (C$5127). In terms of quality of life, self-management resulted in a QALY gain at the 5 years of 0.07. This was due to a modelled reduction in both the number of thromboembolic events and the number of haemorrhagic events. The reported ICER for self-management versus physician-managed care was C$14,129 per QALY gained. The authors concluded that self-management was cost-effective for people receiving long-term anticoagulation therapy.
The methods for calculating the costs and outcomes in this study were not transparent and the time spent in the therapeutic range was derived from the results of a single clinical trial conducted in a Canadian setting. Moreover, the perspective adopted was that of a Canadian health-care payer, which makes the generalisability of these results to a UK setting difficult. In addition, the comparator in this study was physician-managed care relying on laboratory testing, rather than anticoagulation clinic-managed care using point-of-care testing. As such, the result may be less generalisable to contexts where the latter approach is used in standard practice.
Brown 200732
Another Canadian study conducted by Brown and colleagues32 adopted a decision-analytic modelling approach to assess the cost–utility of patient self-testing (52 tests per year), compared with physician-managed laboratory testing (20 tests per year) and physician-managed point-of-care testing (23 tests per year). The 5-year model presented results from both the health-care provider (estimated separately to include and exclude nursing home costs) and a wider societal perspective. The model was similar in structure to other models reported in the literature, with thromboembolic and haemorrhagic events modelled by time spent inside and outside the specified INR therapeutic range. The analysis was conducted for a hypothetical cohort of people on long-term warfarin therapy, with input parameters estimated from the published literature and a meta-analysis of studies assessing TTR. It was assumed that self-testing and physician-managed point-of-care testing were equivalent in terms of clinical effects. Cost parameters were identified from the published literature and were valued using Canadian sources.
Cumulative costs and QALYs were estimated over a 5-year period. From the health service provider perspective, the results indicated that physician-managed point-of-care testing was cost saving compared with usual care. Self-testing, on the other hand, was not found to be cost-effective in comparison with usual care (ICER $57,595 per QALY gained) and was dominated by physician-managed point-of-care testing. However, from a societal perspective self-testing was found to be cost saving over both usual laboratory testing and physician-managed point-of-care testing. A probabilistic sensitivity analysis showed that from the societal perspective, patient self-testing had a 52% probability of being cost saving compared with usual care. An important limitation of this study was that it did not assess the impact of extending the time horizon beyond 5 years, which presumably would have improved the cost-effectiveness of self-testing versus usual care (physician-managed laboratory testing).
Connock 200721
The objective of this UK-based modelling study was to assess the cost-effectiveness of patient self-management of anticoagulation therapy compared with usual care (a mixture of primary and secondary care testing). A Markov model was constructed and analysed over a 10-year time horizon, adopting a NHS and personal social services perspective. The base-case cohort was aged 65 years and was assumed to have an increased risk of death, compared with the age-/sex-matched general population.
Model input parameters were derived from a number of sources. Estimates of time spent in therapeutic range, warfarin monitoring costs and baseline health state utility (measured using the EQ-5D) were derived from a previous RCT conducted in the UK with an accompanying economic evaluation.87 The cost of INR devices (assumed to be paid for by the NHS) were annuitised over a 3-year period, and it was assumed that where patients stopped using these for any reason within 3 years, 75% would be reused by another patient. Risks of thromboembolic, major haemorrhagic and minor haemorrhagic events were estimated from a variety of published sources by time spent in, above and below the specified INR therapeutic range. Following major events, patients could either enter a state of permanent disability with associated costs and utility decrements, or have no long-term consequences.
Following disabling events and minor haemorrhagic events, patients were modelled to be at an increased risk of death from all causes. Within the model, it was assumed that there was a non-specific 2.5% reduction in the risk of adverse events with patient self-management – mediated through patient education and empowerment rather than improved INR control. This was based on the finding that self-management was not found to have a significant impact on TTR in a pooled analysis of results from eight trials where this outcome was available. This was despite it having a significant beneficial impact on the risk of thromboembolic events and mortality (based on the pooled results from 15 trials).21
The base-case results were presented for both a 5- and a 10-year time horizon. Over the 5-year time frame, the incremental cost per QALY for self-management was estimated to be £122,365. The cost-effectiveness of self-management improved over the longer time horizon, with the incremental cost per QALY gained being £63,655 at 10 years. Cost-effectiveness acceptability curves were generated to characterise the uncertainty surrounding the 10-year estimate. Applying a ceiling ratio of £30,000 per QALY, patient self-management was found to have only a 44% chance of being cost-effective. However, the authors also carried out a sensitivity analysis whereby the pooled estimate of effects (on major complications) from all available trials were applied, and under this scenario found the incremental cost per QALY gained to be £19,617 for self-management at 10 years. The authors concluded that patient self-management of anticoagulation therapy was unlikely to be more cost-effective than usual care in the UK, but that it might offer a cost-effective alternative for patients whose therapy could not be satisfactorily controlled in usual care.
Gailly 200933
The objective of this study was to conduct a cost-effectiveness analysis of the use of point-of-care devices for GP-managed care, anticoagulation clinic-managed care, patient self-testing and patient self-management, compared with standard laboratory testing. The analysis focused on a cohort of patients on long-term anticoagulation therapy. A decision-tree model, with a 10-year time horizon, was constructed from the perspective of a Belgian health-care provider. The models input parameters were estimated from a meta-analysis of published studies for clinical effects and Belgian health-care databases for baseline risks and resource use.
As the meta-analysis of clinical effectiveness studies only identified evidence for a significant impact of point-of-care testing on mortality for patient self-management, the cost-effectiveness analysis focused on this modality of monitoring versus usual care (GP-managed testing with analysis of the blood sample in a laboratory). Furthermore, the outcome measure was restricted to the number of life-years gained as it was reported that no reliable quality-of-life data were identified. The annual number of point-of-care tests and the number of GP consultations due to INR tests in usual care and patient self-management were varied in a sensitivity analysis.
Applying the significant beneficial effects of self-management on mortality and thromboembolic events, the results showed self-management to be the dominant strategy compared with usual care, except when 100% of the GP consultations observed in usual care were assumed to be maintained with patient self-management and when the annual number of tests with self-management increased to 52 per year. The probabilistic sensitivity analysis showed patient self-management to have a high chance of being a dominant cost-saving strategy in comparison with usual care.
Medical Advisory Secretariat 200988
This Canadian study assessed the cost–utility of health service point-of-care testing, patient self-testing and patient self-management versus standard care for patients on long-term anticoagulation therapy. A Markov decision-analytic model was developed with a 5-year time horizon, and was analysed from the perspective of the Ministry of Health and Long-Term Care. The model was analysed for a hypothetical cohort of patients, and model inputs were derived from a systematic review of effectiveness, other published literature and expert opinion. Time spent within and outside the therapeutic range was used to estimate the likelihood of patients moving from one health state to another. The results indicated that all of the evaluated point-of-care strategies were cost-effective compared with usual care, and that patient self-management appeared to be the most cost-effective strategy.
Other studies
In addition to the above published evaluations, two abstracts were identified for potential relevance. Visnansky and colleagues90 conducted a rapid HTA to explore the cost-effectiveness of patient self-testing using CoaguChek compared with standard care (laboratory testing). A Markov model was constructed and analysed for hypothetical cohorts (mean age 63 years) on long-term anticoagulation therapy for different indications, applying a lifetime horizon. The authors concluded that patient self-testing was a cost-effective (dominant) strategy compared with usual care for all diagnosis subgroups.
Schmidt and colleagues91 conducted a cost–utility analysis of patient self-management compared with standard monitoring among long-term OAT patients in an Austrian setting. A Markov model was constructed adopting a lifetime horizon with an average baseline age of 67 years. This study found that although self-management incurred higher costs initially, throughout follow-up these costs reduced due to the lower number of health-care contacts over time. Adopting a lifetime perspective, it was found that self-management was the dominant strategy based on both a cost-per-life-year and a cost-per-QALY analysis.
Summary of findings from identified studies
The above overview of existing economic evaluations illustrates that the cost-effectiveness of patient self-testing and self-management versus usual care is uncertain and largely dependent on a number of key factors.
The adopted perspective appears to have a significant impact on estimated cost-effectiveness. Existing studies have estimated costs from different perspectives, including those of health service providers, society as a whole, health-care payers and health insurance funds. When a wider societal perspective has been adopted, self-management and self-testing strategies have generally compared favourably with standard clinic-based testing, as a result of lower time costs associated with fewer health service contacts. The initial costs associated with patient self-management and self-testing also appear to be important determinants of cost-effectiveness.
Variation between the studies in terms of the estimated or assumed effects of self-monitoring (on thromboembolic and bleeding events) also helps to account for the variable findings and conclusions. The two UK-based evaluations of greatest relevance to the scope of this diagnostic assessment report (DAR)21,87 estimated or applied effect estimates consistent with small or negligible differences between self-management and usual care with respect to TTR and adverse thromboembolic and haemorrhagic events. They subsequently found there to be a low probability of patient self-management being cost-effective. Contrary to this, several studies applied large effect estimates favouring self-monitoring in terms of TTR, thromboembolic events and/or mortality, and, subsequently, found self-monitoring strategies to be cost-saving or cost-effective.33,86,88
In relation to the scope of this assessment, the two most relevant studies are those reported by Jowett and colleagues87 and Connock and colleagues.21 These economic evaluations were largely based on the same trial conducted in the UK. Jowett and colleagues adopted a NHS and wider societal perspective and Connock and colleagues21 adopted a health service and personal social services perspective, which was in line with the NICE reference case. Key outcomes were measured directly within the trial-based evaluation, including utility values and complications experienced. Self-testing and self-management strategies do appear to increase the costs of INR monitoring in the short run, as demonstrated by these studies and others. However, other studies have shown that these costs can be offset by future cost-saving and quality-of-life gains, depending on the relative effectiveness of self-monitoring versus usual care in reducing the incidence of mainly thromboembolic events.
The two UK-based economic evaluations suggest that for effect estimates consistent with those observed in the largest UK-based trial of patient self-management, self-monitoring of INR is unlikely to be cost-effective. However, no UK-based trials have been sufficiently powered to detect a significant difference between standard INR monitoring and patient self-monitoring in terms of major thromboembolic or haemorrhagic events. Meta-analysis of similar trials, given their rarity, provides a more powerful means of estimating the true effect of self-monitoring on these clinical outcomes. An updated meta-analysis was described and presented in Chapter 2, and included randomised evidence from a number of recent European trials where standard care is similar to that provided in the UK in terms of approach, frequency and the level of INR control achieved. Therefore, the following section describes the construction and analysis of a new economic model that builds on those described above, and which incorporates all of the available evidence on the clinical effectiveness of self-testing and self-monitoring.
Independent economic assessment
A de novo economic model was developed in TreeAge Pro (TreeAge Software, Williamstown, MA, USA). The model was designed to assess the cost-effectiveness of self-monitoring (self-testing and self-management) using alternative point-of-care devices: CoaguChek XS system, INRatio2 PT/INR monitor and ProTime Microcoagulation system. The model was structured based on the review of published models of INR self-monitoring, and previous models evaluating the cost-effectiveness of new anticoagulant drugs compared with warfarin therapy in people with atrial fibrillation.92,93 A further unpublished economic model of INR self-monitoring was provided by Roche (the manufacturer of CoaguChek XS), and this model was also used to inform the structure of the new economic model (J Craig, York Health Economics Consortium, 2013, personal communication provided by Roche through NICE).
The model was populated using data derived from the systematic clinical effectiveness review, other focused reviews to inform key parameters (e.g. baseline risks), routine sources of cost data,94,95 and, where necessary, some study-specific cost estimates based on expert opinion. The model was built and analysed in accordance with the NICE reference case for the evaluation of diagnostic tests and devices.29
Methods
Relevant patient population(s)
The model compared the alternative monitoring strategies for a hypothetical cohort of people with atrial fibrillation or an AHV. These two groups represent the majority of people on long-term vitamin K antagonist therapy. While self-monitoring of INR is relevant to other patient groups, including those with venous thrombotic embolism, there were insufficient data to explicitly model cost-effectiveness for all groups individually. Furthermore, the majority of studies informing the relative effects of alternative monitoring strategies were derived from trials including predominantly people with atrial fibrillation and/or an AHV. Therefore, the base-case modelling exercise was carried out for a mixed cohort consisting of people with one or other of these two conditions. In the base-case analysis, 60% of the cohort was modelled to have atrial fibrillation, with the remaining 40% having an AHV, in line with the observed proportions of patients with these conditions in self-monitoring trials.
Monitoring strategies to be evaluated
The economic model incorporated the pathways of care that individuals currently follow under standard practice in the NHS, as well as proposed new pathways for self-testing and self-management (informed by a review of current guidelines and expert opinion). Current practice was dichotomised in the model as standard monitoring in primary care and standard monitoring in secondary care. In the base-case analysis, the proportional split between standard primary and secondary care INR monitoring was taken from the manufacturer’s submission for technology appraisal 256 (TA256).96 Based on a survey of providers in England and Wales carried out in 2011, it was estimated that 66.45% and 33.55% of warfarin monitoring appointments were managed in a primary and secondary care setting, respectively. These figures were accepted by the independent evidence review group (ERG) and appraisal committee for NICE TA256.97
In terms of self-monitoring, the model incorporated both self-testing and self-management strategies using the alternative devices identified in the scope. However, the cost-effectiveness of self-monitoring was assessed as a whole, and it was assumed in the base-case analysis that 50% of people would self-test while 50% would self-manage. Self-testing and self-management strategies were costed separately for each device based on the assumption that self-testing people telephone in their results from all tests undertaken, while self-managing people manage their dosing independently. In reality, some self-monitoring people are likely to fall somewhere in between these two strategies, and the potential impact of this was addressed in sensitivity analysis by varying the proportional split between self-management and self-monitoring.
Framework (method of synthesis)
The alternative monitoring pathways, informed by review of previous guidance and expert opinion, were embedded in a Markov model simulating the occurrence of adverse events over time (Figure 16). The adverse events that constituted the model were ischaemic stroke (minor, non-disabling, and major, disabling or fatal), systemic embolism, minor haemorrhage and major haemorrhage [intracranial haemorrhage (ICH), including haemorrhagic stroke, gastrointestinal bleed, and others]. Systemic embolism was treated as a transient event within the model, such that people surviving this event returned to baseline levels of quality of life and did not incur ongoing costs and morbidity. Minor haemorrhage was handled in the same way. Ischaemic stroke and ICH were assigned post-event states associated with additional costs and quality-of-life decrements.

FIGURE 16
Schematic of the model structure. AF, atrial fibrillation; HS, haemorrhagic stroke; M, Markov process.
The model simulated transitions between the discrete health states, and accumulated costs and QALYs on a quarterly (3-month) cycle. Within each 3-month cycle, the simulated cohort was exposed to a risk of the aforementioned events as well as death from other causes. A constraint was applied whereby simulated people could experience only one event per cycle. A further simplifying structural assumption was applied, such that, following a major ischaemic stroke or ICH, no further events were explicitly modelled. However, all-cause mortality was inflated following these events to account for the increased risk of death.
Baseline risks for the modelled events were derived from the observed event rates in cohorts of people being managed under current standard models of care. RRs of these events resulting from improved/reduced INR control, conferred by self-monitoring, were derived from the meta-analysis of RCTs of self-monitoring versus standard practice. Appropriate costs and quality-of-life weights were attached to modelled events and health states, allowing cumulative health and social care costs and QALYs to be modelled over time. Further details of the event risks, transitions, costs and quality-of-life weights applied in the model are provided in the following sections.
Modelled baseline risks for people with atrial fibrillation
Previous economic models relied on a variety of sources to inform the underlying baseline risks of adverse events, ranging from single-centre trials to data pooled from a number of trials. The unpublished model provided by Roche made use of event rates reported by TTR,98–100 based on data from the control arms of large multinational trials comparing new anticoagulant drugs with standard treatment with warfarin for people with atrial fibrillation.
The Randomised Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial of dabigatran etexilate versus warfarin provides a detailed source of event-rate data by centre-level quartiles of mean TTR.99,101 The advantage of these data is that they allow underlying risks to be modelled by the level of anticoagulation control achieved, but there is a question surrounding their generalisability to the atrial fibrillation population on warfarin therapy in the UK. However, a previous study assessed the representativeness of the RE-LY clinical trial population to real-world atrial fibrillation patients in the UK,102 and found that the majority of patients in the UK (65–74%) would have met the inclusion criteria. Furthermore, to assess the generalisability of the annual risks of stroke derived from RE-LY data, these were compared with those derived from a large cohort study of atrial fibrillation patients on warfarin in the UK. Gallagher and colleagues103 analysed longitudinal data from the General Practice Research Database on 27,458 warfarin users with atrial fibrillation, and provided a Kaplan–Meier plot of the probability of being stroke free by different levels of TTR. Points on these plots were extracted using DigitizeIt software (DigitizeIt, Braunschweig, Germany: www.digitizeit.de), and used to estimate the annual risks of stroke by TTR groupings.
These stroke risks were found to be very similar to those for people in the corresponding TTR quartiles of the RE-LY trial control arm. Therefore, the control arm of the RE-LY trial was considered to be an appropriate source for estimating baseline risks by level of TTR in the economic model. The study by Gallagher and colleagues103 also estimated a mean TTR (INR 2–3) of 63% for the UK cohort of people with atrial fibrillation on warfarin, and so the baseline risks in the model were set to those observed in RE-LY trial centres that achieved a mean TTR between 57.1% and 65.5%.
The analysis of RE-LY trial data by TTR quartiles99 provided estimated annual event rates for non-haemorrhagic stroke and systemic embolism, major haemorrhage (including intracranial bleed, haemorrhagic stroke and major gastrointestinal bleeds) and minor haemorrhage. These rates were entered in the model, where they were converted into annual risks (Table 14). Following further adjustment, where appropriate, with RRs, the annual risks were converted into quarterly risks using the following equation:
TABLE 14
Annual baseline event risks for people with atrial fibrillation by level of INR control (TTR)
The events were modelled within each cycle of the model, and were further disaggregated based on the observed numbers of different types of event observed within each composite outcome in the RE-LY trial99,101 (Table 15).
TABLE 15
Disaggregation of modelled composite outcomes
Further adjustments were applied to the risk of stroke in atrial fibrillation patients, to reflect the importance of age as a risk factor. For this purpose, the same approach as used in the model for NICE TA256 (rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation) was applied.96 RRs of stroke by age, compared with a 70- to 74-year-old cohort (the average age of participants in RE-LY trial), were derived from a Framingham-based risk score calculator for patients with atrial fibrillation,106 and applied to adjust the risk of stroke and systemic embolism by 5-year age bands.96 A similar approach was also used to inflate the risk of bleeding with increasing age, using data from Hobbs and colleagues.107
Death following stroke was estimated by applying case fatality rates to these modelled events. Death following stroke utilised the same approach as used in the model of dabigatran versus warfarin for NICE TA249.105 Based on Hylek,104 the hospital case fatality rate was first applied, followed by the reported 30-day mortality by severity of stroke (Rankin score 0–2; 3–5) post discharge (see Table 15).
Modelled baseline risks for people with an artificial heart valve
Less extensive data were identified describing the baseline risk of adverse events for people with AHVs by level of INR control. Previous economic models have tended to use overall event risks for mixed cohorts rather than explicit event risks for individual patient groups included in the modelled cohort. However, the model provided by Roche used a dichotomised cohort with event risks estimated separately for people with atrial fibrillation and an AHV. This approach is useful for modelling subgroups and cohorts with varying proportions of people with the two conditions. Therefore, the same general approach was adopted.
As per the model provided by Roche (J Craig, York Health Economics Consortium, 2013, personal communication provided by Roche through NICE), a recent meta-analysis of individual patient-level data from 11 RCTs of self-monitoring versus standard care provided the source of event data.108 Heneghan and colleagues108 presented a subgroup analysis where they presented the estimated pooled hazard ratio and number needed to treat to prevent one major thromboembolic event (ischaemic stroke and systemic embolism) and one major haemorrhagic event by year of follow-up (up to 5 years) based on 2243 people with an AHV. The formula used by Heneghan and colleagues108 to estimate the number needed to treat was:
Sc(t) is the survival probability in the control group (standard monitoring) at time t, Sc(t)h is the corresponding survival probability in the active treatment group (self-monitoring), and h is the hazard ratio. The 5-year probability of experiencing a thromboembolic (0.089) and major haemorrhagic event (0.169) in the control group were back calculated for people with an AHV, and converted into annual probabilities (Table 16). These were incorporated in the model for subsequent adjustment and conversion into quarterly probabilities for use as baseline risks.
TABLE 16
Annual baseline event risks for people with an AHV
A focused search was undertaken to identify alternative sources of data to inform the baseline risk of thromboembolic events in people with an AHV. A previous meta-analysis estimated a pooled annual linearised risk of 1.6% for people with a mechanical aortic valve. A further large Canadian series (including 1622 people with a mechanical heart valve) estimated linearised embolic stroke risks of 1.4% and 2.3% per year for people with an artificial aortic and a mitral valve, respectively.109 These figures are generally consistent with the baseline estimates used in the model. However, a smaller series from a single centre in the south-west of England reported a lower rate of 1.15% per patient-year based on 2 years’ follow-up of 567 people with a Sorin Bileaflet third-generation prosthesis.110 The impact of applying this lower baseline risk was assessed through sensitivity analysis.
In the absence of more detailed data for people with an AHV, the same proportional splits used to disaggregate thromboembolic and major haemorrhagic events for people with atrial fibrillation were applied (see Table 15). Furthermore, as data on minor bleeds were not available from Heneghan and colleagues108 for people with an AHV, the same baseline risk applied for people with atrial fibrillation was adopted. This was justified on the grounds of the two groups of people facing similar risks of a major bleed (0.405 and 0.363).
Further adjustments to baseline risks
Within the model, a number of simplifying structural assumptions were made. Following the occurrence of a major disabling ischaemic stroke or an ICH/haemorrhagic stroke, no further events were modelled. However, the risk of age-/sex-specific all-cause mortality was inflated following these events using RRs estimated by Sundberg and colleagues.111 Deaths from other causes following minor stroke were also inflated in the model to account for the observed increased risk of death from all causes following this event.111,112
The background risk of death from other causes also was increased for the atrial fibrillation and AHV cohorts using standardised mortality ratios reported by Friberg and colleagues113 and Kvidal and colleagues114 (Table 17).
TABLE 17
Parameters used in the model to adjust rates of death from all and other causes
Baseline rates of death from all and other causes were modelled by age and sex based on interim life tables. For other cause mortality, deaths due to stroke, systemic embolism and ICH were removed.116,117
Incorporation of relative treatment effects
Pooled estimates of RR derived from the meta-analysis of RCTs of self-monitoring versus standard practice were used to adjust the baseline risks of events in the model (Table 18). Given the limitations of the available data, it was not possible to accurately estimate the relative clinical effectiveness of using the alternative self-monitoring devices. Therefore, in the first instance, equivalent effects were assumed on the basis of several studies showing reasonable correlation between the instruments in terms of precision and accuracy. However, it is worth noting that the majority of the clinical effectiveness evidence relates to CoaguChek S, with only one trial included in the systematic review using the INRatio2 PT/INR monitor (although not exclusively), and two trials using the ProTime Microcoagulation system (exclusively).
TABLE 18
Relative effects for self-monitoring applied in the model
For the base-case analysis, relative effects were entered separately for the different types of event (any thromboembolic event, major bleed and minor bleed) by type of self-monitoring strategy (self-management and self-testing) (see Table 18). While not all effects were significant, the point estimates were applied in the model with appropriate distributions assigned to reflect the uncertainty surrounding them. These RRs, which represent pooled estimates obtained from trials with follow-up periods varying between 3 and 24 months, were assumed to apply directly to the 12-month risk of an event. Therefore, they were used to adjust the estimated annual baseline risk of events in the model, from which constant 3-month transition probabilities were derived, assuming constant proportional hazards over time. The RRs were applied only to people continuing on self-monitoring in the model.
Resource use estimation
Data on the resource use and costs associated with the alternative monitoring strategies were informed by published literature, existing guidance, expert opinion, manufacturers’ and suppliers’ prices, and other routine sources of unit cost data.94,95 As noted above, certain costs were informed by expert opinion where suitable data from other sources were not available.
Costs of standard care
Resource use associated with standard monitoring was informed by a number of sources. The model provided by Roche used estimates of monitoring costs (under standard primary and secondary care) based on previous estimates calculated by the independent ERG for NICE technology appraisal TA249, Dabigatran etixilate for the prevention of stroke and systemic embolism in atrial fibrillation.118 These estimates of monitoring costs in standard care, which were later applied in the NICE costing template for dabigatran,119 were derived by the ERG based on previous estimates used in the NICE costing report for clinical guideline CG36 on atrial fibrillation.24 This report summarised the estimated annual resource use required for monitoring people in primary care, assuming 20 monitoring visits per year. These measures of resource use, per visit, are summarised in Table 19.
TABLE 19
Resource use and updated variable cost estimates per standard primary and secondary care INR monitoring visit
An alternative source of standard monitoring costs per visit was identified from the largest UK-based RCT of self-monitoring.64 Jowett and colleagues carried out the economic analysis alongside the Self-Management of Anticoagulation, a Randomised Trial (SMART), where people in the control arm received a mix of standard primary and secondary care monitoring.87 A unit cost per visit (accounting for staff time, equipment, consumables and overheads) was estimated for each care setting from a sample of NHS providers. The resultant cost estimates (per visit) for different types of standard care are presented in Table 20, inflated to 2011–12 prices.
TABLE 20
Alternative unit costs of standard care INR monitoring in different settings, reported by Jowett and colleagues
Updated unit costs have been applied to provide a total cost per patient monitoring visit in 2011–12 GBP. When calculating the variable cost per patient associated with monitoring in a secondary care setting, the ERG in their report on dabigatran etexelate assumed that 33% of secondary care monitoring costs would be fixed and not influenced by changes in the number of people being monitored. This assumption was based on the observed proportional split between fixed and variable costs in the bottom-up calculation of the total cost of INR monitoring in primary care.24 This same assumption was applied in our updated estimates.
When updating the unit costs for practice nurse time in primary care, we used an estimate per hour that incorporates allocated overhead costs (including management and administration) and use of practice space. Some of these allocated costs were not included in previous variable cost estimates for monitoring in primary care. It was considered appropriate to include them here to capture the opportunity cost associated with use of primary care facilities for INR monitoring.120 However, as the allocated costs account for administration, additional administration time per patient visit was not costed separately as it was in previous estimates.24,93,118
Given the slightly different approach to updating the unit costs for standard monitoring services, our cost estimates based on 20 monitoring visits (£235.20 and £306.94 for primary and secondary care monitoring, respectively) differ somewhat from those used in the NICE costing template for dabigatran (£220.90 and £303.43, respectively, for monitoring in primary and secondary care in 2009–10 prices) and also from those applied in the model provided by Roche (£231.33 and £317.90, respectively, for primary and secondary care monitoring in 2012–13 prices).
For primary care monitoring, these unit costs are somewhat higher than those presented in Table 19. However, the cost estimate for monitoring in a secondary care (hospital clinic) is substantially lower. Furthermore, while the proportional mix of standard care service use was not reported in the study by Jowett and colleagues,87 a total mean standard care monitoring cost of only £89.89 (£120.18 in 2011–12 prices) was reported at 12 months. The actual annual monitoring frequency observed in the control arm of the SMART trial was 37.9 days.64
This suggests that an annual number of only ≈ 10 monitoring visits per year was required to achieve the level of control reported for the standard-care arm of this pragmatic UK-based RCT.
The assumption of 20 visits being the average number of monitoring visits required for people on long-term vitamin K antagonist therapy comes from the NICE costing report for the clinical guideline on the management of atrial fibrillation.24,119 This was estimated based on the ratio of second to first attendances at anticoagulation clinics (≈ 19 from reported activity in the 2004–5 NHS reference costs) and a previous study by Jones and colleagues,121 which reported a median frequency of INR testing of 16 days for people receiving warfarin (equating to ≈ 22 tests per year). A repeat of the calculation based on reference costs activity data for 2011–12 yielded a ratio of only 9.5. However, this lower value may merely reflect a trend for more people to be followed up in primary care following initiation of therapy.
Given the uncertainty surrounding the average number of monitoring visits for people under standard primary and secondary care, the DAR specialist committee members were consulted on this parameter. Opinion on the frequency of monitoring suggested that 10–12 visits would be required on average in primary and secondary care, but that the number of visits would be highly variable across participants. It was also noted by one member that more monitoring visits may be required for people managed in secondary care, as it tends to be the people with poorer control who are managed in this setting. A further question was raised about the nursing time requirements for routine monitoring visits used in the previous cost estimates informing TA249 (15 minutes of band 5 nurse time per patient visit). One source suggested that 10 minutes would suffice for this.
Based on consideration of the all of the above evidence, it was assumed in the base-case analysis that, on average, 12 monitoring visits would be required per year for people under standard primary and secondary care monitoring. To retain consistency with previous analyses used to inform NICE guidance, we applied the unit costs per visit based on the figures in Table 19.
The impact of altering the number of standard care monitoring visits per year was also assessed through sensitivity analysis. We also conducted sensitivity analyses where the updated unit costs in Table 20 were applied to cost monitoring visits, and where we assumed only 10 minutes of nurse time per standard care monitoring visit.
Finally, given the reliance of some people on NHS transport for attending secondary care monitoring visits, a cost of transport was applied for a percentage of people modelled to receive this form of monitoring. The percentage of 8.55% was taken from a previous survey of patient pathways used to inform the manufacturer’s model for NICE TA25696 and the return transport cost was taken from the NHS reference costs (£30.96).94
Costs of self-monitoring
An average testing frequency of 35 tests per year (every 10.42 days) was assumed for self-monitoring in the base-case analysis. This number was chosen to be consistent with the trials from which the relative effect estimates for self-monitoring were obtained. In a recent meta-analysis of patient-level data,108 11 of the self-monitoring trials included in our review reported the mean increase in the number of tests performed with self-monitoring versus control. There was an average of 24 additional tests by 12 months for people with atrial fibrillation and 22 additional tests for people with an AHV. The average of these two values was added to the estimated 12 tests per year for standard care, to give an estimate of 35 tests per year for self-monitoring. The impact of altering the difference in testing frequency between standard care and self-monitoring, through the 95% CIs reported by Heneghan and colleagues (13–30 per year), was assessed through sensitivity analysis.108 Furthermore, we assessed scenarios where self-monitoring was not used to increase the frequency of monitoring as a means to improve INR control, but simply used to replace primary and secondary care testing. Under this scenario, we assumed no relative effects of self-monitoring on outcomes. The sections below provide further details on the cost of self-monitoring, with a summary of cost elements provided in Table 21.
TABLE 21
Summary of self-monitoring device, training and testing costs
Equipment
Self-monitoring device costs were obtained from the manufacturers. However, no up-to-date cost could be obtained for ProTime Microcoagulation System. The UK distributor of this device [International Technidyne Corporation (ITC), NJ, USA] was contacted for information, but stated that the device was not marketed for patient self-monitoring in the UK, and that the device was being superseded by the ProTime InRhythm™ System, ELITech (Berkhamsled, Herts, UK), which is being marketed in the UK for professional use only. For completeness, a self-monitoring strategy using the ProTime Microcoagulation System was included in the economic model, by applying a NHS list price from 2008.122 Finally, a new promotional price (of £195) was provided for INRatio2. The impact of using this price was assessed in a sensitivity analysis.
Device costs were treated in the same way that capital investments are normally dealt with in economic evaluation. It was assumed that the NHS would pay for these and loan them out to patients. As such, they were annuitised over their expected use life to provide an equivalent annual/quarterly cost of use. While these devices have a potentially long life span based on the advice of manufacturers, their costs were annuitised over a 5-year period in the base-case analysis to account for the potential for loss and accidental damage.
There was also a degree of uncertainty about the suitability of the devices for reuse following discontinuation of self-monitoring by participants. In the base-case analysis, the same assumption that was used in a previous UK-based economic modelling study21 was applied, i.e. three-quarters of devices are reused by another patient in situations where a patient discontinues self-monitoring (see Training, for details on assumptions about discontinuation).
Consumables
The cost of test strips were provided by the manufacturers, and it was assumed in the base-case analysis that the annual cost of test strips would be equal to the number of tests performed annually multiplied by the cost per strip (i.e. that there would be no wastage). It was further assumed that two more test strips would be used annually to cross-check each device against a quality assured clinic-based machine. This was modelled to take place during biannual assessments for self-monitoring participants (see Biannual routine assessments).
NHS staff time
The staff time input required to oversee self-monitoring relied on expert opinion. People who are self-monitoring can require varying degrees of input from clinical staff to check readings and respond to queries. In the base case, it was assumed that all self-testing people would call in each and every test result on a dedicated telephone line, and that a nurse would later check and enter each patient’s result, and then telephone the patient back with instructions to either maintain or alter their warfarin dose. This was assumed to incur 5 minutes of band 5 nurse time per patient (based on the opinion of the specialist advisory committee), which was valued using nationally available unit costs.95 It was assumed that self-managing people would not require any further support from nursing staff other than biannual routine assessments (see next section).
Biannual routine assessments
It was assumed that quality control of self-monitoring devices would take place at biannual clinic appointments, at the local anticoagulant clinic or practice from where self-monitoring was initiated. It was assumed that this would involve checking the patient’s instrument against an externally validated one, and that it would incur 15 minutes of direct face-to-face contact time with a practice nurse (£45 per hour) or hospital clinic nurse (£85 per hour).95 In line with the base-case assumption that 34% of people are monitored in secondary care under standard practice, it was assumed that 34% of self-monitoring people would return to this setting for routine assessments, while the remainder would return to primary care clinics.
Training
Based on existing literature,123 as well as consultation with members of expert advisory committee, it was assumed that self-testing people would require 2 hours of one-to-one training, while those progressing to self-management would receive 4 hours of one-to-one training prior to initiation. These assumptions are consistent with those applied in the model that was provided by Roche (J Craig, York Health Economics Consortium, 2013, personal communication provided by Roche through NICE) and the literature on training requirements from RCTs of self-monitoring. Training time was costed using hourly unit costs for direct patient contact time (£45 per hour for practice nurse time and £85 per hour for hospital clinic nurse time).
The RCT literature64 and the expert advisory committee were also consulted with respect to training success rates and ongoing adherence to self-monitoring. In light of this, we incorporated a training failure rate of 15% – the mid-point between 5%, suggested by members of the expert advisory committee, and 24%, a pragmatic UK-trial-based estimate64 – and assumed that these people would incur the cost of training but return to standard care without incurring the cost of a monitoring device.
In addition to including a training failure rate in the model, it was considered unrealistic to assume that 100% of participants would continue to self-monitor after initiation. Therefore, we incorporated a discontinuation rate of 10% by 12 months in the model, based on consideration of the views of the expert advisory committee (≈ 5%) and a rate of 14% reported in the largest UK-based trial.64 Beyond 12 months, it was assumed that self-monitoring people would continue to do so unless they experienced a fatal or disabling adverse event.
Warfarin costs
In line with previous evaluations, it was assumed that the quantity and cost of vitamin K antagonist drugs would not vary significantly between self-monitoring and standard monitoring. Therefore, these costs were excluded from the model.
Costs of adverse events
The cost of minor bleed was based on the NHS reference cost for VB07Z: Accident and emergency services, category 2 with category 2 treatment (weighted average). A major non-intracranial bleed was taken as the weighted average reference cost for the Healthcare Resource Group (HRG) codes related to non-elective admissions for gastro-intestinal bleeds (Table 22).
For the cost of a systemic embolism, a weighted average of the reference costs for non-elective admissions relating to the HRG for non-surgical peripheral vascular disease (QZ17A, QZ17B, QZ17C) was applied.
The initial cost of a minor stroke was taken as the weighted average of the 2011–12 non-elective reference costs for the HRG codes AA22A and AA22B (non-transient stroke or cerebrovascular accident, nervous system infections or encephalopathy, with and without complications and comorbidities). This equates to a cost of £3082.
For major stroke, the cost used in the rivaroxaban submission was also updated, whereby the initial treatment cost was taken as the weighted average of AA22A and AA22B (£3082), with the addition of 10.97 additional bed-days costed using the weighted average excess bed-day cost (£236.16 per day) for AA22A and AA22B. The excess bed-days were estimated by subtracting the length of stay accounted for in the reference costs for AA22A and AA22B – up to 24.43 days94 – from the average length of stay in hospital for people suffering a major stroke (34.4 days based on Saka and colleagues124). In addition, 14 days’ rehabilitation was added at a cost per day of £313.41 – based on the HRG VC04Z (rehabilitation for stroke) – to estimate the total cost of a major stroke to 3 months (£10,061). This estimate is lower than that used in the model for NICE TA256 (updated cost of £13,547), as excess bed-day costs were applied only to days above the costing trim-point for AA22A and AA22B, rather than days above the average length of stay for these codes. This is conservative in favour of standard care.
The costs associated with adverse events were adapted from those used in the model informing NICE TA256; rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation.96 These cost estimates were based largely on NHS reference costs, and were considered appropriate by the independent ERG in their critique of the manufacturer’s submission.97 These costs were updated for the current analysis using the National Schedules of NHS Reference Cost, 2011–12,94 where possible, or were otherwise inflated from previously reported 2009–10 prices using the Hospital and Community Health Services (HCHS) pay and prices index.95 These costs are presented in Table 22.
TABLE 22
Health and social care costs associated with adverse events
Further costs were applied on a quarterly basis in the years following ischaemic stroke. These costs were adapted from those applied in NICE clinical guideline CG92, which were initially based on costs reported by Wardlaw and colleagues125 of £11,292 per year for disabling stroke and £876 per year for non-disabling stroke (2001–2) prices. These costs were inflated to 2011–12 values using the HCHS pay and prices index.95
For the acute treatment costs associated with an intracranial bleed, a weighted average of the non-elective reference costs for HRG AA23Z (haemorrhagic cerebrovascular disorders) was applied. In addition, the same rehabilitation costs as applied following major ischaemic stroke were applied following ICH, and the following quarterly health and social care costs were taken as the weighted average of those following minor (0.369) and major (0.631) ischaemic stroke. The cost of minor bleed was based on the NHS reference cost for VB07Z: accident and emergency services, category 2 with category 2 treatment (weighted average). A major non-intracranial bleed was taken as the weighted average reference cost for the HRG codes related to non-elective admissions for gastrointestinal bleeds (see Table 22).
Health measurement and valuation
Time spent in different states of the model was adjusted using utility weights reflecting the desirability of those states on a scale where 0 is equal to death and 1 is equal to full health. With the model structure similar to that of the model used to inform NICE TA256 (rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation), a number of the utility values used in this previous model were applied (acute major and minor stroke, acute major haemorrhage and ICH). These values were considered appropriate by the independent ERG for NICE TA25697 and accepted by the appraisal committee. However, the utility values applied to the states ‘post minor’ and ‘post major stroke’ in TA256, were derived from a Norwegian study where values were elicited directly from participants and the general population.127 Alternative values were identified for these states based on the EQ-5D responses of stroke people in the UK. Dorman and colleagues128 used the EQ-5D to measure the health status of 867 people enrolled in the International Stroke Trial.129 The reported values of 0.31 for dependent health states and 0.71 for independent health states were considered more consistent with the NICE reference case than the directly elicited Norwegian values (0.482 and 0.719, respectively) used in TA256. Further, it was assumed that for people experiencing an ICH or a haemorrhagic stroke, the proportion of people returning to independent living would match that observed for ischaemic stroke, and that the same utilities for minor and major ischaemic stroke would apply to dependent and independent states following ICH. This approach was used as it was noted that the value used in the rivaroxaban submission92,96 was higher than the age-specific UK EQ-5D population norm for people ≥ 75 years of age. Finally, the baseline utility value for people with atrial fibrillation or mechanical heart valve who were stable was taken as the baseline EQ-5D value of patients enrolled in the SMART trial (0.738).87
This value was applied to 65- to 70-year-old people. The difference between the UK EQ-5D population norm for 65- to 70-year-olds and the utility estimate from the SMART trial (0.042) was used to estimate age-specific baseline utilities in the model. The resultant utility values applied to events and health states are provided in Table 23.
TABLE 23
Health state utility values applied to modelled events and states in the model
Utilities associated with acute events were applied for the 3-month period following the event. For post-event states with associated ongoing morbidity, the appropriate health state utilities were applied for all subsequent cycles spent in these states. Half-cycle corrections were applied, by assuming that people experienced events on average at the mid-point of the cycle. Thus, a patient starting off in the well state and experiencing a major stroke in a given cycle of the model would accrue 6 weeks at the utility value for well and 6 weeks at the utility value for major stroke.
Time horizon, and discounting of costs and benefits
Both costs and benefits (QALYs) were discounted at 3.5% per annum, in line with the NICE reference case.29 The model was initially analysed over a 10-year period, but the impact of adopting longer time horizons (including the patient’s lifetime) was explored in sensitivity analyses. It was anticipated that a 10-year time horizon would be sufficient to demonstrate the main health and cost impact of any identified differences in adverse event rates between the alternative monitoring strategies, while avoiding the uncertainty surrounding assumptions about event rates far into the future.
Analysis
The results of the model are presented in terms of a cost–utility analysis (i.e. costs for and number of QALYs generated by each monitoring strategy). Each strategy was compared incrementally with its next less costly, non-dominated comparator, to estimate its incremental cost per QALY gained. In addition, given the uncertainty surrounding the relative effectiveness of the alternative self-monitoring devices, self-monitoring using each device was also compared incrementally with the standard care monitoring strategy (mixed primary and secondary care monitoring).
Further analyses were undertaken to assess cost-effectiveness by age, indication for anticoagulation therapy (atrial fibrillation, AHV), the standard care comparator (primary care monitoring, secondary care monitoring), and the active intervention (self-monitoring, self-management). The impact of altering key parameter values and assumptions was also assessed through extensive sensitivity analysis.
Given the computational burden of running the model probabilistically for each scenario assessed, results of main analyses and sensitivity analyses are presented based on deterministic runs of the model using the point estimates for input parameters. To characterise the joint uncertainty surrounding point estimates of incremental costs and effects, probabilistic sensitivity analysis was also undertaken.133 Each parameter was assigned an appropriate distribution as indicated in the preceding parameter tables. The model was then run iteratively 1000 times, with a value drawn randomly for each input parameter from its assigned distribution for each run. The estimated mean cost and effects for each strategy, based on these 1000 iterations, are presented for comparison with the deterministic results. The results of the probabilistic analysis are also presented in the form of incremental cost-effectiveness scatterplots and cost-effectiveness acceptability curves – for self-monitoring using each device compared with standard practice. As no direct evidence for the relative clinical effectiveness of the alternative monitoring devices could be identified, the strategies have not been compared simultaneously in the probabilistic analysis. Parameters excluded from the probabilistic analysis were self-monitoring training costs; in-hospital fatal stroke costs; post-stroke costs; the proportion of the cohort with atrial fibrillation; the proportion male; the proportional split between primary and secondary standard care monitoring; discontinuation rates; and unit costs of devices, consumables and staff time.
Results
Base-case analysis
This section presents the results of the base-case analysis. The following assumptions were applied:
- 66.45% of standard care monitoring occurs in primary care with practice nurses.96
- 60% of the cohort have atrial fibrillation, 40% have an AHV.108
- Average age of the cohort is 65 years, and 55% are male.108
- 50% of self-monitoring people self-test, 50% self-manage (assumption).
- The increase in the number of tests performed per year with self-monitoring is 23.108
- Relative treatment effects are estimated and applied separately for self-testing and self-management (see Table 10).
- 15% of participants do not commence self-monitoring following training (see Training).
- 10% of participants discontinue self-monitoring within a year of commencing (see Training).
- Self-monitoring device costs are annuitised over 5 years (see Equipment).
- 75% of devices are reused by another patient when a patient discontinues self-monitoring (see Equipment).
Figure 17 indicates the modelled proportion of the cohort (under standard monitoring care) experiencing a stroke, thromboembolic event, major bleeding event, and death by time in years. Figure 18 presents the same outcomes under the self-monitoring strategy. Applying the base-case assumptions, the results indicate that over a 10-year period, the introduction of self-monitoring would reduce the proportion of people suffering a thromboembolic event by 2.5%, while slightly increasing the proportion suffering a major haemorrhagic event by 1.4% (Table 24).

FIGURE 17
Modelled cumulative probability of a first thromboembolic and major haemorrhagic event, and death from all causes (standard-care cohort).

FIGURE 18
Modelled cumulative probability of a first thromboembolic and major haemorrhagic event, and death from all causes (self-monitoring cohort).
TABLE 24
Mean costs and outcomes over a 10-year time horizon
While the predicted monitoring costs are higher with self-monitoring (see Table 24), the total health and social care costs are similar and in some cases lower, and the QALY gains are greater. Thus, under the base-case scenario, the self-monitoring strategies compare favourably with standard care, except for with ProTime, where the incremental cost per QALY gained is £47,640 (Table 25, Figure 19). Furthermore, due to the lower cost of the INRatio2 device and testing strips, coupled with the assumption of equivalent clinical effectiveness of the alternative self-monitoring devices, INRatio2 dominates CoaguChek XS. However, it should be noted that no direct evidence of clinical effectiveness was identified exclusively for INRatio2 from the systematic review.
TABLE 25
Mean and incremental costs and effects over a 10-year time horizon

FIGURE 19
Cost-effectiveness frontier (base case).
Incremental analysis of alternative scenarios
Table 26 shows the results of further scenario analyses. For exclusive self-testing and self-management versus mixed primary/secondary care standard monitoring, and for mixed self-monitoring versus exclusive primary and secondary care clinic testing. Exclusive self-management with INRatio2 and CoaguChek XS was cost saving under the base-case assumptions, whereas self-testing was not cost-effective. The results also showed the mixed self-monitoring strategy (50% self-testing, 50% self-management) to be cost saving with CoaguChek XS and INRatio2 in comparison with exclusive secondary care testing. When applying the pooled RR for adverse events (derived from all self-monitoring studies) to both self-testing and self-managing participants, the cost savings and QALY gains associated with self-monitoring increased (see Table 26, scenario 5). This is because under this scenario self-testing becomes independently more effective. The same pattern of results was identified when self-monitoring was compared with exclusive secondary care anticoagulation clinic testing (see Table 26, scenario 6) using the point estimates of RRs derived only from trials making this comparison (see Figures 6 and 14). Finally, scenario 7 (see Table 26) shows the results when restricting the comparison to CoaguChek XS versus standard monitoring, using the pooled point estimates of RR derived only from trials of CoaguChek versus standard practice.
TABLE 26
Cost-effectiveness by type of self-monitoring and standard-care comparator (primary/secondary care)
Table 27 presents the results of alternative non-base-case scenarios, assessing the impact of using self-monitoring not to increase the number of tests performed annually, but to replace standard monitoring tests (average 12 per year). For these analyses it was assumed that no difference in clinical effectiveness exists between self-management, self-testing and standard care. Under most of these scenarios, standard monitoring was found to be less costly than self-monitoring. However, self-testing and self-management with INRatio2 and CoaguChek XS remained cost saving in comparison with exclusive secondary care anticoagulation clinic monitoring.
TABLE 27
Cost-minimisation scenarios assuming of no difference in the number of monitoring tests or clinical effectiveness between patient self-monitoring and standard monitoring
Differential results for subgroups
Table 28 presents the results for self-monitoring versus standard care by indication (atrial fibrillation and AHVs) and cohort age. Compared with standard monitoring, self-monitoring in a 65-year-old cohort with atrial fibrillation was estimated to cost £2574 and £4160 per QALY gained with INRatio2 and CoaguChek XS, respectively. Self-monitoring with ProTime was estimated to cost £58,584 per QALY gained. For a 65-year-old AHV cohort, self-monitoring with INRatio2 and CoaguChek XS was found to be more effective and less costly (dominant) than standard monitoring.
TABLE 28
Cost-effectiveness results by patient subgroups
A further analysis was carried out for the atrial fibrillation cohort using the baseline risks observed for participants with better INR control in standard care, assuming a constant RR reduction for thromboembolic events associated with self-monitoring. As the INR TTR increased in the control group, and the baseline risk of thromboembolic events consequently dropped, the cost-effectiveness of self-monitoring also decreased. However, the ICERs for CoaguChek XS and INRatio2 rose above £20,000 per QALY only when the baseline TTR was set at > 72.6%.
While cost-effectiveness was found to decrease slightly in a younger mixed cohort (due to the lower baseline risk of thromboembolic events), the ICERs for CoaguChek XS and INRatio2 remained below £20,000 per QALY gained. Self-monitoring was found to be most cost-effective in a 75-year-old cohort.
Further analysis of uncertainty (sensitivity analysis)
Deterministic sensitivity analysis was undertaken to test the robustness of the model, based findings to various parameter and structural assumptions (Table 29). The findings were found to be most sensitive to the baseline risk of thromboembolic events and the effectiveness of self-monitoring for preventing these events (see Table 29, scenarios 14–16). Applying a baseline risk of 1.15% coupled with the upper 95% confidence limit of the RR estimate for self-management (0.69), the ICERs for the mixed self-monitoring strategies rose above £30,000 per QALY gained (see Table 29, scenario 17). The same was found when the lower baseline risk (1.15%) was coupled with the upper confidence limit for the RR (for thromboembolic events) associated with self-monitoring as a whole (0.84 applied for self-testing and self-management). One hundred per cent self-management remained cost saving under the former combined scenario but not the latter.
TABLE 29
Sensitivity analysis scenarios
The cost-effectiveness of self-monitoring improved further when the modelled time horizon was extended to 20 and 30 years, with both CoaguChek XS and INRatio2 dominating standard primary/secondary care-based monitoring. The incremental cost per QALY gained for self-monitoring with CoaguChek XS and INRatio2 also remained below £20,000 when higher training failure and discontinuation rates were applied, and when higher self-monitoring testing frequencies were applied (with no change in effects). The cost-effectiveness findings were also robust to the number of tests performed annually in standard primary/secondary care-based clinic monitoring.
A final sensitivity analysis was conducted to approximate the cost-effectiveness of self-monitoring for a cohort of children with an AHV on long-term vitamin K antagonist therapy. For this analysis, the cohort age was set to 10 years, the baseline risk of thromboembolic events was reduced to 1.4%, and the annual risk of all-cause mortality following a stroke was set at 14.5.134 Under this scenario, the ICERs for self-monitoring with CoaguChek XS and INRatio2 remained favourable. However, it should be noted that no good data were identified to appropriately adjust the risk of death from all causes in children with an AHV, and therefore the standardised mortality ratio estimated for an 18- to 55-year-old cohort of AHV participants was applied.
Probabilistic sensitivity analysis of the base case
Table 30 presents the mean costs and effects, and mean incremental cost-effectiveness results, for the four strategies based on 1000 probabilistic simulations. Compared with the deterministic analysis presented in Table 25, the results are very similar.
TABLE 30
Mean and incremental costs and effects over a 10-year time horizon
Figure 20 shows the scatterplot of the estimated mean incremental costs and effects of self-monitoring with CoaguChek XS compared with standard monitoring, derived from 1000 probabilistic iterations of the model. Approximately 50% of the points lie below zero on the cost axis and above zero on the effect axis, indicating a 50% chance of the self-monitoring strategy (50% self-testing, 50% self-managing) dominating standard care monitoring. The acceptability curve (Figure 21) indicates an 80% chance of self-monitoring with CoaguChek XS being cost-effective compared with standard monitoring at a willingness-to-pay threshold of £20,000 per QALY gained.
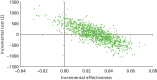
FIGURE 20
Incremental cost-effectiveness scatterplot: self-monitoring with CoaguChek XS vs. standard monitoring.

FIGURE 21
Cost-effectiveness acceptability curves: self-monitoring with CoaguChek XS vs. standard care. WTP, wllingness to pay.
Figures 22 and 23 show the corresponding incremental cost and effect scatterplot, and acceptability curve for self-monitoring with INRatio2 versus standard care. This analysis assumes equivalent effects for INRatio2 compared with CoaguChek XS. Self-monitoring with INRatio2 was estimated to have an 81% chance of being cost-effective at a threshold of £20,000 per QALY gained under these assumptions. However, it should be noted that no direct RCT evidence was identified for the effect of INRatio2 on long-term adverse outcomes, with the majority of RCT evidence relating to versions of CoaguChek.
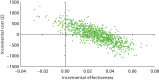
FIGURE 22
Incremental cost-effectiveness scatterplot: self-monitoring with INRatio2 vs. standard monitoring.

FIGURE 23
Cost-effectiveness acceptability curves: self-monitoring with INRatio2 vs. standard care.
Figures 24 and 25 summarise the results of the probabilistic analysis for self-monitoring with ProTime versus standard monitoring. Owing to the higher cost of the device, this strategy was found to have a lower chance of being cost-effective in than standard practice.
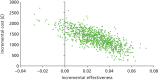
FIGURE 24
Incremental cost-effectiveness scatterplot: self-monitoring with ProTime vs. standard monitoring.

FIGURE 25
Cost-effectiveness acceptability curves: self-monitoring with ProTime vs. standard care.
Finally, Figures 26 and 27 summarise the uncertainty surrounding the cost-effectiveness of self-monitoring with CoaguChek XS versus secondary care anticoagulation clinic testing (applying RR distributions based on the pooled estimates from trials making this comparison) and mixed (primary/secondary care) standard monitoring (using RRs derived from trials using only CoaguChek).

FIGURE 26
Cost-effectiveness acceptability curves: self-monitoring with CoaguChek XS vs. standard monitoring (based on pooled RR estimates from CoaguChek studies only).
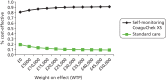
FIGURE 27
Cost-effectiveness acceptability curves: self-monitoring with CoaguChek XS vs. secondary care anticoagulation clinic monitoring (applying RRs for self-monitoring vs. specialised anticoagulation clinic testing).
Summary
Self-monitoring, and in particular self-management, of anticoagulation status appears cost-effective when pooled estimates of clinical effectiveness are applied. However, if self-monitoring does not result in significant reductions in thromboembolic events, it is unlikely to be cost-effective from the NHS and personal social services perspective at the frequency of testing observed in RCTs.
We are most confident in the applicability of the base-case cost-effectiveness findings to self-monitoring strategies using CoaguChek XS. The majority of clinical effectiveness evidence relates to a previous version of CoaguChek (CoaguChek S), to which the current version (CoaguChek XS) has been shown to have very similar or slightly superior performance in terms of accuracy and precision (see Independent economic assessment). While INRatio and ProTime have been shown to have acceptable performance in relation to laboratory testing, very few studies have directly compared CoaguChek XS with the INRatio2 PT/INR monitor and/or ProTime Microcoagulation system. Further studies are needed to assess relative diagnostic and clinical performance.
The main findings and uncertainties are discussed further in Chapter 4.
- Assessment of cost-effectiveness - The clinical effectiveness and cost-effective...Assessment of cost-effectiveness - The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation
- Assessment of clinical effectiveness - The clinical effectiveness and cost-effec...Assessment of clinical effectiveness - The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation
- Drosophila arizonae strain AZTUS-31 hypothetical protein (GI19546) gene, complet...Drosophila arizonae strain AZTUS-31 hypothetical protein (GI19546) gene, complete cdsgi|744515618|gb|KP236482.1|Nucleotide
- Bisphosphonate alternative regimens for the prevention of osteoporotic fragility...Bisphosphonate alternative regimens for the prevention of osteoporotic fragility fractures: BLAST-OFF, a mixed-methods study
Your browsing activity is empty.
Activity recording is turned off.
See more...