Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Pickard R, Starr K, MacLennan G, et al. Use of drug therapy in the management of symptomatic ureteric stones in hospitalised adults: a multicentre, placebo-controlled, randomised controlled trial and cost-effectiveness analysis of a calcium channel blocker (nifedipine) and an alpha-blocker (tamsulosin) (the SUSPEND trial). Southampton (UK): NIHR Journals Library; 2015 Aug. (Health Technology Assessment, No. 19.63.)

Use of drug therapy in the management of symptomatic ureteric stones in hospitalised adults: a multicentre, placebo-controlled, randomised controlled trial and cost-effectiveness analysis of a calcium channel blocker (nifedipine) and an alpha-blocker (tamsulosin) (the SUSPEND trial).
Show detailsPrimary outcome
A primary outcome was attributed to 1136 of the 1150 included participants (99%), with 14 participants (1%) completely lost to follow-up. The occurrence of the primary outcome at any time up to 4 weeks after randomisation (number of participants not requiring further intervention for the symptomatic ureteric stone) was 307 out of 378 (81.2%) in the tamsulosin group and 304 out of 379 (80.2%) in the nifedipine group, compared with 303 out of 379 (79.9%) for those randomised to placebo. These primary results are described in more detail in Table 15 using both raw and adjusted analyses. The full logistic regression model is detailed in Appendix 7.
TABLE 15
Primary outcome: stone passage at 4 weeks (defined as number of participants not requiring further intervention)
We also recorded the number of participants having an intervention planned between 4 weeks and 12 weeks on the 12-week CRF. A further 27 (7.1%) participants in the tamsulosin group, 25 (6.4%) in the nifedipine group and 28 (7.4%) in the placebo group were recorded as having had an intervention between these time points.
Secondary outcomes
Estimated time to stone passage
The outcome for time to passage of stone as measured by clinical report, and confirmed by imaging, was available for 237 (21%) participants and showed no difference between groups (Table 16).
TABLE 16
Time to stone passage
Pain
Participants scored the severity of pain during the day of completion of the baseline and 4-week questionnaire using the NRS.53,54 The duration of pain and use of analgesic medication was recorded as the number of days pain medication was used on the 4-week participant questionnaire. The results are shown in Table 17.
TABLE 17
Pain outcomes
Health status
Generic health profile as measured by the SF-36 and EQ-5D at baseline and 4 weeks and 12 weeks after randomisation is shown in Table 18 and Figure 6.
TABLE 18
Quality-of-life scores
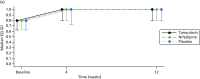
FIGURE 6
Quality-of-life scores measured at baseline, 4 weeks and 12 weeks. Graphs summarise mean and SD for SF-36 physical component summary (PCS) and mental component summary (MCS), median and 25th to 75th centiles for the EQ-5D.
On both the EQ-5D and the SF-36 physical component summary, participants had impaired HRQoL at baseline which returned to population average (SF-36) or full health (EQ-5D) levels by 12 weeks. There was no evidence of a difference between any groups when comparing MET to placebo or tamsulosin to nifedipine at either of the time points. Owing to the high proportion of missing data, the robustness of these results was tested using multiple imputation and pattern mixture models. Younger participants and those with higher baseline SF-36 physical and mental component summary scores were less likely to respond at 4 weeks and 12 weeks. Multiple imputation models gave practically identical treatment effect estimates but with slightly tighter CIs for all treatment effect estimates in Table 18. The results were also robust when varying the pattern of missing data for all but implausible scenarios; for example, missing data in MET group were no different from observed data, but in the placebo group the missing data were over one-third of a SD lower (i.e. 3 points on the SF-36 physical component summary) than observed data. This was the case for all treatment effects summarised in Table 18.
Duration of hospitalisation
The number of participants with further hospital admissions was low and similar in all the groups (see Table 21). The average stay of additional hospital admissions up to 12 weeks after randomisation was 0.17 days (SD 0.64 days) in the tamsulosin group, 0.23 days (SD 1.06 days) in the nifedipine group and 0.25 days (SD 1.13 days) in the placebo group.
Significant clinical events
Participant discontinuation of trial medication was reported from responses to a single question on the 4-week participant questionnaire. Discontinuation rates solely as a result of side effects were 10% (25/247) for tamsulosin, 17% (40/241) for nifedipine and 6% (15/231) for placebo (Table 19). Serious adverse reactions (defined as SAEs that were thought to be possibly or definitely related to trial medication) were recorded by sites using a SAE form. The event rates are shown in Table 19 with further details of the reactions reported in Table 20.
TABLE 19
Discontinuation and serious adverse reaction rates
TABLE 20
Details of reported serious adverse reactions
Subgroup analysis
We explored the potential moderating effect of several factors previously reported in the literature by analysing the following subgroups:
- sex
- stone size (≤ 5 mm, > 5 to 10 mm)
- stone location (upper, mid, lower ureter).
Use of analgesia was considered as a subgroup analysis. However, as 98% of participants had used analgesia prior to randomisation it was therefore felt that any subgroup analysis would be uninformative.
Results of the subgroup analysis are summarised graphically using forest plots in Figures 7 and 8 for MET versus placebo and tamsulosin versus nifedipine, respectively. The forest plots present ORs and 99% CIs. There was no evidence of any subgroup by treatment interaction. The p-values for the interaction terms were 0.59 for sex, 0.23 for stone size, and 0.12 for upper and 0.04 for mid ureter (with lower ureter as the reference location) for stone location comparing MET versus placebo. For tamsulosin versus nifedipine, these p-values were 0.39 for sex, 0.13 for stone size, 0.54 for upper ureter and 0.70 for mid ureter. The full breakdown of primary outcome by subgroup is summarised in Appendix 8.

FIGURE 7
Subgroup analysis: stone size and stone location on stone passage (MET vs. placebo).
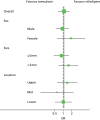
FIGURE 8
Subgroup analysis: stone size and stone location on stone passage (tamsulosin vs. nifedipine).
- Outcomes and results - Use of drug therapy in the management of symptomatic uret...Outcomes and results - Use of drug therapy in the management of symptomatic ureteric stones in hospitalised adults: a multicentre, placebo-controlled, randomised controlled trial and cost-effectiveness analysis of a calcium channel blocker (nifedipine) and an alpha-blocker (tamsulosin) (the SUSPEND trial)
- Clinical effectiveness: pyridoxine/doxylamine combination - Treatments for hyper...Clinical effectiveness: pyridoxine/doxylamine combination - Treatments for hyperemesis gravidarum and nausea and vomiting in pregnancy: a systematic review and economic assessment
- Glossary - Evaluation and validation of social and psychological markers in rand...Glossary - Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme
- Discussion - Management of Asthma in School age Children On Therapy (MASCOT): a ...Discussion - Management of Asthma in School age Children On Therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety
- Clinical effectiveness: vitamin B6 (pyridoxine) - Treatments for hyperemesis gra...Clinical effectiveness: vitamin B6 (pyridoxine) - Treatments for hyperemesis gravidarum and nausea and vomiting in pregnancy: a systematic review and economic assessment
Your browsing activity is empty.
Activity recording is turned off.
See more...