Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Everard ML, Hind D, Ugonna K, et al. Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review. Southampton (UK): NIHR Journals Library; 2015 Aug. (Health Technology Assessment, No. 19.66.)

Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review.
Show detailsRecruitment and participant flow
Participants who were randomly assigned, received intended treatment and were analysed for the primary outcome
Between 26 October 2011 and 23 December 2013, the trial recruited and randomised 317 participants, with 158 patients allocated to the nebulised 3% HS group and 159 allocated to usual care (Figures 1 and 2). There were five patients from the nebulised 3% HS group who did not receive the intended treatment. Of the 317 patients randomised, 26 of these patients were excluded because they were randomised when ineligible and for one patient primary outcome data were unavailable because their medical notes were lost; therefore, 290 patients were included in the primary outcome analysis.

FIGURE 1
Participant recruitment curve.

FIGURE 2
The CONSORT flow diagram. ITT, intention to treat.
Losses and exclusions after randomisation
The number of post-randomisation exclusions together with the reason for exclusion is displayed in Table 4. Overall, 26 patients were excluded from the study: 16 from the intervention group and 10 from the control group.
TABLE 4
Post-randomisation exclusions
Of the 317 randomised patients, 26 were excluded, as described previously. Of the remaining 291 patients, five were not included in the PP analysis for the following reasons: four withdrew before receiving any HS/intervention and one patient’s medical notes were lost (therefore no treatment data were available). Of the remaining patients in the PP analysis, 32 withdrew from treatment having received at least one dose of HS and three withdrew from the study, two from the standard care group and one from the standard care plus intervention group.
Dates defining the periods of recruitment and follow-up
The trial consisted of three recruitment seasons. The study recruited patients during winter 2011/12, winter 2012/13 and from October 2013 to December 2013. Patients were followed up for a period of 28 days after randomisation to collect data on readmissions, duration of respiratory symptoms post discharge, health-care utilisation post discharge and infant and parental quality of life.
Why the trial ended or was stopped
The trial closed to recruitment after reaching the accrual target on 23 December 2013.
Baseline data
Table 5 shows the characteristics of the recruited and non-recruited patients, the former being further subdivided according to whether or not the patient was subsequently excluded. There were no notable differences between these groups in terms of age or sex.
TABLE 5
Characteristics of recruited and non-recruited
Baseline characteristics are shown in Table 6. There were no notable differences between the study groups but the control group was slightly older, heavier and contained more males.
TABLE 6
Demographics (FAS)
Seven out of 10 centres collected RSV test data routinely, if not always completely. The viral status by season is shown in Table 7.
TABLE 7
Viral status by season
Numbers analysed
In the intention-to-treat (ITT) population, in which patients were analysed by their original assigned groups, there were 142 participants in the HS arm and 149 in the control arm. In one patient in the intervention group, date and time of fitness for discharge were not recorded and are not included in any of these analyses, but the patient was known to have been discharged on day 6 (i.e. between 120 and 144 hours); this LoS is similar enough to the LoS of the other trial participants that the missing data is unlikely to have had any material impact. In the PP analysis, five participants did not receive treatment as scheduled and so were removed, leaving 136 participants in the HS arm with complete data; 149 remained in the control arm.
Outcomes and estimation
There was no evidence of any difference between the two treatment arms in the primary outcome, the time to being declared fit for discharge [hazard ratio (HR) 0.95, 95% CI 0.75 to 1.20; p = 0.66], or in the time to actual discharge (HR 0.97, 95% CI 0.76 to 1.23) (Table 8 and Figures 3 and 4). In absolute terms, the median difference between HS and control (adjusted for site) was 2.5 hours (95% CI –13.8 to 18.7 hours) for time to discharge and 0.5 hours (95% CI –18.0 to 19.1 hours) for actual discharge. For both groups, the time to being declared fit for discharge was nearly 76 hours from admission and the time to actual discharge was nearly 89 hours.
TABLE 8
Time to being declared fit for discharge and time to discharge (hours)
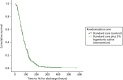
FIGURE 3
Cumulative survival plot for time to being declared fit for discharge.
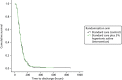
FIGURE 4
Cumulative survival plot for actual time to discharge.
Figures 5 and 6 show the time to discharge by treatment and RSV status [RSV positive (RSV+) vs. RSV negative (RSV–)]. RSV+ was associated with prolonged time to fitness to discharge and (less strongly) with time to actual discharge, but there was no indication that HS had a differential effect in relation to RSV status.
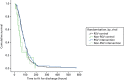
FIGURE 5
Cumulative survival plot for time to being declared fit for discharge by study arm and viral status.

FIGURE 6
Cumulative survival plot for time to being declared fit for discharge.
Admission to HDU/ICU and readmission rates are displayed in Table 9. There was a lack of evidence to suggest that there was a difference between treatment groups in terms of the numbers admitted to HDU/ICU or readmitted within 28 days of randomisation. In addition, as there was little evidence that the effect differed between treatment group by RSV status for readmission rates, there was some evidence of an interaction for admission to ICU/HDU, although the numbers were small and the study was not powered to detect this (RSV+ 3.7%, 95% CI –5.2% to 12.6% vs. non-RSV –26.7%, 95% CI –56.2% to 2.8%; p = 0.07).
TABLE 9
Admission to HDU/ICU, readmission rates and symptoms to 28 days
The ITQoL results are displayed in Table 10. There were no important differences between treatment group scores on any of the ITQoL dimensions and a lack of evidence that outcomes differed between groups according to RSV status (Figure 7).
TABLE 10
The ITQoL results
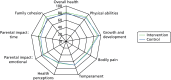
FIGURE 7
The ITQoL dimensions by study group: FAS.
All important harms or unintended effects in each group
Adverse events are reported in Tables 11–13. Six events were probably or possibly related to saline treatment, including one SAE. The SAE was bradycardia and desaturation during administration of the nebuliser, which resolved the following day. The remaining five non-SAEs, each of which occurred in different subjects, were bradycardia (self-correcting), desaturation, coughing fit, increased respiratory rate (all of which were resolved within 1 day) and a chest infection (which resolved after 6 days).
TABLE 11
Adverse events
TABLE 13
Adverse event details: standard treatment group
TABLE 12
Adverse event details: saline group
Impact of post-randomisation exclusions
Of the 26 babies retrospectively excluded from analysis after randomisation, outcome data were available for 24. The time to fit for discharge and to actual discharge are summarised in Table 14. These times were similar to those of the 291 participants included in the trial, and the comparison of HS with control virtually unchanged if these were included.
TABLE 14
Time (hours) to fit for discharge and actual discharge in the analysis population and those excluded post-randomisation
- Trial results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hyper...Trial results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review
- Comparison of the REFLUX trial with other randomised trials of laparoscopic surg...Comparison of the REFLUX trial with other randomised trials of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease - Clinical and economic evaluation of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease: 5-year follow-up of multicentre randomised trial (the REFLUX trial)
- List of abbreviations - SeHCAT (tauroselcholic [75selenium] acid) for the invest...List of abbreviations - SeHCAT (tauroselcholic [75selenium] acid) for the investigation of bile acid diarrhoea in adults: a systematic review and cost-effectiveness analysis
- Process evaluation - A multidomain decision support tool to prevent falls in old...Process evaluation - A multidomain decision support tool to prevent falls in older people: the FinCH cluster RCT
- Introduction - Online remote behavioural intervention for tics in 9- to 17-year-...Introduction - Online remote behavioural intervention for tics in 9- to 17-year-olds: the ORBIT RCT with embedded process and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...