Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Everard ML, Hind D, Ugonna K, et al. Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review. Southampton (UK): NIHR Journals Library; 2015 Aug. (Health Technology Assessment, No. 19.66.)

Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review.
Show detailsComplete hospital data were available for all patients, except for the duration of ward stay for one patient in the intervention group. Consequently, this patient was excluded from the analysis, but this is not thought to have any material effect on the results. Only small, non-statistically significant differences are seen in all measures of hospital stay (Table 15). A difference is seen in the number of doses of saline, with the intervention group receiving on average 10.4 doses of nebulised saline over their hospital stay, while no nebulised saline was used in the control group.
TABLE 15
Resource use by group in hospital
Concomitant medication data were analysed, but it was decided not to incorporate these into the economic evaluation. The two main reasons for this were that the medications were all low-cost treatments, for example salbutamol, and assumptions would be required to convert usage into costs, for example whether the patient was prescribed the full pack of tablets or it was taken from the ward supply, and what size of pack was used. It was decided, therefore, that given that the costs of the medications used were negligible in comparison to overall hospital costs, and additional assumptions were needed in their calculation, their inclusion would provide only spurious accuracy.
Resource use in primary care following discharge was self-completed by guardians. The overall response rate was low, with complete follow-up in 53 (37%) and 54 (36%) participants in the control and intervention groups, respectively. Of the remaining patients, the guardians of 57 (40%) and 72 (48%), respectively, were contacted by telephone and provided basic health resource usage; we include this information in Table 16 but have not included it in the economic analysis. One (control group) remained an inpatient at 28 days and their resource usage is included only in Table 15. The remaining 55 guardians were unable to be contacted after multiple attempts.
TABLE 16
Resource use by group in primary care
Use of primary care services was low and was similar in both patient groups (see Table 15). There were no reported attendances at NHS walk-in centres or minor injury units. In cases for which the brief resource information had been obtained by telephone, the resource use was lower than that obtained via questionnaire response, but again was similar between the randomised groups.
When individual cost components are combined with their unit costs, the mean hospital costs were £2595 in the control and £2727 in the intervention group (Table 17). The 95% CI around the difference of £132 is –£520 to £785. QALYs were calculated using the total LoS in hospital, then transformed into quality-adjusted life-days (QALDs) over the 36 days following randomisation in order to aid the presentation in the table of very small numbers. The difference in mean QALDs was 0.006 greater in the intervention group (or 0.0000175 QALYs) and was not statistically significant.
TABLE 17
Total costs and QALDs
Mean primary care costs relating to the services described previously were £19 and £11 in the control and intervention groups, respectively (see Table 16). The difference in means is £8, with a 95% CI of –£21 to £6 (p = 0.25). Given the large number of missing data, it was decided not to impute missing values or combine them with the hospital costs.
With numerically higher costs (£132) and QALYs (0.0000175), an incremental cost-effectiveness ratio (ICER) can be produced, which is approximately £7.6M per QALY gained. The uncertainty around this is represented by plotting the incremental costs and QALYs on the cost-effectiveness plane from the non-parametric bootstrap of the trial data in Figure 8. This shows a cloud of observations centred, approximately, around the origin (representing zero cost difference and zero QALY difference).
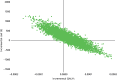
FIGURE 8
Cost-effectiveness plane of nebulised saline.
The estimates used to generate the cost-effectiveness plane are then transformed into a cost-effectiveness acceptability curve (Figure 9). This shows that there is a 34% probability of nebulised saline being cost-effective at a funding threshold of £20,000 per QALY. This probability is constant over the funding range that is typically considered by the NHS (£20,000–30,000).96 One-way sensitivity analyses examining different unit costs for time spent on a ward, ICU or HDU did not alter the probability that nebulised saline would be cost-effective.

FIGURE 9
Cost-effectiveness acceptability curve for the use of nebulised saline.
- Health economic results - Saline in Acute Bronchiolitis RCT and Economic evaluat...Health economic results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review
- Methods - Preoperative intravenous iron for anaemia in elective major open abdom...Methods - Preoperative intravenous iron for anaemia in elective major open abdominal surgery: the PREVENTT RCT
- List of abbreviations - A systematic review and economic evaluation of new-gener...List of abbreviations - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
- Methods - The clinical effectiveness and cost-effectiveness of brief interventio...Methods - The clinical effectiveness and cost-effectiveness of brief intervention for excessive alcohol consumption among people attending sexual health clinics: a randomised controlled trial (SHEAR)
- Results - Magnetic resonance enterography compared with ultrasonography in newly...Results - Magnetic resonance enterography compared with ultrasonography in newly diagnosed and relapsing Crohn’s disease patients: the METRIC diagnostic accuracy study
Your browsing activity is empty.
Activity recording is turned off.
See more...