Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Stein RC, Dunn JA, Bartlett JMS, et al.; on behalf of the OPTIMA Trial Management Group. OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer. Southampton (UK): NIHR Journals Library; 2016 Feb. (Health Technology Assessment, No. 20.10.)

OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer.
Show detailsRecruitment
The first centre opened to recruitment into the OPTIMA prelim study on 5 September 2012. There were 35 centres open to recruitment and, of these, 34 centres recruited participants. Between 16 October 2012 and 3 June 2014, when the database was locked, 350 participants were registered, of whom 313 were subsequently randomised into the study (Figure 4). The final 150 patients were recruited within 6 months at a rate of 28 patients consented (registered) or 25 patients randomised per month. The average recruitment rate was 0.8 patients consented per open site per month in the final 6 months of recruitment (0.7 over the whole recruitment period). The average recruitment rate for randomising patients was 0.7 patients randomised per open site per month in the final 6 months of recruitment (0.6 over the whole recruitment period) (Figure 5). Recruitment rates for recruiting centres ranged from 0.1 to 2.2 patients consenting (registered) per month open.
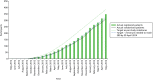
FIGURE 4
Recruitment graph.
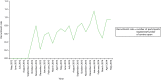
FIGURE 5
Recruitment rate per open centres.
Screening and acceptability
A total of 968 patients were reported on the screening logs, of whom 795 were deemed eligible according to local information; however, 45 of these were excluded by clinicians (Figure 6). Of the 750 patients approached by their clinicians about participating in OPTIMA prelim, 350 consented to join the study. This equates to a 47% patient acceptance rate, which was above the target 40%. The acceptance rate ranged from 0% to 100% across the 35 open centres, with 19 (54%) centres having an acceptance rate above the target 40%.

FIGURE 6
Screening of potential participants.
Patients did not have to give a reason for declining to take part but when a reason was given it was recorded on the screening log. Recorded reasons are summarised in Table 19. Of those who declined to enter the study, 51% did so because they had a strong treatment preference (33% wanted chemotherapy and 18% did not want to have chemotherapy). Some patients who declined to enter the study (8%) chose to pay privately for the Oncotype DX test.
TABLE 19
Patient-reported reasons for declining to participate
Registered patients who were not randomised
Of the 350 patients who consented to participate in the OPTIMA prelim, 313 had been randomised prior to 3 June 2014 and 15 were undergoing central eligibility confirmation on that date (Figure 7).
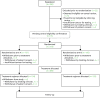
FIGURE 7
The CONSORT diagram.
A total of 22 (6%) registered patients were not randomised (see Figure 7) for the following reasons:
- referring site realised that patient did not meet eligibility criteria (n = 2)
- HRT was being taken at the time of breast cancer surgery (n = 1)
- repeat local receptor testing showed tumour to be ER negative (n = 1)
- insufficient invasive tissue to perform Oncotype DX test and therefore ineligible (n = 1)
- withdrawal of patient by their clinician (n = 2)
- underlying condition that made patient unsuitable for the trial (n = 1)
- axillary surgery had been delayed and needed to begin chemotherapy immediately (n = 1)
- patient withdrew consent (n = 5)
- sample submitted for central review did not contain sufficient invasive tumour for testing and patient did not want to wait for another sample to be sent and tested (n = 3)
- decided to pay for an Oncotype DX test privately (n = 1)
- decided did not want to receive chemotherapy (n = 1)
- deemed ineligible on central review of receptor status (n = 12).
The discrepancy between local and central determination of receptor status was low at 3.7% (95% CI 1.7% to 5.8%), with only 12 of the 325 registered patients for whom central review of receptors was performed deemed ineligible (Table 20).
TABLE 20
Details of registered participants deemed ineligible on central review of receptor status
Randomised participants
The characteristics of the 313 randomised patients are shown in Table 21. Patients were allocated to the partially blinded trial arms (157 on arm A and 156 on arm B) and their characteristics were well balanced across trial arms.
TABLE 21
Participant demographics and tumour characteristics
Withdrawal post randomisation
Four participants withdrew consent for the trial following randomisation but before receiving their treatment allocation (see Figure 7). Of these, two patients withdrew consent because of the delay in treatment allocation. One patient was withdrawn by her clinician because she had received preoperative letrozole and was therefore ineligible. One patient was withdrawn because, prior to treatment allocation, it was found that there was insufficient invasive tumour to obtain an Oncotype DX test and no additional sample was available.
A further three participants withdrew following treatment allocation. One patient was withdrawn by her clinician as a subsequent bone scan revealed metastatic disease and two patients withdrew consent as they chose not to follow the results of the randomisation; one patient decided to pay for Oncotype DX off-study and one did not accept her trial-allocated treatment (chemotherapy).
These seven patients are included in the baseline data, but no further information is available.
Sample handling logistics
The median time between patients signing the OPTIMA prelim consent form to treatment being allocated was 20 days (IQR 16–23 days, range 10–41 days). Treatment allocation took longer than 4 weeks from date of consent for 25 patients (8%). The main reasons for these delays were staffing problems and issues within the central laboratory (10 patients) and obtaining a suitable sample from centres for testing (11 patients). Furthermore, central eligibility testing took substantially longer than the target timeline for two patients and Oncotype DX testing took substantially longer for one patient.
The median time between patients signing the OPTIMA prelim consent form and starting chemotherapy for those allocated to chemotherapy was 4.6 weeks (IQR 4.1–5.3 weeks, range 2.6–11.3 weeks). The proportion of chemotherapy-assigned patients starting treatment within 6 weeks was 91% over the whole recruitment period, but 92% within the last 6 months of recruitment. The delays with allocating chemotherapy because of problems with the tissue samples led to the withdrawal of four registered patients and two randomised patients prior to treatment allocation.
Recruitment conclusions
OPTIMA prelim had three pre-defined success criteria for establishing feasibility, namely that 300 patients would be recruited in no more than 2 years from the first centre opening, and, for the final 150 patients, (1) patient acceptance rate should be at least 40%; (2) recruitment should take no longer than 6 months; and (3) chemotherapy should start within 6 weeks of signing the OPTIMA prelim consent form for no less than 85% of chemotherapy-assigned patients. All of these criteria have been met demonstrating that a large-scale randomised trial to demonstrate the effectiveness of multiparameter test-directed chemotherapy allocation in a high-risk population of patients with ER-positive HER2-negative chemotherapy is feasible in the UK.
Involving patients in developing the study
Three patient focus groups met to discuss the OPTIMA trial; two of these were held shortly before and after the study opened (in August 2012 and February 2013) and the third discussion, which involved a mixed patient group, took place in a non-participating centre in April 2013. Members of ICPV facilitated all three groups. Although the conversations took different directions, it is possible to extrapolate certain themes that recurred throughout and within the groups. These illustrated the ‘big picture’ and explained some of the possible difficulties and opportunities that these groups felt would be involved in making a decision to take part in the OPTIMA study. These are described below.
Personalised chemotherapy
Perceptions of chemotherapy and the move towards more test-guided treatment, such as that evaluated by the OPTIMA trial, were discussed at length in all the groups. Although a small number of participants saw the benefits in receiving no or less chemotherapy, they felt most patients would want to treat breast cancer as thoroughly as possible, which meant having chemotherapy. Focus group participants acknowledged that test-guided treatment was becoming more commonplace now (e.g. those mentioned by group members included HER2 and ER status testing and tests required for treatment of haematological malignancy). However, they felt that there was not always a clear understanding if and when chemotherapy was needed, and that some patients ignored potential side effects in their enthusiasm for treatment.
Overall, it was felt that there was a need for contemporary information, both generally in the media and more specifically, such as in the OPTIMA prelim participant information leaflet, so that the expectation of chemotherapy, and the view that more chemotherapy is always better and that less is somehow deficient, will be challenged by both patients and within society. One group felt that this was a view perpetuated by health professionals and should be confronted through education. They felt that endocrine therapy, such as tamoxifen, was not, as one participant described, ‘painted in the same light’ by health professionals and was therefore seen to be less important and less effective. Participants stated that evidence-based information must be made widely available to make people aware that every person is different; that there is a multiplicity of breast cancer diagnoses and thus every treatment is different and ‘personalised’. They felt this would manage expectations more appropriately and prevent inappropriate comparisons with other women. They also felt that it needed to be explained that best practice changes over time.
Focus group participants felt that there should be more information made available in the news media and magazines about personalised, test-guided treatments to make the public aware and change perceptions generally about cancer always requiring chemotherapy.
It was agreed that the best way to introduce the study to potential OPTIMA participants would be to explain that breast cancer treatment is always tailored to the individual woman and it should be stressed that the OPTIMA trial involves personalised treatment, rather than less treatment, in any written information about the study.
There was some concern from focus group participants that people would think that less treatment, as prescribed in the OPTIMA trial, was simply a cost-saving measure by the NHS. They felt it should be made explicit to potential study participants that this was not the case.
Deciding to take part in research and the OPTIMA study
There was a consensus that the OPTIMA study seemed to be an appropriate and helpful study from a PPI perspective and focus group participants said they would take part in principle (one woman had actually been offered and consented to OPTIMA prelim). The reasons why they would take part were both general to research and specific to the OPTIMA study. For example, being invited to take part in research made one feel valued and important, and it was seen to make a difference to treatment and potentially help others. It also made individuals feel ‘looked after’ and the additional follow-up and ‘active monitoring’ that was involved, even within the control group, was highly valued. Participants felt that this was specifically the case in the OPTIMA study, in which the regular and active monitoring involved was seen as positive and information given about individual risk was seen as constructive.
The hypothetical question of making an informed choice to take part in the OPTIMA study was appreciated to be very difficult; focus group participants in all three groups wished this to be noted. Describing their own experience of being invited to take part in RCTs, some participants had felt under enormous pressure to make a quick decision. A minority felt that deciding to take part would have caused too much personal anxiety and so they would have preferred to leave the decision to, as described by one participant, ‘those that were qualified’. Another minority felt that they would be deterred from taking part because of the wait to get results and consequent delay in starting treatment. There was a common misconception among focus group participants that chemotherapy had to be instigated very quickly to have the most beneficial effect but they were reassured that, in breast cancer, adjuvant chemotherapy could be delayed for up to 12 weeks.180 Participants felt that this should be made very clear to potential OPTIMA study participants. Knowing this, they also felt that the complexity of the OPTIMA trial and the possibility of potentially receiving less treatment would mean that the information and consent process would be likely to take longer and research staff should be made aware and of this and trained, if appropriate.
The information provided for the OPTIMA study
Focus group participants from all three groups stressed the importance of an expert multidisciplinary team to manage the OPTIMA study involvement and treatment in which potential study participants could have ready access. In order for the OPTIMA study participants to have confidence in their treatment allocation and in their decision to consent to the study, they agreed they would need confidence in the information they were given and the person giving it.
All focus groups also agreed that an audio-recording of the initial consultation would be positive for potential OPTIMA study participants to take away with them as discussions and information given in consultations often, as one participant described, ‘went in one ear and out of the other’. It was also felt that it was important for the OPTIMA study information to be made available so that it could be discussed with family members and friends away from the hospital environment. Multiple formats, which would allow patient choice and be appropriate for different people, were preferable and formats described included digital versatile discs, leaflets, websites and podcasts. Independent information was also valued and it was felt that this could be hosted by a neutral organisation such as ICPV or a cancer charity. The provision of full information was felt to be important and would encourage rather than discourage recruitment.
There was also some concern about the partial blinding in the OPTIMA study and the inherent lack of information. For example, would trial participants allocated to no chemotherapy worry that they actually had a borderline score? Focus group participants were reassured that borderline scores of the genomic test had been lowered specifically for the purposes of the trial to ensure safety. Focus group participants felt that if potential OPTIMA participants were told this, they might be more likely to consent knowing that there was less risk of a false-negative score. They also felt strongly that potential study participants should be made aware that chemotherapy could be offered at a later date if necessary, as many had been under the impression that the test would predict chemotherapy use indefinitely, which is not the case.
The additional burdens of chemotherapy
Interestingly, focus group participants felt that the important additional burdens of treatment were often ignored by health professionals and were under-reported and therefore not recognised. These were often related to employment and financial issues. As such, the health economics questionnaire that is included in the OPTIMA study was seen to be particularly useful and important. The additional burdens that were discussed in the groups are reproduced in Box 1. The quality-of-life questionnaires that are included as part of the trial were also perceived as highly relevant and valuable, although some individuals felt that certain important and impactful information would not be gathered by the measures proposed, for example the impact of tamoxifen and AIs on sexuality and sexual function.
BOX 1
Additional costs incurred with breast cancer treatment (as identified by focus group participants) Wigs, scarves and hats.
Patient and public involvement conclusion
Data from the three focus groups and the dissemination of these results have generated invaluable in-depth information about the potential impact of taking part in the OPTIMA study from people who have a direct experience of living with cancer. It has shown us that the study is supported and, in theory, people would be keen to be involved and take part. However, it has highlighted the difficulties involved in accepting randomisation to potentially less treatment than expected. The data that have been generated by the focus group participants have been incorporated into the study design and, although they cannot be truly quantified, have probably contributed to the success of the study.
Qualitative recruitment study results
The integrated QRS undertaken within the OPTIMA prelim study was based on a refinement of the methods developed for the ProtecT study complex recruitment intervention.140 This section reports on the details of the data collected and analysed, and presents the findings from each of the three phases of the QRS. Detailed results from the analysis of phase 1 (including the recruitment difficulties identified through interviews with the OPTIMA staff members and audio-recordings of recruitment consultations) are reported in full in Appendix 8.
A summary of those findings is presented in this section, followed by the full findings from phase 2 (describing the delivery of interventions to address the barriers identified from phase 1) and phase 3 (considering the impact of the QRS interventions on recruitment during OPTIMA prelim).
Qualitative recruitment study data set
In total, 18 of the 32 staff contacted for interview responded: 14 agreed to participate (no withdrawals), two declined outright and two declined the interview at the time, with the offer of being contacted at a later more convenient date. The 14 semistructured interviews were conducted with participants across six centres and included eight oncologists (three of whom were also members of the TMG), four research nurses, one surgeon and a non-recruiting member of the TMG. Six interviews were conducted face to face and eight were conducted via telephone. Interviews were conducted with staff from ‘mid-range’ centres by chance rather than by design. One of the centres included in the QRS had failed to recruit any patients; the remaining centres’ recruitment rates ranged from 0.32 to 1.85 patients per month compared with an overall range of 0 to 2.20 in the study. At least one site from each of the OPTIMA prelim’s geographical clusters participated in interviews.
Of the eight research nurses invited to take part in structured telephone interviews about patient pathways, all agreed to participate and none withdrew.
A total of 36 audio-recordings were obtained from 29 patients’ consultations. First and second oncology consultations were recorded for 7 of the 29 patients. First oncology consultations were available for 21 patients and the second oncology consultation was available for one patient. Twelve different recruiters (10 oncologists and two registrars) led the consultations and audio-recordings were obtained from all geographical clusters.
Phase 1: understanding recruitment issues
Detailed findings are reported in Appendix 8. In summary, the recruitment issues identified through the QRS fell under three overarching themes:
- eligibility processes and patient pathways
- missed opportunities for discussing the trial
- opportunities for improving communication and information provision.
The sources of data outlined earlier (see Qualitative recruitment study data set) were considered in tandem to identify key recruitment challenges in the OPTIMA prelim. Although there was some overlap in identified recruitment challenges, which emerged through different methods of data collection, there was also a tendency for certain methods to be better suited for uncovering certain challenges to recruitment. For instance, challenges relating to communication were largely uncovered through audio-recorded consultations, while issues relating to patient pathways were largely based on information derived from interviews. Some themes, such as recruiters’ levels of equipoise, were supported through both interview data and audio-recorded consultations.
Eligibility processes and patient pathways
‘Eligibility processes’ relate to clinicians’ and triallists’ decision-making about patients’ eligibility for the OPTIMA prelim study, while ‘patient pathways’ refers to the series of OPTIMA prelim-related events patients experienced in the lead up to entering (or declining) the trial. These processes had potential to hinder recruitment and so provided an initial focus for phase 1. Obtaining an overview of these processes from OPTIMA prelim staff also provided a useful framework for placing specific barriers to recruitment into context. A summary of these processes is presented in the paragraphs below, with more detailed descriptions shown in Appendix 8.
Although trial eligibility processes appeared straightforward, systems were not always in place to map eligibility from the first point at which patients were considered for the study [i.e. within multidisciplinary team (MDT) meetings]. At the commencement of the trial, screening logs recorded patients from only the point of registration and randomisation, thus making it difficult to elucidate the full patient pathway. This missing information pertaining to the screening process probably led to potentially eligible patients not being offered the opportunity to consider the study.
Patient pathways were clear, simple and appeared successfully integrated into research nurses’ services. However, patients sometimes gave decisions about participation via telephone conversations with research nurses rather than in face-to-face consultations. Consequently, some patients may not have discussed their thoughts or concerns about the OPTIMA prelim study with an oncologist. This had implications for clinicians’ opportunities to resolve patient misunderstanding and/or concerns (if appropriate) and limited scope for the QRS to capture discussions for or against trial participation (i.e. through audio-recordings).
Missed opportunities for discussing the OPTIMA prelim with patients
Interviews and early TMG meetings showed recurring concern among staff that fewer than anticipated patients were being approached about OPTIMA prelim. Regular telephone conversations with research nurses frequently suggested that no eligible patients had been identified. Six interview informants from five different centres talked about the limited flow of eligible patients they had encountered at the time of interview. This was the most frequently reported issue raised by informants when asked about their views on the main barriers to recruitment:
I don’t know why we’re not actually identifying (any); it’s not that we’re deciding that we’re . . . no, it’s not that we’re approaching patients and they’re saying ‘No’.
Research nurse (RN) 1
With the starting pool of potential eligible patients unknown, little could be deduced with regards to the numbers of patients deemed ineligible or the associated underlying reasons for ineligibility. This led to TMG members producing broad, non-specific explanations of why the number of patients approached for OPTIMA prelim was lower than expected when the study was conceived:
[Dr A] will probably tell you but at the meeting yesterday [they] said how surprised [they were] about how small the pool of eligible patients is. It’s much smaller than (they) thought it would be [. . .]; that’s like a change of tune, really, from before we started recruiting (they) were very positive about how big the pool was [. . .]. So I think (their) experiences in trying to identify eligible patients have shown that perhaps the expectation of how many eligible patients there would be, were a bit, perhaps slightly over-enthusiastic.
TMG member
Further evidence supporting the theory that the full pool of eligible patients was not being offered participation in the study came in the form of recruitment figures and acceptance rates. Actual recruitment figures trailed behind target figures throughout the recruitment period, while ‘acceptance’ rates consistently exceeded targets. Interviews with recruiters gave insight into potential attitudinal factors that may have played a role in restricting the pool of eligible patients. In summary, these factors related to the following.
Discomfort surrounding eligibility criteria
Recruiters’ unease surrounding various aspects of the eligibility criteria was widespread, reported by staff members from every centre participating in interviews. OPTIMA prelim staff varied in their readiness to accept increasing risk (in terms of disease status). Increasing lymph node involvement, tumour size and grade caused discomfort surrounding the upper thresholds of the eligibility criteria stated in the protocol.
Concern about patients’ ability to handle information
Some clinical informants gave general or specific examples of refraining from offering the trial to patients on the basis of their clinical judgement. Failure to approach patients at a clinician’s discretion would not breach the study protocol; the eligibility criteria clearly stated:
Patient must be fit to receive chemotherapy and other trial-specified treatments with no concomitant medical, psychiatric or social problems that might interfere with informed consent, treatment compliance or follow up.
However, an issue that arises is whether or not the decision not to inform patients about the opportunity to take part in the OPTIMA prelim study was always appropriate or well founded. Such judgements, if applied differently across different centres, could have had implications for the numbers of patients being approached for the OPTIMA prelim.
Priority of the OPTIMA study at multidisciplinary team meetings and among surgeons
Although research nurses were well versed in the inclusion and exclusion criteria, some felt that they did not always have the necessary expertise or voice to flag a patient as an OPTIMA prelim candidate at a MDT meeting. This suggested that potentially eligible patients were at risk of falling through the net at the stage of initial eligibility screening.
Summary of missed opportunities to discuss the OPTIMA prelim study
To summarise, findings from QRS interviews and informal discussion/observation in meetings suggested that there had been missed opportunities to approach eligible patients about the OPTIMA prelim study. This was a key finding from the QRS and suggested that there would be clear opportunities to increase levels of recruitment. These findings were supported through the observation that, although numbers of eligible participants were falling short of targets, the study seemed very acceptable to patients who were informed about it, as the percentage of eligible patients agreeing to randomisation consistently exceeded targets (see Screening and acceptability).
Opportunities for improving communication and information provision
Communication and information provision were largely explored through audio-recorded consultations, although data from staff interviews sometimes provided a useful backdrop for exploring particular challenges. Recruitment consultations varied in length and structure across and between centres, with some formats more conducive to explaining the study than others. Difficulties emerged in relation to how trial-specific processes were explained to patients, and staff attitudes also played a role in influencing the direction consultations took – particularly when it came to exploring patients’ views and perspectives on OPTIMA prelim participation.
Explaining randomised controlled trial processes and concepts of uncertainty
A fundamental challenge to recruitment identified by interview informants was the perceived difficulty in explaining the OPTIMA prelim trial design. Having an arm that split into two further arms, combining random and test-directed treatment allocations, and the partially blinded design were all thought of as potentially confusing to patients. Some interview informants had directly experienced patients dismissing the trial on this basis:
This particular lady just didn’t go into any detail at all she said, I just didn’t know what it was talking about, and I said, and I tried to explain bits of it to her, but no, she said, I just, I don’t, I just want to, I don’t understand it, she just said locked her mind off completely to it.
RN4
In the light of the above, analysis of audio-recorded consultations focused on recruiters’ explanations of trial processes and scrutinised information exchange that led up to evidence of patient misconceptions or confusion. Particular practices were identified as being potentially detrimental to patient understanding. These included absent or incomplete explanations of ‘randomisation’, ‘blinding’ and unclear descriptions of the treatment(s) provided in each trial arm. Explanations of the trial arms also revealed examples of loaded terminology, including use of the words ‘standard’ and ‘experimental’ (see Appendix 8).
The issue of uncertainty was an important theme to arise from audio-recorded consultations, particularly in relation to the discussions about chemotherapy benefits and the accuracy of the test. There were differences in how recruiters framed chemotherapy provision at the start of the consultation, which in turn had implications for patient understanding. Recruiters’ explanations ranged from presenting chemotherapy as a treatment with definite benefits, to framing it as an uncertainty that required careful balancing of benefits and side effects/complications. There were occasional examples in which patients still believed that they would definitely benefit from and/or receive chemotherapy even after the trial had been explained. Uncertainty surrounding use of the Oncotype DX test in treatment decision-making was another difficult concept to communicate according to recruiters’ interview accounts (i.e. oncologists and research nurses). Overselling the benefits of the test could prompt patients to pay for the test privately, whereas an overly cautious approach ran the risk of obscuring any incentives for participating in OPTIMA prelim. These considerations raised the dilemma of how best to reassure patients while conveying the inherent risk of participating in a study in which treatment allocation may be determined by a test that requires further research:
The trouble is if in our initial interview we sort of really down play the test as not being useful, then nobody would want to go in the trial in the first place. And if we play it too much they think ‘well it’s such a great test, I don’t want to risk not having it in the trial’ and go and find out the information. So again, it’s that fine balance.
Oncologist (Onc) 4
The TMG meetings revealed that a particular point of concern was a line in the PIS that highlighted the uncertainty surrounding the test’s accuracy. Based on TMG members’ experiences (not audio-recorded), patients had reportedly refused the trial once they had read the following statement:
The test might not work so well for patients with larger tumours or involved lymph glands. We do not believe this to be the case. However if it was, then in the future we might realise that we should have given you chemotherapy.
It was noted that this issue reportedly materialised after (rather than within) the first oncology consultation (i.e. once the patients had read the information sheets). This prompted us to consider how the Oncotype DX test was described in consultations, with a focus on how uncertainty was communicated and responded to (see Appendix 8 for details). Overall, recruiters varied in how they conveyed uncertainty, which in turn had implications for patients’ understandings of the trial aims and design.
Tailoring consultations to the OPTIMA prelim study
Recruiters varied in the extent to which they presented OPTIMA prelim as the primary focus of the discussion. This was influenced by the extent to which they covered diagnostic information and non-OPTIMA prelim-related treatments within the first oncology consultation. Some consultations were particularly long (up to 52 minutes), covering non-trial-related information in some detail prior to introducing the OPTIMA prelim study. In some ways, it could be considered that many appointments were not ‘recruitment consultations’ at all. Although there was the opportunity to present the OPTIMA prelim study, this opportunity was not always fully utilised in some consultations. In other cases, the study was presented as a ‘next step’ in a series of ‘information blocks’, rather than the heart of the consultation. Some recruiters successfully tailored information provision around explanations of the trial, whereas other struggled to do this, resulting in the aims and key details of the trial becoming somewhat lost.
Patient preferences
Screening logs and interview informants’ accounts suggested that patients’ preference either for or against chemotherapy was the most common reason for declining the trial (50% of reasons for decline in final screening logs: 33% for and 17% against):
We have a very good functioning wider unit [. . .]. There’s nothing that’s blocking us from doing it other than patient preference.
Onc3
There were few (if any) examples in consultations where patients expressed strong preferences, but there were some examples where patients appeared to be influenced by other clinicians that they had seen prior to the oncologist (e.g. surgeons). There were also examples of patients showing misunderstandings about trial participation while explaining their decision to refuse the trial; however, the recruiters did not always address these misconceptions, instead accepting the patients’ decisions without further discussion. A common thread that ran throughout recruiters’ interviews was that breast cancer patients in particular were thought likely to have set preferences given the influence of the media, peers and the well-publicised nature of the disease. As such, some recruiters completely disassociated themselves from patients’ decisions about trial participation and many indicated that they would accept patients’ preferences with no further exploration. Recruiters’ discomfort in exploring patients’ decisions and/or preferences stemmed from concern that raising these issues would jeopardise their relationships with patients or leave them susceptible to accusations of coercion.
Delay
Patients’ concern about the delay attributed to waiting for the Oncotype DX test result was a dominant theme to emerge from audio-recorded consultations. Almost every consultation analysed supported this, with most patients asking about how long the testing process would take. Although some recruiters reassured patients that the delays associated with OPTIMA prelim had no implications for treatment safety, this was not consistently done across consultations. This, therefore, also represented a missed opportunity to address patients’ concerns and provide reassurance.
Summary of opportunities for improving communication and information provision
Analysis of audio-recorded consultations revealed opportunities to clarify and refine recruiters’ explanations of trial-specific processes to patients, reconsider use of terminology and frame explanations of treatment options around the OPTIMA prelim trial design. Both interview data and audio-recorded consultations revealed recruiters’ discomfort and reluctance to explore patients’ decisions to decline the trial. These attitudes stemmed from concerns of being seen to coerce and the belief that patients’ views are fixed at the outset of consultations. We found no evidence of clear ‘fixed’ patient preferences in the consultations we listened to, but there were examples of patient expectations for chemotherapy/no chemotherapy that had stemmed from previous interactions with clinicians (e.g. surgeons) prior to the first oncology consultation.
Summary of findings from QRS phase 1 and opportunities for intervention
Phase 1 of the QRS identified a number of challenges to recruitment from the analyses of interviews and recruitment consultations, including opportunities for improvement to discuss with the CfI and TMG with a view to developing a plan of action to be implemented in phase 2. The potential opportunities included:
- Considering introducing screening logs at the stage of eligibility assessment. Screening logs could then record the recruitment process in greater detail, from the first point of identifying a potentially eligible patient, through to the patient’s decision to accept or decline the trial. This would reveal if as many potentially eligible patients as possible were being approached and informed about OPTIMA prelim, and help to identify centre-specific barriers to recruitment.
- Discussing ‘on the ground’ perceptions of equipoise – including discomforts around particular eligibility criteria – to reach a consensus about the need to change the criteria or to discuss these issues more widely.
- Considering whether or not the basis of decisions not to approach patients could be standardised or measured and whether or not they were appropriate, with a view to increasing opportunities for potentially eligible patients to decide for themselves whether or not to participate.
- Considering how to raise the profile of OPTIMA prelim at MDT meetings and among surgeons.
- Providing recruiters with support to explain the study design, justification and details more clearly; promoting early mention of OPTIMA prelim in consultations and encouraging recruiters to frame their explanations of treatment options around the OPTIMA prelim study design.
- Exploring with recruiters how best to conceptualise issues of uncertainty and discuss those with patients in the context of the OPTIMA prelim trial.
- Raising issues about the use of terminology that might not help recruitment, including the use of the words ‘standard’ and ‘experimental’, which suggest the trial arms are not equivalent.
- Exploring with recruiters how best to describe advantages and disadvantages of chemotherapy and the Oncotype DX test so that patients could understand the key issues.
- Exploring with recruiters how best to present issues of delays with Oncotype DX.
- Exploring with recruiters opportunities to discuss the origin of patient preferences for or against chemotherapy and, in appropriate cases, to provide additional information that might alleviate inappropriate concerns – all with the aim of ensuring more informed decision-making about participation in the study.
Phase 2: developing interventions to address recruitment challenges
Identification of potential barriers to recruitment was an iterative process that spanned the entire recruitment period. As themes emerged from data collection, interventions were designed in collaboration with the TMG and delivered to centres as recruitment proceeded. This section will outline the timing and nature of these interventions, and discuss their function relative to the recruitment challenges identified in the previous section. A summary of the interventions delivered is shown below in Table 22.
TABLE 22
Timeline of QRS interventions
Addressing issues of discomfort surrounding eligibility: Trial Management Group discussion, circulation of interview findings and clinician-to-clinician centre visits
The QRS interviews provided strong evidence of discomfort surrounding the OPTIMA prelim eligibility criteria, suggesting that the full pool of eligible patients was not being approached. The knowledge acquired from interviews was fed back to the CfI and ‘core TMG’ over a prolonged period, initially through comments on ‘preliminary’ findings from interviews and finally through more formalised written reports of interview findings produced for the CfI (18 October 2013). Findings were also shared generically in wider TMG meetings attended by oncologists from centres around the UK. TMG members’ responses to these findings suggested familiarity with the issues raised and opened up discussions about how clinicians’ discomfort surrounding higher-risk status patients could be addressed. One early suggestion generated by the core TMG was to reassure individual oncologists about high-risk cases by publicising their peers’ readiness to include these patients in the study. This suggestion was put into practice on the premise that awareness of what oncologists around the country were doing might alleviate individual oncologists’ concerns – particularly those in ‘isolated’ centres. Following this, the QRS team revisited recruiters’ discomfort surrounding eligibility criteria at a wider TMG meeting, suggesting that open discussion with non-recruiting centres might be beneficial (17 June 2013). In particular, the team felt it was important that these took the form of peer-to-peer discussions among clinical experts who fully understood the realities of embedded clinical practice. Clinical members of the core TMG made a series of centre visits following this, targeting non-recruiting centres. The function of these meetings was to openly address recruitment difficulties in a supportive and confidential environment. Centre visits continued throughout the duration of the recruitment period.
Amendments to the patient information sheet
Members of the TMG expressed concern that the PIS section outlining the ‘disadvantages’ of OPTIMA prelim participation prompted patients to decline the trial. This prompted the QRS team to review the PIS. This opportunity was used to review the PIS in its entirety, with a view to considering how patients could interpret the information. Elements of the study arm descriptions were clarified, but most amendments were concentrated on the ‘advantages’ and ‘disadvantages’ sections of the document. These sections needed to provide an honest and clear description of the trial-specific advantages and disadvantages of participation, in as balanced a way as possible. Visually, the disadvantages section of the document far outweighed the advantages section, yet it became apparent that some of the details stated as ‘disadvantages’ were repetitions of trial processes described elsewhere. Through discussion with the TMG, it was agreed that some of this information was not a specific disadvantage of trial participation and it was removed.
The particular line in the PIS that had created most concern among recruiters informed patients that the Oncotype DX test might not work and that they might find out that they ‘should have received chemotherapy’. Audio-recordings of recruiters’ descriptions of uncertainty surrounding the test were used to inform how this disadvantage could be conveyed. It was evident that some recruiters successfully communicated the same idea stated in the PIS, but preceded this with information about the current use of the test (i.e. in other countries and in different subgroups of breast cancer patients). Audio-recordings also showed examples of recruiters reassuring patients that the test had not been ‘plucked out of the air’. It was on this basis that information surrounding the test’s current use, and intended extended use, were added to the PIS. Similarly, we found evidence of recruiters tentatively stating that they believed the test would work, balancing this with the assertion that concrete evidence was not yet available. Similar information was added to the ‘disadvantages’ section of the PIS. Patient representatives and the Research Ethics Committee approved these amendments made to the PIS and the updated version was circulated in October 2013.
Tips and guidance for recruiters
The challenges associated with structuring consultations and explaining trial-specific processes were addressed through circulation of a tips and guidance sheet formulated by the QRS team (see Appendix 9). This document provided recommendations on how recruiters might explain difficult concepts such as randomisation, blinding and the events that occur in each treatment arm. Other details pertinent to the study rationale were also included to help recruiters structure their consultations.
All information stated within the tips and guidance document was grounded in the data collected from audio-recordings and based on the potential opportunities listed above. Suggestions about how to explain concepts were based on clear, effective examples derived from the collection of recorded consultations. The CfI contributed some key clinical concepts and the document as a whole was reviewed by the CfI prior to finalising. A letter from the CfI accompanied the document, with the option for recruiters to contact the QRS team if they had any questions. Importantly, the letter framed the tips and guidance as suggestions that recruiters may wish to incorporate into their current practices. The document was circulated to all open OPTIMA prelim centres in October 2013.
Group feedback sessions
A series of group feedback sessions was delivered to a range of QRS and non-QRS participating centres around the UK. The purpose of these feedback sessions was to spark discussion to generate acceptable solutions to recruitment challenges, using findings from the interviews and audio-recordings as prompts. The QRS team guided this process by showing extracts from consultations deemed more or less effective, with recruiters encouraged to comment on and evaluate the extracts themselves. Group feedback sessions were particularly targeted to addressing broad topics relevant to incorporating the OPTIMA prelim study into clinical practice rather than specific technical challenges (e.g. explaining difficult concepts, which were better addressed by the tips and guidance sheet). Topics addressed included discomfort surrounding eligibility criteria, approaches to structuring consultations and how to respond to patient preferences. Presentations were adapted to each feedback session, using updated recordings or focusing in on topics that were problematic for the centre in question. TMG members, particularly the CfI, assisted in the formulation and delivery of presentations; for instance, the CfI contributed a number of vignettes designed to generate discussion about eligibility and took the lead for this part of the presentation at one of the meetings.
Exploring patient preferences was covered in all feedback presentations. The widespread discomfort surrounding this was still apparent in meetings, but the QRS team attempted to show the merits of exploring preferences (through actual consultation data) and offered suggested ‘lead-ins’ to how recruiters might respond to a patient preference.
An attempt was made to deliver group feedback to as many regional clusters as possible by organising meetings that could be attended by representatives from multiple centres. Secondary to this option was to target centres that requested feedback and centres that appeared to be struggling with recruitment.
Four group feedback sessions were conducted in total: two attended by multiple centres and two directed at single centres. Meetings attended by multiple centres were conducted in Scotland and the south-west of England. Both regional meetings had at least one representative from local centres present. Centre-specific feedback meetings were conducted in a centre that requested feedback and a centre that had been struggling with recruitment.
Individual feedback sessions
In addition to group feedback sessions, recruiters who provided at least three audio-recorded consultations were offered confidential individual feedback, which was conducted over the telephone or in person. A summary sheet of key points discussed was provided to recruiters following this session. Individual feedback sessions were useful for discussing specific challenges individual recruiters faced, which might not have been appropriate to discuss in a public context (e.g. concerns of coercion). Furthermore, these sessions were an ideal opportunity to discuss challenges that were specific to the individual, rather than ‘recurring’ challenges addressed in group meetings. All guidance provided by the QRS team was offered in the form of suggestions that were discussed with the recruiters, making it clear that the individual should proceed as they feel best. In all cases, it was emphasised that recruiters needed to remain comfortable and confident while speaking with patients about OPTIMA prelim. In total, individual feedback was provided to four oncologists. These were delivered in the later stages of recruitment, given the need to base feedback on a sufficient number of recordings and the relatively low numbers of recordings that were made.
Phase 3: impact of qualitative recruitment study interventions
There were a number of approaches taken to evaluate the impact of the QRS, although no single method could definitively show the influence interventions had on recruitment. As a consequence, the discussions that follow should be viewed as speculative, although viewing the problem from multiple angles could contribute to a clearer picture than relying on one method alone. In the light of this, three approaches were used to explore the potential impact of the QRS interventions: analysis of how recruitment figures changed relative to interventions; analysis of recruitment figures for centres receiving group feedback versus those that did not; and a qualitative analysis of changes in practice for individual recruiters.
Changes in recruitment figures with respect to qualitative recruitment study interventions
Figures 4 and 5 show overall recruitment with time and monthly recruitment rate adjusted for the number of open centres. Recruitment rate fluctuated substantially from month to month but there was an overall upwards trend. The month of early October to early November 2013 coincide with a series of general QRS interventions that could have impacted all centres or large clusters of centres. It is apparent that the recruitment figures immediately following these interventions changed the trajectory, as rates of increase sped up and the gap between ‘target’ and ‘actual’ figures began to close. This trajectory continues for the remainder of the recruitment period, while the difference between ‘target’ and ‘actual’ recruitment figures continued to narrow. One would not expect recruitment rates to increase at the exact point at which QRS interventions were disseminated. A lag is to be expected, to account for the time needed for knowledge/advice to diffuse and for eligible patients to emerge.
The observations described above are far from conclusive evidence that the QRS had a positive impact on recruitment. Causality cannot be established based on these data, owing to the countless confounding factors that could have influenced recruitment and/or uptake of the interventions. For instance, we cannot prove definitively that recruiters took note of the interventions, and could speculate that the slight increase in recruitment could be attributed to any number of reasons. It should be noted, however, that this slight boost in recruitment could be considered unusual given the time of the year (December/Christmas shut-down).
Comparison of recruitment figures for centres with and without group feedback
Comparing centres that did/did not receive non-generic interventions was problematic, given that feedback sessions occurred at various points in time within each centre’s ‘recruiting trajectory’. Centres had been open to recruitment for different lengths of time, resulting in different levels of experience and (possibly) confidence among clinical staff. Comparing ‘feedback’ versus ‘no feedback’ centres was also problematic as there were difficulties in grouping ‘feedback centres’ in a single category: some centres received both individual and group feedback, while others received one or the other; some had their full clinical team present at feedback meetings, while others had one or two individuals present; some centres were visited by the QRS team or TMG, whereas others sent representatives to regional meetings; and, finally, the professional roles and numbers of those attending feedback meetings varied by centre (sometimes oncologists were present, sometimes research nurses, and sometimes both). Comparing recruitment rates for centres with/without feedback was further complicated by extraneous factors that could influence recruitment rates (e.g. centres size, location), including more elusive factors that were harder to measure (e.g. ‘commitment’ to the trial). None of these factors could be controlled or accounted for. The distribution of ‘generic guidance’ to all centres also raised the question of whether or not any ‘no feedback’ centres truly existed, as generic guidance often overlapped with the advice and recommendations offered in feedback meetings.
Despite these difficulties, there were some examples where QRS interventions or ‘clinician-to-clinician’ discussions had clear impact. For example, two centres that had not yet recruited, despite being open for numerous months, consistently recruited immediately after ‘clinician-to-clinician’ visits took place. Looking at changes in recruitment before and after group feedback sessions, it was noted that recruitment rates (patients per month) were almost always higher after these meetings took place (9 out of 11 centres). Once again, though, it is difficult to distinguish the impact of the QRS from the effects of ‘natural increasing momentum’; crude comparisons of change in recruitment rates could have been made with ‘no feedback’ centres if there was a single point of QRS intervention, but as mentioned above, variable timings of feedback delivery meant this was not possible.
Qualitative analysis of audio-recordings: do recruiters’ practices change?
One relatively straightforward approach to assessing the impact of QRS interventions was to look at whether or not suggestions in the tips and guidance sheet and feedback presentations were adopted by recruiters. Three recommendations were consistently covered in ‘generic’ QRS interventions (i.e. widespread advice to all centres), and were, therefore, ideal points to evaluate across centres before and after intervention. These were:
- advice to draw out the arms of the trial and explain each route separately (e.g. ‘option 1′ and ‘option 2′)
- recommendations to specify that randomisation means there is no control over allocation
- advice to reassure patients that treatment delays associated with OPTIMA prelim were safe.
These points were also deemed the most important advice generated by the QRS team, having considered factors that most frequently appeared to lead to patient confusion or concern. Table 23 sets out whether or not each of these suggestions were detected in consultations occurring before (no shading) and after (green shading) the date of intervention. Examples occurring before intervention indicate that the recruiter was already doing this. The date of intervention was defined as the first point at which these recommendations were distributed (October 2013 – the ‘tips and guidance’ document). All feedback meetings also covered these points, all of which took place after October 2013. The data summarised in the table are based on targeted analysis of consultations (arranged by date) to focus specifically on the topics of interest. The word ‘Yes’ showed that the recruiter spontaneously demonstrated the behaviour/recommendation, while ‘Yesa/Yesb’ indicate that the behaviour was demonstrated in response to patient confusion (a) or questioning (b).
TABLE 23
Recruiters’ demonstration of QRS recommendations by centre before and after intervention
There is a general pattern suggesting change in centres’ practices before and after the QRS intervention, although there are a few exceptions where practices were present before intervention and not adopted after intervention. Evidence to support real changes in practice was particularly strong where recruiters very clearly altered their approaches to communicating concepts. This was most apparent in recruiters’ descriptions of the study design before and after QRS intervention (specifically, their tendencies to draw out arms and describe each separately as two ‘options’). This is demonstrated through extracts from the following oncologist’s pre- and post-intervention explanations (Box 2). The changes apparent were similar for other recruiters who adopted the technique of drawing out the study arms.
Not all elements of the QRS guidance and advice could be evaluated through qualitative analysis. Recruiters’ readiness to explore patient decisions and preferences was a clear opportunity for change, but actual practices could not be assessed owing to the limited numbers of audio-recorded consultations where patients voiced their decisions. On a more general level, there were limited opportunities to discuss pre-/post-intervention changes in practices for individual recruiters, as few (if any) had contributed enough recordings that could be compared before and after intervention.
Qualitative recruitment study conclusions
Phase 1 of the QRS provided considerable information about the process of recruitment, as it was enacted in clinical centres participating in OPTIMA prelim. Interviews, audio-recordings and screening logs all provided insights that enabled a detailed and nuanced understanding of the challenges faced by recruiters in explaining the complex issues inherent in the OPTIMA prelim study and its design. These understandings were transmitted to the CfI, TMG and recruiters in a range of ways, including direct discussion of eligibility and information issues with the CfI and TMG, and through individual feedback, group discussions, tips and guidance documents and a revised PIS.
The methods used in this QRS were developed in a number of other RCTs.140,181,182 Several issues identified as important barriers to recruitment in other trials were also found in OPTIMA prelim, such as recruiters’ discomfort in exploring patient preferences, and the use of problematic terminology in recruitment consultations and trial documentation.182,183 However, there were also some original barriers to recruitment that were specific to the OPTIMA prelim trial, including recruiters’ discomfort in eligibility criteria and patients’ difficulties understanding the trial design.
The QRS was facilitated by a supportive CfI and TMG, but was hampered by clinical centres being given the opportunity to opt out of the QRS, and by recruiters’ unwillingness to audio-record consultations. These issues have also been found previously.184 If OPTIMA prelim proceeds to a main trial, clearer support for the QRS as a fully integrated and study-wide recruitment support initiative should be considered and attention given to encouraging (or perhaps expecting) greater commitment to recording consultations. In particular, it was noted that most of the centres participating in the QRS in the current study were mid-range recruiters. Future work would benefit from targeting centres for participation in the QRS on the basis of purposeful sampling methods, where screening logs are used to select centres deemed as ‘high’ and ‘low’ recruiters. This approach depends on clear, complete screening logs that record the entire recruitment process, from initial screening to trial participation. A more detailed discussion of the limitations of the QRS methods, including ideas for optimising future integration of the QRS in the OPTIMA main study, is presented in the Chapter 6, Qualitative recruitment study and Appendix 10.
- Results: recruitment and study conduct - OPTIMA prelim: a randomised feasibility...Results: recruitment and study conduct - OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer
- List of abbreviations - Is whole-colon investigation by colonoscopy, computerise...List of abbreviations - Is whole-colon investigation by colonoscopy, computerised tomography colonography or barium enema necessary for all patients with colorectal cancer symptoms, and for which patients would flexible sigmoidoscopy suffice? A retrospective cohort study
- Discussion - Feasibility of a RCT of techniques for managing an impacted fetal h...Discussion - Feasibility of a RCT of techniques for managing an impacted fetal head during emergency caesarean section: the MIDAS scoping study
- Research methods - Prognostic models of survival in patients with advanced incur...Research methods - Prognostic models of survival in patients with advanced incurable cancer: the PiPS2 observational study
- Protocol changes - A high-dose preparation of lactobacilli and bifidobacteria in...Protocol changes - A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE)
Your browsing activity is empty.
Activity recording is turned off.
See more...