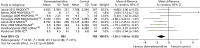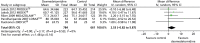Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cruickshank M, Henderson L, MacLennan G, et al. Alpha-2 agonists for sedation of mechanically ventilated adults in intensive care units: a systematic review. Southampton (UK): NIHR Journals Library; 2016 Mar. (Health Technology Assessment, No. 20.25.)

Alpha-2 agonists for sedation of mechanically ventilated adults in intensive care units: a systematic review.
Show detailsThis chapter reports the evidence of the clinical effectiveness of dexmedetomidine compared with clonidine and of dexmedetomidine or clonidine compared with propofol or benzodiazepines (midazolam or lorazepam) in mechanically ventilated adults admitted to ICUs.
Methods for assessing the outcomes arising from the use of the intervention
The methods for this assessment were prespecified in a research protocol (www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014014101).105
Identification of studies (search strategy and information sources/dates)
Highly sensitive literature searches, using an appropriate combination of controlled vocabulary and text word terms, were developed to identify reports of published, ongoing and unpublished studies reporting the clinical effectiveness of dexmedetomidine or clonidine in comparison with propofol and benzodiazepines (e.g. midazolam, lorazepam and diazepam) in mechanically ventilated adults admitted to ICUs. Literature searches were carried out from 12 to 15 November 2014 for publications from 1999 onwards. Details of the search strategies are reported in Appendix 1. Major electronic databases were searched including MEDLINE without revisions, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Science Citation Index, Bioscience Information Service and the Cochrane Central Register of Controlled Trials. Reports of relevant evidence synthesis were sought from the Cochrane Database of Systematic Reviews and Database of Abstracts of Reviews of Effects. The World Health Organization International Clinical Trials Registry Platform, metaRegister of Controlled Trials and ClinicalTrials.gov were searched for evidence of ongoing studies.
Websites of regulatory bodies and Health Technology Assessment agencies were checked for relevant unpublished reports, while websites of relevant pharmaceutical companies and professional organisations were searched for further pertinent information and reports.
In addition, reference lists of all included studies were perused for further citations.
Inclusion and exclusion criteria
Types of studies
Evidence was considered from RCTs comparing dexmedetomidine with clonidine or dexmedetomidine or clonidine with propofol or benzodiazepines such as midazolam, lorazepam and diazepam.
The following types of reports were excluded:
- narrative reviews, editorials and opinions
- case reports
- conference abstracts for which a full publication or further methodological information could not be found
- non-English-language reports for which a translation could not be organised
- studies that focused predominantly on people with primary brain injuries.
Types of participants
The types of participants considered were critically ill adults in ICUs who required MV. We did not prespecify definitions for ‘critically ill’ or ‘adults’, so any study population described as such was deemed suitable for inclusion.
Interventions
The sedative interventions considered were dexmedetomidine and clonidine.
Comparator interventions
The comparator interventions assessed were propofol and benzodiazepines such as midazolam, lorazepam and diazepam.
Outcomes
The following primary outcomes were considered:
- mortality
- duration of MV
- ventilator-free days
- length of ICU stay
- adverse events as reported by trial investigators and including the rate of:
- hypotension
- hypertension
- bradycardia
- respiratory depression
- delirium
- coma
- non-planned or accidental removal of lines (e.g. extubation) or catheters
- unpleasant side effects as reported by trial investigators (e.g. unpleasant memories, constipation or diarrhoea).
Secondary outcomes considered were:
- duration of weaning
- time spent in target sedation range
- proportion of patients in target sedation range
- discharge readiness
- extubation readiness
- length of hospital stay
- quality of life
- cost.
Data extraction strategy (study selection and data collection)
One reviewer (MC) screened all titles and abstracts identified by the search strategies. A second reviewer (MB) independently double-screened the first 100 abstracts and titles of the 2011–14 list. Agreement between the two reviewers was 100%.
All potentially relevant reports were retrieved in full and assessed independently by one reviewer (MC). A total of 40 reports were double-assessed by a second reviewer (Pawana Sharma or MB). Any disagreements were resolved by consensus. The full-text screening form is presented in Appendix 2. A data extraction spreadsheet (Microsoft Excel®, 2013; Microsoft Corporation, Redmond, WA, USA) was developed specifically for the purpose of this assessment, piloted and amended as necessary. From each study, one reviewer (MC) extracted information on geographical location, sponsor, study design, participants’ characteristics, setting and characteristics of ICU practice, characteristics of sedative intervention and outcome measures. Data extraction was double-checked by a second reviewer (MB). Any disagreements were resolved by discussion.
Critical appraisal strategy
The risk of bias of included RCTs was initially assessed by one reviewer (MC) using Cochrane’s risk-of-bias tool106 and, subsequently, cross-checked by a second reviewer (MB). The following domains were assessed: sequence generation, allocation concealment, blinding of participants and medical personnel, blinding of outcome assessors, incomplete outcome data and selective outcome reporting. Assessment of ‘other bias’ was based on the funding source, and a study was judged to be at high risk of bias if it was funded by the manufacturer(s) of the sedative agent(s) under investigation. Individual outcomes were judged as being at ‘high’, ‘low’ or ‘unclear’ risk of bias. Overall, risk of bias for each study was based on the findings of three key domains: sequence generation, allocation concealment and blinding of outcome assessor.
Studies were classified as follows: (1) high risk of bias if one or more key domains were at high risk; (2) unclear risk of bias if one or more key domains were judged to be at unclear risk; and (3) low risk of bias if all key domains were judged to be at low risk. Any disagreements between reviewers were resolved by discussion.
Method of analysis/synthesis
The general approach recommended by Cochrane was used for data analysis and synthesis.106 For binary outcomes, the Mantel–Haenszel approach was used to pool risk ratios (RRs) derived from each study. A random-effects model was used to calculate the pooled estimates of effect. For continuous outcomes (duration of MV, ICU length of stay, hospital length of stay, time to extubation, time in target sedation range and ventilator-free days), mean differences between groups were pooled when possible using the inverse variance weighted mean difference method and a random-effects model. Random-effects methods, rather than fixed-effects methods, as outlined in the original protocol, were chosen because of the clinical and statistical heterogeneity observed among included studies.
For each continuous outcome, an initial analysis was conducted using only studies where the mean and standard deviation (SD) were provided. In studies that did not report a mean and SD [and we could not derive these summary measures from reported p-values, standard errors or confidence intervals (CIs)], we tried to impute these from the data reported. The imputation strategy was as follows:
- Where the median, range and n for each group were available, we used the formulae reported by Hozo and colleagues107 to estimate the mean and SD.
- Where this method proved unfeasible, we imputed a SD from the available data using the methods outlined by Furukawa and colleagues.108
- In studies where a median and interquartile range were reported, we used two methods to calculate the mean. If the sample size was < 25, then first the median was used and second the value midway between the lower quartile and upper quartile was used. If the two methods yielded results that reversed the direction of treatment effect for a certain outcome within a study, then the study was excluded from the pooled analysis of that outcome.
For each outcome where the above provided extra data, a second analysis was done using the imputed data.
Heterogeneity across studies was explored by visual inspection of forest plots and using the chi-squared test and I2-statistics.
When data were available, subgroup analyses were performed according to type of comparator intervention.
Results of the evidence synthesis
Quantity of the evidence (studies included and excluded)
The literature searches identified 1182 potentially relevant citations, of which 83 were selected for full-text assessment and 107 for background information. Of these, 59 were subsequently excluded because the patient population, study design, outcomes reported or publication type were not eligible. A total of 18 RCTs published in 24 papers with a total of 2489 people were included in this assessment.5,52,69–72,102,109–125 It is worth noting that the results of the two large multicentre trials of PROpofol compared with DEXmedetomidine (the PRODEX trial) and of MIdazolam compared with DEXmedetomidine (the MIDEX trial) were published in a single report by Jakob and colleagues70 for the Dexmedetomidine for Long-Term Sedation investigators. Figure 2 presents the flow chart of the selection process. Appendix 3 provides the details of the 18 included trials and related secondary publications. Appendix 4 categorises the excluded studies according to the main reasons for their exclusion.
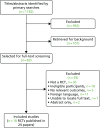
FIGURE 2
Flow chart of the study selection process.
Study characteristics
Appendix 5 details the study characteristics of the 18 included trials. All 18 trials were published in full. Four different comparators were assessed. One trial, with a total of 70 randomised patients, compared the effects and safety of dexmedetomidine with clonidine;55 nine trials, with a total of 1134 randomised patients, compared dexmedetomidine with propofol;70,109–111,114,117,120,122,123 four trials, with a total of 939 randomised patients, compared dexmedetomidine with midazolam;70,71,112,116 one trial, with a total of 118 randomised patients, compared dexmedetomidine with propofol or midazolam (three arms);72 two trials, with a total of 122 randomised patients, compared dexmedetomidine with ‘standard care’ (i.e. propofol and/or midazolam);69,121 and one trial with a total of 106 randomised patients, compared dexmedetomidine with lorazepam.102 A total of 2446 patients were analysed in the 18 included trials.
Six trials assessed dexmedetomidine in patients admitted to ICUs following elective surgery,72,110,111,114,120,123 whereas the remaining trials included general ICU patients.
Four trials were conducted in the USA,72,102,110,116 two in India,55,120 three in Turkey,112,117,122 two in Egypt,109,111 one in the UK,123 one in North America (USA and Canada),114 one in Finland and Switzerland,69 and one in Australia and New Zealand.121 The MIDEX multicentre trial70 was conducted in nine European countries; the PRODEX multicentre trial70 was conducted in six European countries and in Russia; and the SEDCOM71 (Safety and Efficacy of Dexmedetomidine COmpared with Midazolam) multicentre trial was conducted in the USA, Argentina, Brazil, Australia and New Zealand. All included trials involved prospective collection of data.
Three trials assessed patients up to 45 days69,70 and one up to 90 days.121 In one trial,102 participants were observed in the hospital from enrolment until discharge from hospital or death, and survivors were observed for vital status until 1 year after enrolment using hospitals’ electronic record systems and a commercial version of the Social Security Death Master File (http://ssdi.rootsweb.com). One trial114 followed up patients for 24 hours after discharge from ICUs and another trial71 for 48 hours after study drug cessation. One trial72 reported that patients were followed up for 3 days post operatively. In one trial,109 length of follow-up was reported to be 6 hours, in two trials55,120 it was 24 hours and in another trial123 it was 48–72 hours. Two trials reported follow-up in terms of time post extubation: one trial110 assessed patients at least 24 hours post extubation and another trial116 at least 72 hours post extubation. Length of follow-up was not reported in four trials.111,112,117,122
Appendix 6 presents details of dosage and route of administration of the respective sedative agents.
In general, dexmedetomidine was initiated with a loading dose of 1 µg/kg, administered intravenously over a period of 10–20 minutes.109,110,112,114,117,120,122 Some trials involved lower55,72,102,116 or higher111,123 loading doses, four trials did not use a loading dose69,70,121 and, in one trial, the loading dose was optional.71 Dexmedetomidine maintenance doses were fixed in two trials: 0.4 µg/kg/hour110 or 0.7 µg/kg/hour.112 The remaining trials specified lower and upper limits for maintenance doses, with lower limits ranging from 0 µg/kg/hour121 to 0.015 µg/kg/hour,102,116 0.2 µg/kg/hour,55,70,72,111,114,117,120,122,123 0.4 µg/kg/hour,110 0.5 µg/kg/hour109 and 0.7 µg/kg/hour.112 The maximum allowable dose was 2.5 µg/kg/hour.117,122,123
Clonidine was used in one trial. Patients received an infusion of clonidine at 1 µg/kg/hour. Titration was achieved with dosage increments up to 2 µg/kg/hour.55
Of the 12 trials that included a propofol arm, four trials reported a loading dose: an initial bolus dose of 1 mg/kg in one trial111 and 1 mg/kg over 10–15 minutes in three trials.117,122,123 Six trials did not use a loading dose69,70,72,109,110,120 and two trials did not provide information on dosage.114,121 Maintenance infusions of propofol ranged from 0.5–1 mg/kg/hour111 to 4 mg/kg/hour across trials.69,70
Out of the seven trials that included a midazolam arm, one trial reported a loading dose of 0.05 mg/kg112 and another trial reported an optional loading dose of the same level.71 The remaining trials did not use a loading dose. One trial did not specify dosage of midazolam.121 Maintenance doses of midazolam were between 0.03 mg/kg/hour70 and 10 mg/hour across trials.116
In one trial, lorazepam infusion started at 1 mg/hour and was titrated to a maximum of 10 mg/hour.102
All trials titrated sedatives to a target sedation level.55,69–72,102,109–112,114,116,117,120–123 Target sedation level was measured by means of the RSS score in 11 trials,55,72,109–112,114,117,120,122,123 the RASS in six trials69–71,102,121 and the Riker SAS score in one trial.116
The main characteristics of the 18 included studies are shown in Table 1.
TABLE 1
Summary of the main features of the included studies
Participant characteristics
The 18 included trials randomised a total of 1283 participants to dexmedetomidine and 1206 participants to a control intervention. The sample sizes of included studies ranged from 23 to 501 participants.
There was some doubt whether or not the trial by Memis and colleagues (40 patients in total)117 and that by Tasdogan and colleagues (40 patients in total)122 were mutually exclusive with regard to participants. Even though a number of similarities between the two trials were observed, the characteristics of the two patient populations were clearly not identical and, therefore, we treated them as two separate trials. Correspondence with the trials investigators (Dr Dilek Memis named as corresponding author for both trials) proved unsuccessful and did not elicit any response.
The mean age was reported in 12 trials.71,72,109–112,114,116,117,120–122 With the exception of one trial112 that focused exclusively on young pregnant women (mean age 25.1 years in the dexmedetomidine group and 26.8 years in the control intervention group), the 11 remaining trials mean age ranged from 43 to 65 years for dexmedetomidine and from 40 to 67 years for the comparator interventions. The median age was reported in six trials55,69,70,102,123 and ranged from 49 to 65 years for dexmedetomidine and from 46 to 67 years for the comparator interventions.
Sixteen studies reported information regarding the sex of participants.55,69–72,102,109,110,112,114,116,117,120–122 Study populations tended to involve more men than women, with the exception of one trial that involved only pregnant women112 (see Appendix 5 for further details).
The severity of illness at baseline was reported in eight trials55,71,102,112,117,121–123 by means of the APACHE II scores or APACHE III scores (one trial).116 The APACHE II scores have a possible range of 0–71, whereas the APACHE III scores can range from 0 to 299. In both cases, higher scores indicate more severe disease and a higher risk of death.104 Across the eight trials that used APACHE II, scores ranged from a mean of 5.1112 to a mean of 22 for dexmedetomidine117 and a mean of 6112 to a mean of 20117 for the control sedative intervention. One trial102 reported a median APACHE II score of 29 for dexmedetomidine and of 27 for the control sedative intervention. The trial116 that assessed severity of disease using the APACHE III scores reported mean scores of 74.1 for dexmedetomidine and of 70.4 for midazolam.
Table 2 presents an overview of the participants’ characteristics of the 18 included trials. It is worth noting that not all trials provided the same participant details or used the same measures to assess them.
TABLE 2
Summary of main participants’ characteristics (for trials that reported this information)
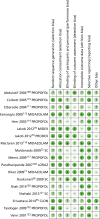
Risk-of-bias assessment of included studies
Figure 3 presents the summary of the risk-of-bias assessments for all included trials. The risk of bias of individual studies is presented in Figure 4.
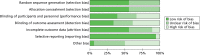
FIGURE 3
Summary of risk-of-bias assessments of all included trials.
Overall, out of the 18 included trials, four were judged to be at low risk of bias70,71,102 and seven at high risk of bias.72,111,114,117,120–122 For the remaining seven trials, there was not sufficient information to make an overall judgement.55,69,109,110,112,116,123
With regard to the assessment of selection bias, around half of the trials were judged to be at low risk (i.e. adequate sequence generation and allocation concealment),55,70,71,102,110,112,116,117,122 whereas the remaining eight trials did not provide sufficient information to formulate a proper judgement.69,72,109,111,114,120,121,123
In eight of the included trials, participants were reported to be blinded to the intervention received,69–71,102,109,111,116 whereas in six trials they were not.72,114,117,120–122 The remaining four trials did not report information on blinding of participants.55,110,112,123 Blinding of outcome assessor was addressed adequately in five trials,69–71,102 not adequately in seven trials72,111,114,117,120–122 and not reported in six trials.55,109,110,112,116,123
With regard to ‘incomplete outcome data’,10 trials had low withdrawal/discontinuation rates, which were balanced between intervention groups and, therefore, judged to be at low risk of bias.69,71,72,102,112,116,117,120–122 Two trials reported significantly higher discontinuation rates, owing to lack of efficacy, among people treated with dexmedetomidine, and were judged to be at high risk of bias.70 The remaining six trials did not provide sufficient information on which to make a definitive judgement.55,109–111,114,123
There was no evidence of selective reporting in any of the included trials, with the exception of one trial111 in which data on hypotension and bradycardia were mentioned only in the discussion section of the published paper and not properly reported in the results section. For this reason, the study was judged to be at high risk of selective reporting.
With regard to ‘other sources of bias’, nine trials declared financial support by manufacturers of sedative agents and were, therefore, judged to be at high risk of bias.69–71,102,116,120,121,123 One trial was judged at low risk of bias, as the authors clearly stated that no funding was received from manufacturers.55 The remaining eight studies were judged to be at unclear risk of bias, as the authors did not explicitly report their source of funding.72,109–112,114,117,122
Summary of clinical effectiveness
Random-effects meta-analyses of relevant clinical outcomes were performed when appropriate.
We had initially planned to perform subgroup analyses according to the type of clinical setting (patients admitted to ICUs following elective surgery compared with general ICU patients) if enough data had been available. However, only 6 of the 18 studies included patients who were admitted to the ICU after elective surgery, and not all of them provided data for all efficacy outcomes. Therefore, because of the dearth of suitable data, subgroup analyses according to the type of clinical setting were deemed unfeasible. As patients admitted to the ICU after elective surgery represent a distinct type of patient population (short duration of sedation and MV, and lower mortality rate), we deemed it inappropriate to combine trials that included patients after elective surgery with those that enrolled more general, critically ill ICU patients. The results of trials that enrolled patients after elective surgery were instead summarised narratively.
It is worth pointing out that there was considerable variation among included trials in the choice, definitions and measurements of outcomes, especially with regard to measures of ventilator dependence such as duration of MV, ventilator-free days, time to extubation or duration of weaning. Often, trials that assessed duration of MV did not report ventilator-free days as an outcome. The number of ventilator-free days was available from three trials, but details on measurement were lacking.69,118,121 Information on time to extubation was reported in six trials,70,71,111,114,123 but definition and criteria for extubation were not consistent across trials. Two large trials (MIDEX and PRODEX)70 reported both duration of MV and time to extubation, but did not provide a clear definition or measurement criteria for time to extubation and failed to discuss the clinical difference between the two measures. Similarly, duration of weaning was reported by two trials,69,114 but only one provided a proper outcome definition and a description of the measurement criteria.114
Clonidine compared with dexmedetomidine
One trial, at unclear risk of bias, randomised a total of 70 general ICU patients requiring MV to dexmedetomidine (35 patients) or to clonidine (35 patients).55 Both clonidine and dexmedetomidine produced effective sedation. Target sedation was achieved in 86% of observations among patients who received dexmedetomidine and in 62% of observations among patients who received clonidine (p = 0.04). Additional sedation was needed by more patients treated with clonidine than those treated with dexmedetomidine (14 patients and 8 patients, respectively; p = 0.034). Hypotension was observed significantly more frequently among patients who received clonidine (11 out of 35 patients) than among patients who received dexmedetomidine (3 out of 35) (p = 0.02). Rebound hypertension was seen only in four patients receiving clonidine. The authors concluded that both clonidine and dexmedetomidine produced effective sedation. However, the haemodynamic stability provided by dexmedetomidine makes it a preferable option over clonidine for short-term sedation of ICU patients.
Propofol and benzodiazepines (i.e. midazolam and lorazepam) compared with dexmedetomidine
Primary outcomes
Mortality
Nine trials reported mortality data (Figure 5).69–71,102,116,117,120,121 A total of 196 out of 909 (22%) patients who received dexmedetomidine and 162 out of 783 (21%) of patients who received a control intervention died. Compared with alternative sedative agents, dexmedetomidine had no significant effects on mortality (RR 1.03, 95% CI 0.85 to 1.24, I2 = 0%; p = 0.78).
Two trials assessing patients after elective surgery reported mortality data.72,123 In one trial,72 two deaths not attributable to sedation occurred among patients who received the control intervention (propofol), whereas in the other trial123 two patients receiving dexmedetomidine died, compared with one patient receiving the control intervention (propofol).
Duration of mechanical ventilation
Two trials reported mean duration of MV (Figure 6).70 There were no significant differences in the duration of MV between dexmedetomidine and control interventions (mean difference –0.36, 95% CI –1.59 to 0.86, I2 = 0%; p = 0.56).
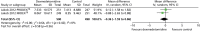
FIGURE 6
Meta-analysis for duration of MV. df, degrees of freedom; IV, inverse variance.
Similarly, there was no difference (mean difference –0.30, 95% CI –1.70 to 1.11; p = 0.68) in the duration of MV between dexmedetomidine and control interventions (Figure 7) when all available data suitable for the analysis were considered (including transformed and imputed data). Statistical heterogeneity was observed among trials (I2 = 70%).

FIGURE 7
Meta-analysis for duration of MV: all available data (including transformed and imputed data). df, degrees of freedom; IV, inverse variance; PROPOFOL, propofol compared with dexmedetomidine; SC, standard care compared with dexmedetomidine.
One trial that assessed patients after elective surgery110 reported no difference between dexmedetomidine and propofol (p > 0.05) with regard to length of intubation.
Ventilator-free days
One trial provided suitable data for ventilator-free days (Figure 8).121 There was no evidence of a statistically significant difference (mean difference 1.20, 95% CI –5.12 to 7.52; p = 0.71) between patients who received dexmedetomidine and those who received standard care (propofol or midazolam).
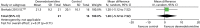
FIGURE 8
Meta-analysis for ventilator-free days. IV, inverse variance; SC, standard care compared with dexmedetomidine.
When all available data suitable for the analysis were considered (including transformed and imputed data) (Figure 9), the mean difference was 3.28 ventilator-free days (95% CI 0.06 to 6.49 ventilator-free days, I2 = 0%; p = 0.046) favouring dexmedetomidine.

FIGURE 9
Meta-analysis for ventilator-free days: all available data (including transformed and imputed data). df, degrees of freedom; IV, inverse variance; LORAZ, lorazepam compared with dexmedetomidine; SC, standard care compared with dexmedetomidine.
Intensive care unit length of stay
One trial provided mean length of ICU stay data (Figure 10).117 There was no evidence of a significant difference between sedative agents (mean difference 2.00 days, 95% CI –3.12 to 7.12 days; p = 0.44).

FIGURE 10
Meta-analysis for ICU length of stay. IV, inverse variance; PROPOFOL, propofol compared with dexmedetomidine.
However, Figure 11 shows that when all available data suitable for the analysis were considered (including transformed and imputed data), ICU length of stay was significantly shorter among patients who received dexmedetomidine than among those who received an alternative sedative agent (mean difference –1.26 days, 95% CI –1.96 to –0.55 days, I2 = 31%; p = 0.0004).
Hypotension
Five trials provided suitable data to assess the incidence of hypotension (Figure 12).69–71,116 There were no statistically significant differences between participants who received dexmedetomidine (232 out of 789, 29%) and those who received an alternative sedative agent (137 out of 675, 20%) (RR 1.28, 95% CI 0.93 to 1.75, I2 = 55%; p = 0.12).

FIGURE 12
Meta-analysis for incidence of hypotension. IV, inverse variance; MIDAZOLAM, midazolam compared with dexmedetomidine; SC, standard care compared with dexmedetomidine.
The proportion of patients who developed hypotension was reported in two trials that assessed patients after elective surgery.110,114 No statistically significant differences were found. In one trial,110 35 out of 43 patients who received dexmedetomidine experienced severe hypotension, compared with 31 out of 46 of those who received propofol (p = 0.132). In the other trial,114 hypotension occurred in 36 out of 148 (24%) participants who received dexmedetomidine and in 24 out of 147 (16%) participants who received propofol (p = 0.111).
Hypertension
Three trials reported the incidence of hypertension during sedation (Figure 13).70,71 There was no evidence of statistically significant differences (RR 1.09, 95% CI 0.89 to 1.33, I2 = 21%; p = 0.43) between dexmedetomidine (211 out of 737, 29%) and alternative sedative agents (143 out of 619, 23%).
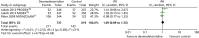
FIGURE 13
Meta-analysis for incidence of hypertension. IV, inverse variance; MIDAZOLAM, midazolam compared with dexmedetomidine.
In one trial, in which patients were sedated after elective coronary artery bypass graft (CABG) surgery,114 hypertension occurred more frequently among patients who received dexmedetomidine than among those who received propofol (p = 0.018).
Bradycardia
Six trials assessed the incidence of bradycardia during sedation (Figure 14).69–71,102,116 Significantly more participants who received dexmedetomidine (189 out of 841, 22%) experienced bradycardia than those who received alternative sedative agents (70 out of 726, 10%) (RR 1.88, 95% CI 1.28 to 2.77, I2 = 46%; p = 0.001).

FIGURE 14
Meta-analysis for incidence of bradycardia. IV, inverse variance; LORAZ, lorazepam compared with dexmedetomidine; MIDAZOLAM, midazolam compared with dexmedetomidine; SC, standard care compared with dexmedetomidine.
In one trial, which enrolled patients after elective coronary artery bypass graft surgery,114 the frequency of bradycardia was similar between intervention groups [5 out of 148 (3%) in the dexmedetomidine group, compared with 2 out of 147 (1%) in the propofol group; p = 0.448].
Delirium
Seven trials reported the proportion of patients who experienced episodes of delirium during sedation.69–71,102,116,121 A total of 234 out of 862 (27%) participants who received dexmedetomidine and 209 out of 742 (28%) participants who received an alternative sedative agent experienced delirium (Figure 15). The difference between sedatives was not statistically significant (RR 0.83, 95% CI 0.65 to 1.06, I2 = 60%; p = 0.14). Statistical heterogeneity was observed among trials (I2 = 60%).

FIGURE 15
Meta-analysis for incidence of delirium. IV, inverse variance; LORAZ, lorazepam compared with dexmedetomidine; MIDAZOLAM, midazolam compared with dexmedetomidine; SC, standard care compared with dexmedetomidine.
Two trials, which enrolled patients after elective surgery, reported the proportion of patients with episodes of delirium.72,110 In one trial,110 the number of patients with episodes of delirium was similar in both intervention groups (1 out of 43 in the dexmedetomidine group compared with 1 out of 46 in the propofol group). In the other trial,72 the incidence of delirium was 10% (4 out of 40) among patients who received dexmedetomidine, 44% (16 out of 36) among those who received propofol and 44% (17 out of 40) for those who received midazolam.
Self-extubation
Four trials reported episodes of self-extubation during sedation (Figure 16).70,102,121 Self-extubation occurred in 12 out of 566 (2%) of patients who received dexmedetomidine and 3 out of 564 (< 1%) of those who received an alternative sedative agent. There was no clear evidence of a statistically significant difference between sedative interventions (RR 2.95, 95% CI 0.96 to 9.06, I2 = 0%; p = 0.06).

FIGURE 16
Meta-analysis for episodes of self-extubation. IV, inverse variance; LORAZ, lorazepam compared with dexmedetomidine; SC, standard care compared with dexmedetomidine.
One trial, which assessed patients after elective surgery,110 reported one episode of self-extubation among participants who received propofol (1 out of 46) and none among those who received dexmedetomidine (0 out of 43).
Tachycardia
Five trials assessed the incidence of tachycardia among patients receiving sedation (Figure 17).70,71,102,116 There was no evidence of a significant difference (RR 0.93, 95% CI 0.63 to 1.39; p = 0.73) between sedative interventions [187 out of 800 (23%) of those who received dexmedetomidine compared with 178 out of 682 (26%) of those who received alternative sedative agents]. Substantial statistical heterogeneity was observed among trials (I2 = 82%).

FIGURE 17
Meta-analysis for incidence of tachycardia. IV, inverse variance; LORAZ, lorazepam compared with dexmedetomidine; MIDAZOLAM, midazolam compared with dexmedetomidine.
Rate of respiratory depression
Rate of respiratory depression was not reported by any of the included trials. However, respiratory rate was reported by two trials.109,120 In both trials, no significant differences were observed between sedatives. One trial109 recorded mean breaths per minute of 28 (SD 4 breaths per minute), 28 (SD 3 breaths per minute) and 29 (SD 4 breaths per minute) among patients who received dexmedetomidine and 29 (SD 3 breaths per minute), 30 (SD 3 breaths per minute) and 30 (SD 4 breaths per minute) among those who received propofol at 2 hours, 4 hours and 6 hours after infusion of study drug, respectively. The other trial120 reported mean respiratory rate per minute pre and post operatively. For patients who received dexmedetomidine, the pre- and post-operative values were 16.53 (SD 3.83) and 17.07 (SD 3.47) breaths per minute, whereas for those who received propofol the values were 17.25 (SD 3.58) and 20 (SD 4.0) breaths per minute, respectively.
Incidence of coma
One trial assessed the incidence of coma during a 12-day evaluation period.102 Significantly fewer patients who received dexmedetomidine (63%) than those who received lorazepam (92%) experienced coma (p < 0.001).
Secondary outcomes and other reported outcomes
It is worth noting that no data were available from the included trials for extubation readiness, discharge readiness and quality of life.
Duration of weaning
Two trials reported duration of weaning.69,114 Ruokonen and colleagues69 did not observe any difference (p = 0.27) between patients who received dexmedetomidine (median 59.4 hours) and those who received propofol and/or midazolam (median 78 hours). Similarly, Herr and colleagues,114 who enrolled patients after elective surgery, found that there was no difference between sedative interventions in median times to weaning. Median time to the start of weaning was 259 minutes (25th–75th percentiles 215–410 minutes) for dexmedetomidine and 300 minutes (25th–75th percentiles 210–482 minutes) for propofol.
Time in target sedation range
Three trials provided data on percentage of total time in target sedation range (Figure 18).70,71 There was no evidence of a significant difference between sedative interventions (mean difference 1.94% of total time in target sedation range, 95% CI –1.70 to 5.57% of total time in target sedation range, I2 = 0%).
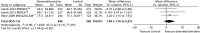
FIGURE 18
Meta-analysis for time in target sedation range. IV, inverse variance; MIDAZOLAM, midazolam compared with dexmedetomidine.
Similarly, Figure 19 shows that no significant differences were evident between dexmedetomidine and alternative sedative agents (mean difference 2.53% of total time in target sedation range, 95% CI –0.82 to 5.87% of total time in target sedation range, I2 = 0%; p = 0.14) when all available data suitable for the analysis (including transformed and imputed data) were considered.
Two trials, which enrolled patients after elective surgery, assessed time in target sedation range.111,123 Both trials showed that the proportion of time spent at adequate depth of sedation was similar for sedative interventions (46.3% for dexmedetomidine and 49.1% for propofol in one trial,123 and 93% for dexmedetomidine and 92% for propofol in the other trial).111
Hospital length of stay
Three trials reported overall length of hospital stay and did not find any significant difference between dexmedetomidine and alternative sedative interventions.70,121 In the MIDEX trial, the median duration of study hospital stay was 35 days (range 14–45 days) for dexmedetomidine and 27 days (range 17–45 days) for midazolam (p = 0.370). In the PRODEX trial, the median duration of study hospital stay was 25 days (range 13–45 days) for dexmedetomidine and 28 days (range 14–45 days) for propofol (p = 0.760).70 Shehabi and collegues121 reported a median of 16.1 days (interquartile range 9.3–33.3 days) for dexmedetomidine and 17 days (interquartile range 4.0–29.0 days) for standard sedative treatments (p = 0.49).
Time to extubation
Two trials reported time to extubation (Figure 20).70 Time to extubation was significantly shorter among patients who received dexmedetomidine than among those who received an alternative sedative agent (mean difference –1.83 days, 95% CI –2.70 to –0.95 days, I2 = 0%; p < 0.0001).
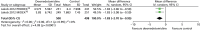
FIGURE 20
Meta-analysis for time to extubation. IV, inverse variance.
Similarly, time to extubation was significantly shorter for patients who received dexmedetomidine than for those who received an alternative sedative agent (Figure 21) when all available data suitable for the analysis (including transformed and imputed data) were considered (mean difference –1.85 days, 95% CI –2.61 to –1.09 days, I2 = 0%; p < 0.00001).
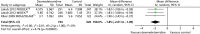
FIGURE 21
Meta-analysis for time to extubation: all available data (including transformed and imputed data). IV, inverse variance; MIDAZOLAM, midazolam compared with dexmedetomidine.
Three trials, which enrolled patients after elective surgery, assessed time to extubation.111,114,123 All three trials showed that times to extubation were similar between sedative interventions. Elbaradie and colleagues111 reported mean times to extubation of 30 minutes (SD 15 minutes) for dexmedetomidine compared with 35 minutes (SD 12 minutes) for propofol. Herr and colleagues114 reported median times to extubation of 410 minutes (25th–75th percentiles 310 to 584 minutes) for dexmedetomidine and 462 minutes (25th–75th percentiles 323–808 minutes) for propofol. In the trial by Venn and Grounds,123 mean extubation times were 29 minutes (range 15–50 minutes) for dexmedetomidine and 28 minutes (range 20–50 minutes) for propofol (p = 0.63).
Cost of care
Three trials71,72,102 reported costs related to sedation. The trial by Pandharipande and colleagues, published in 2007,102 reported median costs of US$4675 for dexmedetomidine and US$2335 for lorazepam. The median total hospital cost was approximately US$22,500 higher, but not significantly higher, for dexmedetomidine. This difference was attributed to costs that occurred prior to enrolment and randomisation.
The trial by Maldonado and colleagues, published in 2009,72 reported an average total cost for post-operative care of US$7025 for dexmedetomidine, compared with US$9875 and US$9570 for propofol and midazolam, respectively. There were no significant differences between sedative interventions. For patients who developed delirium, the average cost was US$12,965, compared with an average cost of US$6763 for those who did not (p = 0.004).
The SEDCOM trial by Riker and colleagues, published in 2009,71 reported overall economic costs (expressed in Canadian dollars) of CA$7022 for dexmedetomidine and of CA$7680 for midazolam; medication costs of CA$1929.57 for dexmedetomidine and CA$180.10 for midazolam; costs associated with delirium of CA$2127.49 for dexmedetomidine and CA$3012.30 for midazolam; and MV costs were CA$2938.62 for dexmedetomidine and CA$4447.64 for midazolam.
Co-operation and communication
In four multicentre trials with a total of 1461 patients69–71 that compared dexmedetomidine with midazolam or propofol, secondary efficacy outcomes included nurses’ assessment of arousal, co-operation and ability to communicate pain using visual analogue scales. In all four trials,69–71 patients who received dexmedetomidine were significantly more arousable, more co-operative and better able to communicate their pain than those who received an alternative sedative agent (propofol or midazolam) (p ≤ 0.001 in all cases).
Neuropsychological testing
In the trial by Pandharipande and colleagues102 (103 patients in total), neuropsychological tests were administered within 72 hours of discharge from the ICU. A higher proportion of patients who received dexmedetomidine (42%), but not significantly higher, were able to complete the post-ICU neuropsychological testing than those who received lorazepam (31%) (p = 0.61). The median Mini-Mental State Examination score, which evaluates global cognitive ability, was 28 for dexmedetomidine and 27 for lorazepam (p = 0.23), whereas the median Trails-B scores, which assesses motor speed and attention functions corrected for age and level of education, were 18 for dexmedetomidine and 19 for lorazepam (p = 0.75).
Anxiety and depression
The trial by MacLaren and colleagues116 assessed the rates of post-ICU anxiety, depression and acute stress disorder manifestations among 23 mechanically ventilated patients admitted to ICUs. Validated assessment scales were administered 72 hours after extubation but before hospital discharge. Eight patients in each intervention group (midazolam compared with dexmedetomidine) completed the questionnaires. Manifestations of anxiety and depression were similar between sedative interventions. Five patients (62.5%) who received dexmedetomidine and one patient (12.5%) who received midazolam manifested acute stress disorder (p = 0.063).
Memory of intensive care unit experience
Three trials provided information on patients’ ICU recall.110,116,123 MacLaren and colleagues,116 who assessed a total of 23 patients, reported that the median number of ICU experiences remembered by patients who received dexmedetomidine was significantly higher than that of patients who received midazolam (18.5 compared with 8.5; p = 0.015).
Venn and Grounds123 enrolled a total of 20 patients after elective surgery and assessed recall 48–72 hours after discharge from ICUs. The majority of patients who received dexmedetomidine remembered their length of stay in ICU accurately, compared with those who received propofol (8 out of 10 compared with 2 out of 10 remembered their length of stay in the ICU; p = 0.023), but few remembered the duration of MV (3 out of 10 compared with 2 out of 10). Sleeping difficulty and noise were more often reported by patients who received propofol and discomfort on the ventilator by those who received dexmedetomidine. No patient recorded pain.
Corbett and colleagues,110 who enrolled a total of 89 patients after elective surgery, evaluated patients’ perception regarding their ICU experience. A validated questionnaire was administered after ICU discharge [mean time between discharge and administration of 46.5 hours (SD 24.5 hours) for dexmedetomidine and 45.5 hours (SD 20.7 hours) for propofol; p = 0.847]. Level of overall awareness as a marker to amnesia was similar between sedative interventions as well as the overall level of discomfort and pain. Participants who received dexmedetomidine perceived a significantly shorter length of intubation than those who received propofol (p = 0.044). Perceptions in length of stay were similar between patient groups (p = 0.767). Patients who received dexmedetomidine reported greater difficulty in resting or sleeping than those who received propofol (p = 0.051).
Subgroup analyses
We were able to perform subgroup analyses of primary and secondary outcomes according to type of comparator (see Appendix 7). Generally, results of subgroup analyses were consistent with those of the overall population. However, subgroups were usually too small to provide reliable conclusions and caution should be applied in their interpretation.
No subgroup analyses were possible for age, severity of disease, different duration of MV, type of clinical setting and nurse/patient ratio because of the paucity of suitable data.
Duration of MV was significantly longer for participants treated with dexmedetomidine than for those treated with propofol, but it was significantly shorter than for those who received standard care. There were no differences between participants who received dexmedetomidine and those who received midazolam. Overall, duration of MV was significantly different across the various subgroups. A high level of heterogeneity was evident in the analyses (I2 = 78.1%).
Incidence of delirium was significantly lower in participants treated with dexmedetomidine than among those treated with propofol or midazolam. There were no differences between participants treated with dexmedetomidine than for those treated with standard care or lorazepam. Overall, there were significant differences in the incidence of delirium across the comparator subgroups and there was evidence of high heterogeneity (I2 = 76.9%). The incidence of tachycardia was significantly lower for participants treated with propofol than for those treated with dexmedetomidine. There were no differences between participants who received dexmedetomidine and those who received midazolam or lorazepam. Overall, there were significant differences in the incidence of tachycardia between the comparator subgroups and, again, there was evidence of substantial heterogeneity (I2 = 77.6%).
Table 3 presents an overview of all meta-analyses results including both main analyses and subgroup analyses.
TABLE 3
Summary of meta-analyses results
- Assessment of clinical effectiveness - Alpha-2 agonists for sedation of mechanic...Assessment of clinical effectiveness - Alpha-2 agonists for sedation of mechanically ventilated adults in intensive care units: a systematic review
- Methods - BREATHER (PENTA 16) short-cycle therapy (SCT) (5 days on/2 days off) i...Methods - BREATHER (PENTA 16) short-cycle therapy (SCT) (5 days on/2 days off) in young people with chronic human immunodeficiency virus infection: an open, randomised, parallel-group Phase II/III trial
- PREDICTED: Homo sapiens activity dependent neuroprotector homeobox (ADNP), trans...PREDICTED: Homo sapiens activity dependent neuroprotector homeobox (ADNP), transcript variant X1, mRNAgi|2462580049|ref|XM_054323292.1|Nucleotide
- Preventing blood-borne virus infection in people who inject drugs in the UK: sys...Preventing blood-borne virus infection in people who inject drugs in the UK: systematic review, stakeholder interviews, psychosocial intervention development and feasibility randomised controlled trial
- Enhanced psychological care in cardiac rehabilitation services for patients with...Enhanced psychological care in cardiac rehabilitation services for patients with new-onset depression: the CADENCE feasibility study and pilot RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...