Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Jones-Hughes T, Snowsill T, Haasova M, et al. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. Southampton (UK): NIHR Journals Library; 2016 Aug. (Health Technology Assessment, No. 20.62.)

Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model.
Show detailsMethods for reviewing effectiveness
The project was undertaken in accordance with a predefined protocol. There were no major departures from this protocol.
The aim was to systematically review the effectiveness of immunosuppressive therapies in adult renal transplantation and determine the effect on patient survival; graft survival; GRF; time to, and incidence of, AR; severity of AR; the effectiveness in improving HRQoL and the impact of AEs. The review was undertaken following the principles published by the NHS Centre for Reviews and Dissemination.66
Identification of studies
Bibliographic literature searching was conducted on 14 April 2014. The effectiveness searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a study design limit to RCTs or controlled trials). The search was date limited to 2002 to current, in line with the previous assessment, and the searches were updated on 18 November 2014. The search was not limited by language or human-only studies to ensure that records were not missed in error. Instead, these exclusion criteria were implemented during the screening process.
The following databases were searched for randomised controlled trials (RCTs) MEDLINE (via Ovid), EMBASE (via Ovid), Cochrane Central Register of Controlled Trials (via Wiley Online Library) and Web of Science (via ISI; including conference proceedings). The following trials registries were hand-searched: ClinicalTrials.Gov (https://clinicaltrials.gov/) and Controlled Trials (www.controlled-trials.com/). The search strategies (including web-searching) are recorded in Appendix 1.
A separate search was undertaken to identify systematic reviews. These searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a pragmatic limit to systematic reviews). The search was run from database inception in the following databases: MEDLINE (via Ovid), EMBASE (via Ovid), Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Health Technology Assessment (HTA; The Cochrane Library via Wiley Online Library) and Health Management Information Consortium (via Ovid). The search was not limited by language and it was not limited to human-only studies. The search strategies are recorded in Appendix 1.
In addition, the following websites were searched for background information:
Renal societies (UK)
- British Renal Society www.britishrenal.org/.
- Renal Association www.renal.org/.
- UK Renal Registry www.renalreg.com/.
- Kidney Research UK www.kidneyresearchuk.org/.
- British Kidney Patient Association www.britishkidney-pa.co.uk/.
- National Kidney Federation www.kidney.org.uk/.
Renal societies (international)
- American Society of Nephrology www.asn-online.org/.
- American Association of Kidney Patients www.aakp.org/.
- National Kidney Foundation (USA) www.kidney.org/.
- Canadian Society of Nephrology www.csnscn.ca/.
- Kidney Foundation of Canada www.kidney.ca/.
- Australian and New Zealand Society of Nephrology www.nephrology.edu.au/.
- Kidney Health Australia www.kidney.org.au/.
- Kidney Society Auckland www.kidneysociety.co.nz/.
The database search results were exported to, and deduplicated using EndNote (X5) (Thomson Routers, CA, USA). Deduplication was also performed using manual checking. The search strategies and the numbers retrieved for each database are detailed in Appendix 1. After the reviewers completed the screening process, the bibliographies of included papers were scrutinised for further potentially includable studies.
Studies included in the previous adult and child HTA reviews65,67 were screened against the inclusion criteria for the Peninsula Technology Assessment Group (PenTAG) review for includable studies. Reference lists of included guidelines, systematic reviews and clinical trials were scrutinised for additional studies.
Ongoing studies
A search for ongoing trials was also undertaken. The terms used to search the ClinicalTrials.gov and Controlled Trials [International Standard Randomised Controlled Trial Number (ISRCTN)] trial registers for the interventions are included in Appendix 1.
Trials that did not relate to immunosuppressive therapies for kidney transplantation in adults were removed by hand-sorting. Finally, duplicates, identified via their study identification numbers, where possible, were removed. Searches were carried out on 19 September 2014.
Inclusion and exclusion criteria
Study design
Only RCTs were included. Systematic reviews of RCTs were also included in order to ensure all relevant clinical trials were identified.
Population
Adults who were undergoing kidney transplantation only, and receiving immunosuppressive therapy, were included in this review. Multiorgan transplantation, the treatment of episodes of AR and individuals who have previously received a renal transplant and immunosuppression (i.e. individuals who were not undergoing the process of a new renal transplant) are outside the scope of this appraisal.
Interventions
Studies evaluating the use of the following immunosuppressive therapies for renal transplantation were included (further details in Chapter 1, Induction therapy and Maintenance therapy).
Induction therapy regimens containing:
- BAS
- rATG.
Maintenance therapy regimens containing:
- MMF
- MPS – EC-MPS
- immediate-release TAC
- TAC-PR
- BEL
- SRL
- EVL.
Under an exceptional directive from the Department of Health, these interventions can be assessed outside their existing marketing authorisation (to reflect their use in clinical practice) where there was compelling evidence of safety and effectiveness.
Comparators
The comparators of interest for induction therapies were regimens without monoclonal or polyclonal antibodies or one of the other interventions under consideration.
For maintenance therapies, the comparators were a CNI with or without an antiproliferative agent and/or CCSs or a regimen including one of the other interventions under consideration.
Outcomes
Outcomes sought from the studies fell into four main categories: mortality, graft-related outcomes, AEs data and HRQoL outcomes. Owing to the variability in evidence available and in order to ensure consistency with the modelling, measurements were restricted as follows:
- Mortality
- Graft-related outcomes:
- graft survival – when graft loss is defined as return to chronic dialysis, retransplant, graft removal or death
- GRF – (estimated) eGFR, which is an estimate of actual GFR; a number of formulae are available for eGFR, which may require age, weight, sex and serum creatinine level
- time to, and incidence of, biopsy-proven acute rejection (BPAR)
- severity of AR according to the Banff classification (grades I–III).
- AEs:
- malignancy and post-transplant lymphoproliferative disorder (PTLD)
- diabetes mellitus
- infections
- cytomegalovirus (CMV).
- HRQoL, including data on validated quality-of-life measures, for example the European Quality of Life-5 Dimensions (EQ-5D), the SF-36 and the Kidney Transplant Questionnaire (KTQ-25).
Selection of studies
Studies retrieved from the searches were selected for inclusion according to the inclusion/exclusion criteria specified in Inclusion and exclusion criteria. Initially, titles and abstracts returned by the search strategy were screened for inclusion independently by two researchers, with TJ-H as first reviewer and LC, MHa, MB or HC as second reviewer. Disagreements were resolved by discussion, with involvement of a third reviewer (MHa or HC). Full texts of identified studies were obtained and screened in the same way.
In addition, studies included in the reviews conducted by Woodroffe et al.65 and Yao et al.67 were screened for inclusion against the eligibility criteria for this review.
Data extraction strategy
Included full papers were split between five reviewers (TJ-H, MHa, LC, MB and HC), with TJ-H as first reviewer for the purposes of data extraction using a standardised data extraction form, and checked independently by another reviewer. Discrepancies were resolved by discussion with the involvement of an additional review team member (MHa or HC) if necessary. Information extracted and tabulated included details of the study’s design and methodology, baseline characteristics of participants, and results, including HRQoL and any AEs, if reported.
If several publications were identified for one study, the data were extracted from the most recent publication and supplemented with information from other publications.
For studies comparing both induction and maintenance, we assigned a separate reference for each study arm, with the author and publication year of the main publication, and added the suffixes ‘a’ and ‘b’.
Critical appraisal strategy
Four reviewers (TJH, MHa, MB and HC) independently assessed quality for the newly identified studies (2002 onwards) according to criteria based on Centre for Reviews and Dissemination guidance (Table 8).66
TABLE 8
Quality assessment
Methods of data synthesis
Where data permitted the results of individual studies were pooled using Stata SE 13.1 (StataCorp LP, College Station, TX, USA) to investigate:
- estimation of overall treatment effect
- assessment of heterogeneity
- subgroup analysis
- assessment of publication bias.
Owing to the heterogeneity of population and study characteristics, a random-effects model was assumed for all meta-analyses. For binary data, odds ratio (OR) was used as a measure of treatment effect and the DerSimonian–Laird method was used for pooling. For continuous data (eGFR), mean differences (MDs) were calculated if the outcome was measured on the same scale in all trials.
If a study had two intervention arms that were separately compared with the control arm, when pooling ORs the number of events and the total sample size in the control arm were divided equally across the comparisons, and when pooling MDs the total sample size in the control arm was adjusted and divided equally across the comparisons. However, if only one experimental arm was eligible for the analysis then all participants assigned to the control arm were included.
A narrative synthesis accompanies all included data.
Network meta-analyses
Network meta-analyses (NMAs) were undertaken within a Bayesian framework in WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). Where prior distributions were required, they were intended to be vague.
For all NMAs assessing the effectiveness of induction therapy, the reference treatment was no induction/placebo (PBO). For networks evaluating the effectiveness of maintenance therapy, the reference treatment was CSA + AZA. For the outcomes graft loss, mortality and BPAR, fixed- and random-effects models having a binomial likelihood with logit link were used (see code in Appendix 6). For the outcome of GRF, models with a normal likelihood and identify link were used (see code in Appendix 6). All models account for the fact that some RCTs have more than two arms.68
Trials reporting zero events for all arms for a particular outcome were excluded from the analysis, as these trials would not contribute information to the network. Where a trial had a zero event in at least one, but not all, treatment arms, 0.5 was added to all cells to allow the model to run within WinBUGS version 14 (MRC Biostatistics Unit, Cambridge, UK).68
Analyses were run with three chains, a burn-in of 40,000 iterations followed by an additional 100,000 iterations, with thinning of every fifth iteration to help convergence. Convergence of the models was assessed by visual inspection of autocorrelation and trace plots for all monitored variables.
Fixed- and random-effects NMAs were analysed and compared using the deviance information criteria (DIC). Models with the lowest DIC were assumed to have a better fit to the data. The posterior medians and 95% credibility intervals (CrIs) are reported.
To assess inconsistency in the network, the inconsistency degrees of freedom (ICDF) were calculated (reflecting the number of independent loops in the network) and inconsistency networks (where only direct evidence for a comparison between treatments is used) were modelled.69 Results from the inconsistency models were compared with those from the consistency models (where direct and indirect evidence were combined) to help identify inconsistencies within the network. The model with the lowest DIC was assumed to be a better fit to the data.
The NMAs that have been conducted to satisfy relevant items on the Decision Support Unit’s Evidence Synthesis Checklist.70
Systematic review results
Owing to the number of regimens for both the interventions and comparators, the assessment of effectiveness will be reported separately for induction and maintenance. All RCT evidence identified for each intervention is presented.
Identified research for induction and maintenance therapies
We screened the titles and abstracts of 5079 unique references identified by the searches, with 750 papers retrieved for detailed consideration. As highlighted in Figure 7, a total of 715 papers were excluded (a list of these, with reasons for their exclusion, can be found in Appendix 2). Overall. 107 studies met the inclusion criteria. At both stages, initial disagreements were easily resolved by consensus.

FIGURE 7
Flow chart: clinical effectiveness review. SR, systematic review.
We then reassessed included studies from the review conducted by Woodroffe et al.65 (43 studies) (TA85). Of these, 20 studies were considered eligible for inclusion in the update review.71–90 The scope for the adult review by Woodroffe et al.65 differed from the final scope issued by NICE; the induction therapy originally included DAC [European Union (EU) marketing authorisation withdrawn in January 2009] and not rATG, the maintenance therapy did not include BEL or EVL, and treatment of AR was included but is outside the scope of this appraisal. Reasons for exclusion from this review include data that were available only in abstract format, population (either participants receiving multiorgan transplant or mixed population of age groups) or duplicate (studies also retrieved in the update searches).
Citations of the included systematic reviews were also searched by two reviewers (HC and MHa). This process revealed an additional two papers.
Update searches were conducted on 18 November 2014 using the same methodology as described earlier. A total of 375 records were screened by three reviewers (TJH, HC and MHa) and 99 records were selected for full-text retrieval. Four papers were judged to be eligible on full-text appraisal. A list of these items, with reasons for their exclusion, can be found in Appendix 2.
The process is illustrated in detail in Figure 7. Note, for the sake of clarity, the figures for the initial and update searches have been combined.
Quality of included studies
We appraised the newly identified trials and those included in the previous HTA review. The reason for reappraising trials from the previous HTA review were twofold: first, to ensure consistency with appraisal of the newer studies, and, second, because we have access to new information from papers that were published after the inclusion date for the previous review. Only primary studies were appraised. Secondary analyses of previously published data were not assessed. Similarly, if a trial was reported in multiple publications, only one quality assessment of the trial was conducted (all publications for that trial were assessed together). In total, 86 trials were assessed (11 induction studies, 73 maintenance studies and two studies of both induction and maintenance treatment). Quality assessments of included trials are presented in Appendix 4. The two trials of both induction and maintenance treatment are repeated in both of these tables.
Overall assessment
The 86 included RCTs49,51,58,59,71–152 were of variable quality, but all appear to be flawed. However, as a result of reporting omissions, for most of the trials it was difficult to make a general assessment regarding quality. The quality appraisal should, therefore, be noted with caution. In fact, six72,73,95–98 of the 14 induction trials, 4075–85,91–94,99–122,153 of the 74 maintenance trials, and one123 of the two trials of both induction and maintenance either did not report, or lacked clarity on, at least five of the 10 items constituting the quality appraisal assessment.
Only four induction studies71–74 and six maintenance studies58,124–127,150 adequately addressed five or more of the 10 items of the quality appraisal assessment. However, even the reports of these trials omitted important information relating to quality, with six71–74,124,125 of the seven failing to clearly describe the procedure used for allocation concealment, and one58 failing to include an intention-to-treat (ITT) analysis.
Seven of the maintenance studies75,76,78,91–94 and two of the induction studies95,96 did not adequately address any of the items in the quality appraisal assessment. Further details of the quality of included studies, according to individual quality appraisal items, are described as follows.
Treatment allocation
Random allocation
The method of random allocation,71,86,128 including the method of sequence generation, was clearly stated and adequate in only two induction studies71,128 and 18 maintenance studies,86,103,110,112,119,122,124,126,127,129–136,150 whereas 65 studies (nine induction studies72–74,87,95,98,137 and 54 maintenance studies51,58,59,75–85,88,89,91–94,99–102,104–109,111,113–118,120,121,125,138–147,152–155) and both of the studies of induction and maintenance treatment123,148 did not clearly specify the method used. The remaining maintenance study149 used a minimisation technique that included a random element.
Concealment of allocation
The method of concealment of allocation was clearly reported in 12 trials (two induction studies,97,128 nine maintenance studies,58,114,129,130,133,140,147,150,152 and one study136 of both induction and maintenance treatment). Fifty-four trials51,72–74,76–79,81–85,87–89,91–93,95,96,98–100,102–106,108–113,115–120,124,127,131,134,135,139,141,143–145,153–155 did not report any information on allocation concealment, whereas 20 trials71,75,80,86,94,101,107,121–123,125,126,132,136–138,142,146,149,156 provided some information pertaining to allocation concealment but lacked sufficient detail or clarity to demonstrate that allocation was adequately concealed.
Similarity of groups
Baseline characteristics
Fifty-seven trials (48 maintenance studies,51,58,77,80–82,84,86,94,99,100,102,104–109,113–117,119–121,124–127,131,132,134,138,139,141–147,149,150,152,154,156,157 eight induction studies71,72,74,87,97,128,137 and one study123 for induction and maintenance) fully reported baseline characteristics. Nine trials (eight maintenance studies88,89,92,110–112,122,148 and one study148 of both induction and maintenance) reported significant baseline between-group differences for key factors, including PRA grade, number of previous transplants, patient age, pretransplant diabetes mellitus, HLA mismatches and ECD donor kidneys. A further six maintenance studies91,101,130,133,140,155 were rated as ‘partial’ because they reported a baseline difference in patient sex.
The remaining trials (four induction studies,71,95,96,98 26 maintenance studies59,75–80,83–85,93,94,103,107,114,115,118,126,127,129,131,132,142,150,152,153 and one study123 of both induction and maintenance) did not provide sufficient information for a judgement to be made about baseline similarity of groups, either by omitting to report sufficient statistical information, by reporting on a very limited range of patient baseline characteristics or by not reporting any patient baseline characteristics.
Implementation of masking
Treatment allocation masked from participants
Five induction studies,87,96,98,128,137 47 maintenance studies51,59,76,78–80,82–84,86,88,92–94,103,105–108,111,113,116,118,125,126,129–135,138–142,144–149,151–153,155 and both of the studies of induction and maintenance treatment123,148 did not blind participants to treatment allocation.
Only two maintenance studies89,124 and four induction studies71–74 made clear that the participants were blinded to treatment allocation. A further four maintenance studies58,77,143,150 were rated as ‘partial’ because it was reported that participants were blinded for a limited period of time only (until 24 weeks for one study58 and until 12 months for the other three studies.77,143,150
One further induction study95 was rated as ‘unclear’ because, despite being PBO controlled, no further details were reported about blinding. The remaining trials (one induction study97 and 20 maintenance studies75,81,85,91,99–102,104,109,110,112,114,115,117,119–122,127) did not report any information about blinding participants to treatment allocation.
Treatment allocation masked from clinicians
All of the trials that did not blind participants from treatment allocation also failed to mask treatment allocation from clinicians.51,59,76,78–80,82–84,86–88,92–94,96,98,103,105–108,111,113,116,118,123,125,126,128–135,137–142,144–149,151–153,155 An additional induction study97 also stated that treatment allocation was not masked from clinicians (participant blinding was not reported). Similarly, the four induction studies71–74 and two maintenance studies89,124 that reported blinding participants to treatment allocation also masked treatment allocation from clinicians. Again, four maintenance studies58,77,143,150 were rated as ‘partial’ for clinician blinding because blinding occurred for only a limited time, and one induction study95 was rated as ‘unclear’ because, although it was a PBO-controlled trial, no further details were reported about blinding. The other 20 maintenance studies75,81,85,91,99–102,104,109,110,112,114,115,117,119–122,127 did not report any details about clinician blinding.
Treatment allocation masked from outcome assessors
The majority of trials (52 maintenance studies,51,75–77,79–84,86,89,91–94,99–102,104–106,108,109,111–114,116–122,130,131,133,138,140,141,144–149,151–153,155 nine induction studies,71–73,87,95–98,128 and both of the studies123,148 of induction and maintenance treatment) did not report whether outcome assessors were blind to treatment allocation.
One induction study137 and five maintenance studies78,132,134,135,139 made it clear that the outcome assessors were not blinded to treatment allocation. For fifteen trials58,59,74,85,88,103,107,110,115,124–127,129,142 (one induction study74 and 14 maintenance studies58,59,85,88,103,107,110,115,124–127,129,142) it was clear that outcome assessors were blinded for at least one outcome, and a further two maintenance studies143,150 were given a ‘partial’ rating because the outcome assessors were blinded for the first 12 months of the study.
Completeness of trials
Reporting of all a priori outcomes
All trials were rated as ‘unclear’ with regard to reporting of a priori outcomes.51,58,59,71–89,91–135,137–153,155 This was because the trial reports failed to explicitly state whether or not all outcomes defined in the study protocol were reported.
Reporting of loss to follow-up, withdrawals and dropouts
Fifty-four trials adequately reported loss to follow-up, withdrawals and dropouts (by providing numbers and reasons by treatment group). Of these, 45 were maintenance studies,51,58,59,80,81,83,84,88,102,104,106–108,111–114,116,118–120,124–127,130–135,138,139,141,142,144–152,155 eight were induction studies,71–74,87,98,128,137 and one148 was a study of both induction and maintenance treatment. In 22 trials (20 maintenance studies76,85,86,91–94,99–101,103,105,109,110,115,121,122,129,140,143 and two induction studies95,96), the reporting of loss to follow-up, withdrawals and dropouts was inadequate, with key information omitted. A further four trials75,79,97,123 (one induction study,97 two maintenance studies75,79 and one study of both induction and maintenance treatment123) were rated as ‘unclear’. For the study of both induction and maintenance, this was because, despite all of the relevant information being provided, the numbers did not appear to tally. For the other three trials,75,79,97 this was because of the fact that all participants appeared to complete the study but this was not explicitly stated. For the remaining six maintenance studies,77,78,82,89,117,153 information regarding loss to follow-up, withdrawals and dropouts was not reported.
Intention-to-treat analysis
Primarily, a strict definition of ITT was used (all randomised and transplanted participants). According to this definition, 48 trials (seven induction studies71–74,87,98,137 and 41 maintenance studies51,59,77,79,80,84,86,88,89,100–102,104,106–108,110,113,115,117,120,121,124–127,129–131,134,135,139,141–143,146,149–153) were rated as adequately performing an ITT analysis, with 19 trials (three induction studies,128,158,159 14 maintenance studies,58,83,91,114,119,132,133,138,140,144145,147,148,155 and both studies123,148 of induction and maintenance treatment) not performing an adequate ITT analysis. In 16 cases (two induction studies96,97 and 14 maintenance studies75,76,81,82,92–94,99,103,105,109,111,112,116) there was a lack of clarity regarding whether or not an ITT analysis had been conducted. The other five trials (one induction95 and four maintenance studies78,85,118,122) did not report any relevant information regarding whether or not an ITT analysis had been conducted.
A secondary definition of ITT analysis was also used (all randomised and transplanted participants or < 10% excluded). When this definition was applied, 13 of the trials previously rated as inadequate were instead rated as adequate (11 maintenance studies58,83,114,119,132,133,138,140,147,148,155 and both of the studies123,148 of induction and maintenance treatment). Thus, only four trials91,128,144,145 did not perform an adequate ITT analysis. The number of trials rated as ‘unclear’ or ‘not reported’ did not change when this definition of ITT was used.
Applicability of trials to the NHS
Applicability to the current NHS in England
Only 11 trials (one induction study,74 nine maintenance studies51,58,86,114,124,125,132,133,155 and one study123 of both induction and maintenance) were adequately applicable to the current NHS in England. The majority of trials (seven induction studies,71,87,95,97,98,128,137 41 maintenance studies,59,75,77–82,84,85,88,89,93,94,99,101,109,112,115–118,120,129–131,134,135,138,139,141,142,144–152 and one study148 of both induction and maintenance) were limited in some way with regard to applicability to the current NHS in England. In all except one of these trials this was primarily as a result of the fact that patients, donors or organ characteristics were not representative of the current NHS in England (e.g. > 90% deceased donors or ‘suboptimal transplants’ or ‘high risk of rejection population’). In the other trial135 this was primarily owing to a lack of statistical power.
The remaining three induction studies72,73,96 and 23 maintenance studies76,83,91,92,100,102–108,110,111,113,119,121,122,126,127,140,143,153 were rated as ‘unclear’ regarding applicability to the current NHS in England. The primary reason for this was as follows: the study lacked clarity regarding key demographic or patient–donor characteristics (two induction studies73,96 and 10 maintenance studies76,83,91,92,102–104,107,113,140); the study was based on a non-EU population (two induction studies72,159 and 13 maintenance studies100,105,106,108,110,111,119,121,122,126,127,143,153).
Study characteristics
Induction therapies
Thirteen studies71–74,87,95–98,123,128,137,148 were identified focusing on induction therapies.
Details of study characteristics can be found in Appendix 5.
The majority of trials report outcomes up to 1 year, with the period of induction therapy generally continued for up to 14 days. No data for HRQoL were identified. It should be noted that, for some studies, the dose no longer reflects clinical practice; however, there were insufficient data for further analysis. Where a higher and lower dose was used in the RCT, the lower dose was selected for investigation.
Overall, no new evidence has been identified for BAS vs. PBO and additional data has been added to both rATG vs. no induction and BAS vs. no induction (Table 9).96,148,158,160 All data for rATG compared with no induction has been identified by the PenTAG search.
TABLE 9
Overview of included studies for induction therapies
Maintenance therapies
Seventy-five studies were identified focusing on a combination of 30 maintenance therapy comparisons (Table 10). Details of study characteristics can be found in Appendix 5.
TABLE 10
Studies identified for maintenance therapy
Outcomes are reported up to a maximum of 5 years, although the majority of data available is reported at 1 year. No data for HRQoL were identified. As for induction therapy RCTs, in some cases the dose no longer reflects clinical practice; however, there were insufficient data for further analysis. When a higher and lower dose was used in the RCT, the lower dose was selected for investigation.
Other than for the TAC + AZA against CSA + AZA combination, the majority of data were identified by the PenTAG search.
Population characteristics
Induction therapies
Baseline characteristics of trial participants for induction therapy are summarised in Table 11.
TABLE 11
Population baseline characteristics for induction therapies
Mean age across studies ranges from 30.3 to 51.3 years. Men generally represented a higher proportion of the participants (57.5–76.3%) other than in the study reported by Mourad et al.,98 in which men constituted 28.6% and 30.5% of the BAS and rATG arms, respectively.
Earlier papers tended to record cadaveric donors, with no further details; however, newer trials report deceased donors as DCD, DBD and ECD. Four studies71,87,128,148 used only cadaveric donors and one study97 used only living donors. In the remainder of the studies, the donors were either mixed or not reported.
The majority of studies had a high proportion of white participants: 60.3–96.2%. Brennan et al.167 and Kahan et al.72 report a comparatively high percentage of black participants in the BAS and rATG arms, respectively (28.5% and 29.1%; 27% and 34%, respectively).
The mismatching of HLAs ranges from 2.13 to 4 (see Chapter 1, Management of kidney transplant). Although a close antigen match is no longer considered to be critical because immunosuppressive therapy is more effective, a better HLA match may lead to longer the graft survival.
Maintenance therapies
Baseline characteristics of trial participants for maintenance therapy are summarised in Table 12.
TABLE 12
Population baseline characteristics for maintenance therapies
Mean age across studies ranges from 29.6 to 57.1 years. Men represented 50–80% of participants for the bulk of the studies. The studies by Baboolal et al.82 and Campos et al.83 fell slightly below this, with men at 48–49%, whereas Chen et al.121 recruited only 24% and 35% in treatment arms and Grinyo et al.51 recruited 33% and 38%.
As for induction therapies, earlier papers tended to record cadaveric donors, with no further details. Fifteen studies75,77,80–82,88,89,99,116,129,130,134,136,138,148 used only cadaveric donors and no studies used only living. For the remainder of the studies, the donors were either mixed or not reported.
The majority of studies had a high proportion of white participants; however, Jarzembowski et al.99 recruited all African American participants, Ciancio et al.106 recruited Hispanic (29.3% and 30.7%) and African American (26.7% and 32.0%) participants, Chadban et al.152 reported Asian participants to be 38.8%, 46.7% and 40.4% in each arm, Anil Kumar et al.110 recruited 59% and 60% African American participants, and Anil Kumar et al.122 recruited 50–54% African American participants in each arm.
For the maintenance studies, HLA is reported in a variety of formats, making any comparisons between studies difficult. As previously mentioned, the matching of HLAs is no longer considered critical, but may have an impact on graft survival.
Study results
The following outcomes have been addressed for each combination of therapies for both induction and maintenance, with meta-analysis performed where possible:
- mortality
- graft loss
- BPAR
- GRF
- time to BPAR
- severity of BPAR
- adverse effects of treatment
- HRQoL.
We also sought HRQoL outcome data from included RCTs. However, none was reported, so we do not have a section for this outcome.
Furthermore, because of an insufficient number of RCTs within each comparison for induction and maintenance therapies (i.e. 10 or more, as recommended by the Cochrane Handbook201), publication bias has not been investigated with funnel plots.
For severity of BPAR, reporting is generally very poor and it is unclear if all of the people with BPAR have received a Banff classification. Therefore, the results as reported are presented with no further analysis.
Induction therapies
BAS compared with PBO/no induction
The 2005 review identified four RCTs71–74 investigating the effectiveness of BAS compared with PBO. One RCT95 was identified in the review by Yao et al.67
No additional studies were identified in the PenTAG search. No data were identified for HRQoL and time to BPAR.
For BAS compared with no induction, one RCT97 was identified in TA99 and two further RCTs123,128 were identified by the PenTAG search.
Mortality
Participant mortality was recorded at 6 months by three studies.73,74,123 Six studies71–74,97,128 report mortality at 1 year.
As displayed in Table 13 and Figure 8, the OR at 0.5 years for the studies by Ponticelli et al.,73 Albano et al.123 and Lawen et al.74 indicates that BAS is associated with lower odds of mortality, although the results are not statistically significant (OR 0.36, 95% CI 0.13 to 1.01).
TABLE 13
Mortality for BAS vs. PBO/no induction

FIGURE 8
Forest plot: mortality for BAS vs. PBO/no induction.
Pooled results at 1 year for the studies by Lawen et al.74 and Sheashaa et al.97 also display no statistically significant difference between BAS and PBO/no induction up to 1 year, which is in agreement with the previous HTA65 (OR 0.95, 95% CI 0.49 to 1.87). The effect estimate for the Sheashaa et al.97 study at 3, 5, 7 and 10 years also shows no difference between arms.
Graft loss
Of the seven studies in this group,71–74,97,123,128 three studies73,74,123 recorded graft loss at 6 months and six studies71–74,97,128 at 1 year (Table 14 and Figure 9).
TABLE 14
Graft loss for BAS vs. PBO

FIGURE 9
Forest plot: graft loss for BAS vs. PBO/no induction.
At both time points the OR may indicate some benefit of BAS compared with PBO or no induction in reducing graft loss (0.5 years: OR 0.78, 95% CI 0.50 to 1.22; 1 year: OR 0.82, 95% CI 0.56 to 1.21). However, this estimate must be treated with caution because of the wide CIs indicating a lack of statistical significance.
The one study97 reporting results at 3, 5, 7 and 10 years showed no statistically significant difference between arms (see Table 14).
Graft function
Pooled analysis for GRF measured as CRC (Table 15 and Figure 10) implies no beneficial effect of BAS compared with PBO [0.5 years: weighted mean difference (WMD) –1.38 ml/minute/1.73 m2, 95% CI –5.96 to 3.20 ml/minute/1.73 m2; 1 year: WMD 1.93 ml/minute/1.73 m2, 95% CI –0.97 to 4.83 ml/minute/1.73 m2].71–73,97,123 In particular, results for 0.5 years must be treated with caution because of the substantial heterogeneity across studies (I2 = 83.4%). It should also be noted that, at 1 year, the study reported by Kahan et al.,72 which indicates an improved GRF for participants on BAS, had a higher percentage of African American participants (34% and 27%) who generally exhibit poor long-term graft survival compared with other ethnic groups.72
TABLE 15
Pooled analysis for BAS vs. PBO/no induction: GRF

FIGURE 10
Forest plot: GRF for BAS vs. PBO/no induction. SD, standard deviation; WMD, weighted mean difference.
Data up to 10 years reported by Sheashaa et al.97 (Table 16) indicate no statistically significant difference between BAS and no induction.
TABLE 16
Graft function for BAS vs. no induction (unpooled)
Biopsy-proven acute rejection
The results of BPAR at 0.5 years are inconclusive because of the substantial heterogeneity across studies (I2 = 80.7%).71,73,74,123 In contrast, at 1 year, BAS statistically significantly reduced BPAR compared with PBO/no induction (OR 0.53, 95% CI 0.40 to 0.70, I2 = 0.0%) (Table 17 and Figure 11).72–74,97,128 Furthermore, the report by Sheashaa et al.97 indicates this effect is maintained up to 10 years (OR 0.41, 95% CI 0.18 to 0.96).97
TABLE 17
Pooled analysis for BAS vs. PBO: BPAR

FIGURE 11
Forest plot: BPAR for BAS vs. PBO.
Severity of biopsy-proven acute rejection
Six studies71–74,97,123 report severity of BPAR (Table 18). Overall, Table 18 indicates that BAS may be associated with less severe exacerbations of BPAR.
TABLE 18
Severity of BPAR for BAS vs. PBO
Time to biopsy-proven acute rejection
Only one study128 reported time to BPAR (Table 19). In general, the results seem similar between arms, although no induction has a broader range (BAS 35–267 days, no induction 10–364 days).
TABLE 19
Time to BPAR for BAS vs. no induction
Summary of results for BAS compared with PBO/no induction
Pooled results indicate no statistically significant difference between BAS and PBO/no induction for mortality up to 1 year (six studies71–74,97,128) (OR 0.95, 95% CI 0.49 to 1.87).
The effect estimate for the Sheashaa et al.97 study at 3, 5, 7 and 10 years also shows no difference between arms.97
No statistically significant difference is found between BAS and PBO/no induction for graft loss (six studies71–74,97,128) (0.5 years OR 0.78, 95% CI 0.50 to 1.22; 1 year OR 0.82, 95% CI 0.56 to 1.21). This is also the case for the single study when follow-up continues up to 10 years.97
Pooled analysis for GRF measured as CRC implies no beneficial effect of BAS compared with PBO (0.5 years, WMD –1.38 ml/minute/1.73 m2, 95% CI –5.96 to 3.20 ml/minute/1.73 m2; 1 year, 1.93, 95% CI –0.97 to 4.83 ml/minute/1.73 m2).71–73,97,123
The results of BPAR at 0.5 years are inconclusive because of the substantial heterogeneity across studies71,73,74,123 (I2 = 80.7%). In contrast, at 1 year, BAS statistically significantly reduced BPAR compared with PBO/no induction (OR 0.53, 95% CI 0.40 to 0.70; I2 = 0.0%).72–74,97,128 Furthermore, the report by Sheashaa et al.97 indicates that this effect is maintained up to 10 years (OR 0.41, 95% CI 0.18 to 0.96).97 In general, severity of BPAR appeared reduced with BAS.
rATG vs. no induction
Both RCTs for this comparison were identified via the PenTAG search.96,148
Mortality
Two trials96,148 provided data on mortality for rATG vs. no induction (Table 20). Follow-up data are provided to only 1 year.96 No clear evidence of a difference between arms is visible, as the OR is close to ‘1’ and the CIs are wide.
TABLE 20
Mortality for rATG vs. no induction
Graft loss
Two trials96,148 provide graft loss data for rATG vs. no induction (Table 21). For both studies,96,148 CIs are extremely wide, crossing an OR of 1, indicating no statistical difference between arms.
TABLE 21
Graft loss for rATG vs. no induction
Graft function
No studies reported GRF.
Biopsy-proven acute rejection
Two studies96,148 report on BPAR for rATG vs. no induction for 0.5 years and 1 year (Table 22). The data at 1 year suggest a statistically significant beneficial effect for rATG (OR 0.41, 95% CI 0.24 to 0.52).96
TABLE 22
Biopsy-proven acute rejection for rATG vs. no induction
Severity of biopsy-proven acute rejection
One study148 reports severity of BPAR at 0.5 years (Table 23). For people identified with BPAR, the occurrence of the most severe classification was 10.7% for rATG and 6.4% for no induction. For Banff classification II, there is a greater association with no induction (rATG 25%, no induction 36.2%).
TABLE 23
Biopsy-proven acute rejection for rATG vs. no induction
Time to biopsy-proven acute rejection
Time to BPAR is reported by one study96 (Table 24), in which more participants experience BPAR at 7–10 days with no induction than with rATG.
TABLE 24
Time to BPAR for rATG vs. no induction
Summary of results for rATG vs. no induction
Only two studies96,148 report rATG vs. no induction. No statistically significant difference was seen for mortality, graft loss or GRF. For BPAR, the data at 1 year suggest a statistically significant beneficial effect for rATG (OR 0.41, 95% CI 0.24 to 0.52) and for severity of BPAR; at Banff classification II, there are greater odds of association with no induction (1 year: OR 0.09, 95% CI 0.01 to 0.73).
BAS vs. rATG
The RCT reported by Lebranchu et al.87 was identified in the 2005 review. The PenTAG search retrieved a further two RCTs: Brennan et al.137 and Mourad et al.98 All three RCTs87,98,137 had a maintenance therapy comprising CSA, MMF and CCSs.
Mortality
The comparison between BAS and rATG for mortality is reported by three studies87,98,137 (Table 25 and Figure 12). Two studies are pooled with 1-year results where no statistically significant effect is seen between arms (OR 1.03, 95% CI 0.35 to 3.00).98,137
TABLE 25
Mortality for BAS vs. rATG
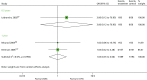
FIGURE 12
Forest plot: mortality for BAS vs. rATG.
Graft loss
Data from three trials87,98,137 were pooled at the 1-year time point (Table 26 and Figure 13). Although the OR indicates lower odds of graft loss associated with rATG, the effect is not statistically significant (OR 1.36, 95% CI 0.61 to 3.03). There was no evidence of heterogeneity across studies. For the individual study87 at 0.5 years there was no statistically significant effect for BAS or rATG.
TABLE 26
Graft loss for BAS vs. rATG

FIGURE 13
Forest plot: graft loss for BAS vs. rATG.
Graft function
Only Lebranchu et al.87 report GRF, with results at 0.5 years and 1 year (Table 27). The MD for CRC of 6.10 ml/minute/1.73 m2 at 1 year in favour of BAS is not statistically significant (p = 0.1103).
TABLE 27
Graft function for BAS vs. rATG
Biopsy-proven acute rejection
A total of three studies87,98,137 report on BPAR for BAS vs. rATG (Table 28 and Figure 14). At both 0.5 years and 1 year, the 95% CIs imply a lack of statistically significant difference between treatments (0.5 years, OR 1.00, 95% CI 0.24 to 4.24; 1 year, OR 1.57, 95% CI 0.95 to 2.61). For Brennan et al.,137 as a much larger study with narrower CIs, rATG appears to reduce BPAR, although this effect is lost when pooled with the smaller studies.
TABLE 28
Biopsy-proven acute rejection for BAS vs. rATG
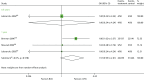
FIGURE 14
Forest plot: BPAR for BAS vs. rATG.
Severity of biopsy-proven acute rejection
Two studies87,98 report on severity of BPAR, although results are not provided for all Banff classifications (Table 29). No difference is seen between treatments.
TABLE 29
Severity of BPAR for BAS vs. rATG
Time to biopsy-proven acute rejection
Time to BPAR is reported by two studies87,98 (Table 30). Neither of the studies87,98 revealed a statistically significant difference between BAS and rATG, despite the study by Mourad et al.98 reporting a mean time for BAS of 155 days (SD 196.27 days) and for rATG of 35 days (SD 30.19 days).
TABLE 30
Time to BPAR for BAS vs. no rATG
Maintenance therapies
TAC + AZA vs. CSA + AZA
Fourteen studies75,76,79–85,88,99,100,104,148 were identified using this combination. Where possible, meta-analysis has been performed. Results are presented for all outcomes, other than HRQoL where no evidence was reported.
Mortality
Ten studies76,79,80,83,84,88,99,100,104,148 report mortality, with meta-analysis possible at the 0.5- and 1-year time points (Table 31 and Figure 15). All studies76,79,80,83,84,88,99,100,104,148 are presented graphically on the forest plot to provide a visual overview (see Figure 15). At 0.5 years, pooled results of only two studies84,148,164,165 generate an OR of 0.54 (95% CI 0.18 to 1.62), indicating lower odds of mortality for TAC; however, the large CIs indicate a low level of precision, and, as they all overlap, the null value (OR = 1) there is unlikely to be a significant difference between treatments. Although the OR at 1 year, which includes eight studies,76,80,83,84,88,99,100,104 has shifted to 1.51, indicating reduced odds of mortality in the CSA arm, the 95% CI of 0.75 to 3.06 also suggests no significant difference between treatments. Heterogeneity across studies for the 1-year time point is low and may not be important at this level according to the Cochrane Handbook201 (I2 = 14.8%). Mayer et al.88 report mortality up to 5 years; however, the results are consistent with earlier time points and indicate no difference between arms (OR 1.20, 95% CI 0.69 to 2.07).
TABLE 31
Mortality for TAC + AZA vs. CSA + AZA

FIGURE 15
Forest plot: mortality for TAC + AZA vs. CSA + AZA.
Graft loss check
Graft loss is reported for 10 trials76,79,80,83,84,88,99,100,104,148 (Table 32 and Figure 16). Results were pooled for the 0.5-, 1- and 2-year time points. The pooling of trials reported by Margreiter et al.84 and Charpentier et al.148 at 0.5 years gives an OR of 0.45 (95% CI 0.24 to 0.84), which is statistically significant in favour of TAC.84,148 The 1-year time point is more reliable, at which seven studies are pooled (see Table 32), generating an OR of 1.18 (95% CI 0.72 to 1.93). However, as with mortality, the results for graft loss suggest no difference between TAC and CSA. This lack of statistical significance for either treatment remains at 5 years (OR 0.92, 95% CI 0.61 to 1.40).
TABLE 32
Graft loss for TAC + AZA vs. CSA + AZA

FIGURE 16
Forest plot: graft loss for TAC + AZA vs. CSA + AZA.
Graft function
Graft function was measured and reported by four studies,75,76,79,84 with effects measured from 0.08 to 3 years. No meta-analysis is provided for GRF, as the results are presented in a number of ways and are not appropriate for pooling. In general, Table 33 shows some variation between arms with large SDs; for example, results presented by Margreiter et al.84 at 1 year imply an improved GRF for TAC as opposed to CSA [68.9 (SD 23.2) ml/minute/1.73 m2 and 61.8 (SD 23.2) ml/minute/1.73 m2, respectively], which is in contrast with the study of Van Duijnhoven et al.,75 who report 60.2 ml/minute/1.73 m2 (range 11.5–86.2 ml/minute/1.73 m2) and 64.9 ml/minute/1.73 m2 (range 29.5–84.5 ml/minute/1.73 m2), respectively. This conflict between studies is seen at all time points.
TABLE 33
Graft function for TAC + AZA vs. CSA + AZA
Biopsy-proven acute rejection
All time points from 0.08 to 4 years reveal ORs of < 1 for BPAR, indicating that TAC is more effective than CSA in reducing this outcome (Table 34 and Figure 17).76,79–84,88,99,100,104,148 BPAR outcomes were reported by nine studies76,81–84,88,99,100,104 at 1 year, where pooled analysis gives an OR of 0.50 and 95% CI 0.39 to 0.64. Minimal heterogeneity is indicated across the studies at year 1 (I2 = 8.1%). Mayer et al.88 report BPAR at 4 years, when the beneficial effect of TAC appears to be maintained (OR 0.38, 95% CI 0.25 to 0.57).
TABLE 34
Biopsy-proven acute rejection for TAC + AZA vs. CSA + AZA

FIGURE 17
Forest plot: BPAR for TAC + AZA vs. CSA + AZA. Note: only the low-dose TAC arm is used for Laskow et al., as this is closest to the dose used in practice.
Severity of biopsy-proven acute rejection
Four trials82,84,100,148 report on severity of BPAR from 6 months to 2 years (Table 35). For the studies by Baboolal et al.82 and Hardinger et al.,100 at 1 year, no participants with BPAR experienced Banff grade III for either arm.82,100 At 6 months, Charpentier et al.148 report the proportion of people with BPAR classified as Banff III as 10.7% for TAC and 15.4% for CSA and by 2 years Margreiter et al.84 report 6.4% and 16.8% of people with BPAR experiencing Banff III, for TAC and CSA, respectively.
TABLE 35
Severity of BPAR at 6 months for TAC + AZA vs. CSA + AZA
Time to biopsy-proven acute rejection
Time to first BPAR is reported by only two studies,82,83 with contrasting results (Table 36). The results reported by Baboolal et al.82 indicate that BPAR is achieved more quickly for participants receiving TAC (35 days, SD 13 days) rather than CSA (59 days, SD 38 days).
TABLE 36
Time to BPAR for TAC + AZA vs. CSA + AZA
Summary of results for TAC + AZA vs. CSA + AZA
- Ten studies76,79,80,83,84,88,99,100,104,148 report mortality, with meta-analysis possible at the 0.5- and 1-year time points. At 0.5 years, pooled results of only two studies84,148 generates an OR of 0.54, 95% CI 0.18 to 1.62, indicating lower odds of mortality for TAC; however, the large CIs overlap the null value (OR = 1) therefore there is unlikely to be a significant difference between treatments. Although the OR at 1 year, which includes eight studies,76,80,83,84,88,99,100,104 has shifted to 1.51, indicating reduced odds of mortality in the CSA arm, the 95% CI of 0.75 to 3.06 also suggest no significant difference between treatments. Heterogeneity across studies for the 1-year time point is low and may not be important at this level according to the Cochrane Handbook201 (I2 = 14.8%).
- Graft loss is reported for 10 trials.76,79,80,83,84,88,99,100,104,148 Results were pooled for the 0.5-, 1- and 2-year time points. The pooling of trials reported by Margreiter et al.84 and Charpentier et al.148 at 0.5 years give an OR of 0.45 (95% CI 0.24 to 0.84), which is statistically significant in favour of TAC.84,148 The 1-year time point, where seven studies76,83,84,88,99,100,104 are pooled, generates an OR of 1.18 and a 95% CI 0.72 to 1.93, which is not statistically significant, and this remains the case at 5 years.
- Graft function was measured and reported by four studies,75,80,84,98 with effects measured from 0.08 to 3 years. No meta-analysis is possible, as the results are presented in a number of ways and are not appropriate for pooling. In general, there is some variation between arms with large SDs, for example results presented by Margreiter et al.84 at 1 year imply an improved GRF for TAC as opposed to CSA [68.9 ml/minute/1.73 m2 (SD 23.2 ml/minute/1.73 m2) and 61.8 ml/minute/1.73 m2 (SD 23.2 ml/minute/1.73 m2), respectively], which is in contrast with Van Duijnhoven et al. 2002, who report 60.2 ml/minute/1.73 m2 (range 11.5–86.2 ml/minute/1.73 m2) and 64.9 ml/minute/1.73 m2 (range 29.5–84.5 ml/minute/1.73 m2), respectively. This conflict between studies is seen at all time points.
- All time points from 0.08 to 4 years reveal ORs of < 1 for BPAR, indicating that TAC is more effective than CSA in reducing this outcome. BPAR outcomes were reported by nine studies76,81–84,88,99,100,104 at 1 year, where pooled analysis gives an OR of 0.50 and a 95% CI of 0.39 to 0.64. Low heterogeneity is indicated across the studies at year 1 (I2 = 8.1%). Mayer et al.88 report BPAR at 4 years, where the beneficial effect of TAC appears to be maintained (OR 0.38, 95% CI 0.25 to 0.57).
- Four trials82,84,100,148 report on severity of BPAR from 6 months to 2 years. For the studies by Baboolal et al.82 and Hardinger et al.,100 at 1 year, no participants with BPAR experienced Banff grade III for either arm.82,100 At 6 months, Charpentier et al.148 report the proportion of people with BPAR classified as Banff III as 10.7% for TAC and 15.4% for CSA and, by 2 years, Margreiter et al.84 report 6.4% and 16.8% of people with BPAR experiencing Banff III, for TAC and CSA, respectively.
- Time to first BPAR is reported by only two studies, with contrasting results. However, the difference between arms for Campos et al.83 is not statistically significant (p = 0.6631). The results reported by Baboolal et al.82 indicate that BPAR is achieved more quickly for participants receiving TAC (35 days, SD 13 days) rather than CSA (59 days, SD 38 days).
CSA + MMF vs. CSA + AZA
Seven studies77,78,86,89,101,104,138 report on this combination of immunosuppressive therapies, with a follow-up of 5 years. All outcomes have been reported other than HRQoL.
Mortality
Seven studies77,78,86,89,101,104,138 report on mortality for CSA + MMF compared with CSA + AZA. Pooling results of five studies78,86,101,104,138 for this combination imply no difference between arms at 1 year, with no evidence of heterogeneity across studies (Table 37 and Figure 18). The ORs switch from > 1 to < 1 for the pooled results at 1 and 3 years; however, the CIs cross ‘OR = 1’ in both cases, suggesting that there may be no difference between MMF and AZA (OR 1.19, 95% CI 0.47 to 3.02 and OR 0.56, 95% CI 0.26 to 1.23, respectively). The study reported by Tuncer et al.78 provides data at 5 years, which also indicates no preference for either MMF or AZA (OR 0.73, 95% CI 0.15 to 3.50).
TABLE 37
Mortality for CSA + MMF vs. CSA + AZA

FIGURE 18
Forest plot: mortality for CSA + MMF vs. CSA + AZA.
Graft loss
Five studies77,86,89,104,138 report on graft loss, with results pooled at 0.5- and 1-year time points (Table 38 and Figure 19). However, the 0.5-year time point has only two studies77,89 and a substantial level of heterogeneity (I2 = 72.2%), therefore the OR of 0.58 (95% CI 0.04 to 0.59), which indicates that MMF is more effective at reducing graft loss, must be treated with caution.201 The results for 1 year suggest no difference between arms (OR 0.76, 95% CI 0.38 to 1.50). Merville et al.138 appear to show more of an effect in favour of MMF; however, the population is much smaller than that for the Tricontinental study89 and Sadek et al.86 and Weimer et al.104 found no evidence of graft loss in either arm.
TABLE 38
Pooled results of graft loss for CSA + MMF vs. CSA + AZA

FIGURE 19
Forest plot: graft loss for CSA + MMF vs. CSA + AZA.
Graft function
Only Merville et al.138 reported on this outcome. At both 6 months and 1 year there was no statistically significant difference in mean GRF (0.5 years; p = 0.7236 and 1 year; p = 0.6584) (Table 39).
TABLE 39
Graft function for CSA + MMF vs. CSA + AZA
Biopsy-proven acute rejection
Six studies77,86,89,101,104,138 report on BPAR. Unlike mortality and graft loss, BPAR analysis reveals that MMF is more beneficial than AZA at 0.5 and 1 year (0.5 years OR 0.50, 95% CI 0.35 to 0.72; 1 year OR 0.47, 95% CI 0.36 to 0.62) (Table 40 and Figure 20).104
TABLE 40
Pooled results of BPAR for CSA + MMF vs. CSA + AZA
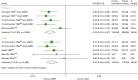
FIGURE 20
Forest plot: BPAR for CSA + MMF vs. CSA + AZA.
Severity of biopsy-proven acute rejection
Two studies were available for 0.5 years77,89 and one study138 for 1 year, although sample numbers are low for this study (Table 41). Overall, at 0.5 years the more severe classification of Banff III appears to be more likely in the AZA arm for people with BPAR (CSA 9.1%, AZA 15.9% for Sollinger et al.;77 CSA 5.9%, AZA 11.9% for the Tricontinental group 199689).
TABLE 41
Severity of BPAR at 6 months for CSA + MMF vs. CSA + AZA
Time to biopsy-proven acute rejection
Insufficient data are provided for analysis on this outcome. Merville et al.138 report 48.5 days for MMF and 43.7 days for AZA.138
Summary of results for CSA + MMF vs. CSA + AZA
- Seven studies77,78,86,101,104,138,202 report on mortality for CSA + MMF vs. CSA + AZA. Pooling results of five studies78,86,101,104,138 for this combination imply no difference between arms at 1 year, with no evidence of heterogeneity across studies. The ORs switch from > 1 to < 1, for the pooled results at 1 and 3 years; however, the CIs cross ‘OR = 1’ in both cases, suggesting that there may be no difference between MMF and AZA (OR 1.19, 95% CI 0.47 to 3.02; and 0.56, 95% CI 0.26 to 1.23). The study reported by Tuncer et al.78 provides data at 5 years, which also indicate no preference for either MMF or AZA (OR 0.73, 95% CI 0.15 to 3.50).
- Five studies77,86,104,138,202 report on graft loss, with results pooled at 0.5- and 1-year time points. However, the 0.5-year time point has only two studies77,89 and a substantial level of heterogeneity (I2 = 72.2%); therefore, the OR of 0.58 (95% CI 0.04 to 0.59), which indicates that MMF is more effective at reducing graft loss, must be treated with caution.201 The results for 1 year suggest no difference between arms (OR 0.76, 95% CI 0.38 to 1.50). The study by Merville et al.138 appears to show more of an effect in favour of MMF; however, the population is much smaller than that for the Tricontinental study89 and Sadek et al.86
- Two studies were available for 0.5 years77,89 and one study138 for 1 year. Overall, at 0.5 years the more severe classification of Banff III appears to be more likely in the AZA arm for people with BPAR (CSA 9.1%, AZA 15.9% for Sollinger et al.;77 CSA 5.9%, AZA 11.9% for the Tricontinental group89). Insufficient data are provided for analysis on time to BPAR. Merville et al.138 report a slightly more rapid rate of 48.5 days for MMF and 43.7 days for AZA.
TAC + MMF vs. CSA + AZA
Two studies129,139 compared these combinations. GRF and time to BPAR are not reported.
Mortality
Wlodarczyk et al.139 report mortality at 0.5 years and Vacher-Caponat et al.129 report at 1 year (Table 42). At 1 year, there are twice as many deaths for TAC + MMF as for CSA + AZA; however, although the OR is > 1, the wide 95% CIs imply no statistically significant difference between arms.
TABLE 42
Mortality for TAC + MMF vs. CSA + AZA
Graft loss
As with mortality, there is only one study for each time point of 0.5 years139 and 1 year129 (Table 43). The wide CIs highlight the low precision and indicate no difference between arms.
TABLE 43
Graft loss for TAC + MMF vs. CSA + AZA
Biopsy-proven acute rejection for TAC + MMF vs. CSA + AZA
Only two studies129,139 have reported BPAR: one study at 0.5 years139 and one study at the 1-year time point129 (Table 44). In both cases the OR is < 1, indicating that TAC + MMF is associated with lower odds of BPAR (OR 0.64, 95% CI 0.42 to 0.98; OR 0.35, 95% CI 0.15 to 0.83, respectively).
TABLE 44
Biopsy-proven acute rejection for TAC + MMF vs. CSA + AZA
Severity of biopsy-proven acute rejection
This outcome is reported only by Vacher-Caponat et al.,129 with no participants experiencing Banff II and III in the TAC + MMF arm, but with 14.3% and 4.8% reported in the CSA + AZA arm, respectively (Table 45).
TABLE 45
Severity of BPAR at 1 year for TAC + MMF vs. CSA + AZA
Summary for TAC + MMF vs. CSA + AZA
- Wlodarczyk et al.139 report mortality at 0.5 years and Vacher-Caponat et al.129 report mortality at 1 year. In both cases the OR is > 1, indicating that TAC + MMF is associated with greater odds of mortality; however, the 95% CIs cross ‘OR = 1’, implying no statistically significant difference between arms.
- Severity of BPAR is reported by only one study,129 with the greater proportion of people experiencing Banff II and III in the CSA + AZA arm.
TAC + MMF vs. CSA + MMF
This combination of immunosuppressive therapy was identified in five RCTs,51,102,103,130,203 with all outcomes other than HRQoL reported. The RCT reported by Grinyo et al.51 is also known as the SYMPHONY study.
Mortality
The effect estimate of five pooled studies51,102,103,130,203 at 1 year suggests that TAC + MMF is associated with higher odds of mortality (OR 1.62, 95% CI 0.77 to 3.44; Table 46 and Figure 21). However, although there is no evidence of heterogeneity across studies (I2 = 0.0%), the CIs are wide and cross ‘OR = 1’, indicating low precision and a lack of statistical significance. Results for 2 years and 5 years also demonstrate no statistically significant difference between treatments.
TABLE 46
Mortality for TAC + MMF vs. CSA + MMF

FIGURE 21
Forest plot: mortality for TAC + MMF vs. CSA + MMF.
Graft loss
Graft loss is reported for five studies.51,102,103,130,153,203 The OR for pooled results at 1 year and 2 years (1.43 and 1.63, respectively) implies greater odds of graft loss for TAC + MMF; however, the CIs cross ‘OR = 1’, indicating no difference between arms (Table 47 and Figure 22).
TABLE 47
Graft loss for TAC + MMF vs. CSA + MMF

FIGURE 22
Forest plot: graft loss for TAC + MMF vs. CSA + MMF.
Kumar et al.203 report graft loss up to 5 years, with similar results of no difference between arms.
Graft function
Graft function as CRC is reported by three studies51,103,130 up to 3 years (Table 48 and Figure 23). Pooling of results for 1- and 2-year data demonstrated a statistically significant difference in GRF in favour of TAC (WMD 4.22 ml/minute/1.73 m2, 95% CI 1.23 to 7.20 ml/minute/1.73 m2; WMD 5.75, 95% CI 2.76 to 8.74 ml/minute/1.73 m2, respectively). There is low evidence of heterogeneity across the 1-year studies (I2 = 9.8%).
TABLE 48
Graft function for TAC + MMF vs. CSA + MMF
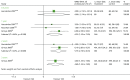
FIGURE 23
Forest plot: GRF for TAC + MMF vs. CSA + MMF.
Biopsy-proven acute rejection for TAC + MMF vs. CSA + MMF
Biopsy-proven acute rejection was reported by five studies.51,102,103,153,203 One-year outcomes provided by four of these studies51,103,153,203 were pooled (Table 49 and Figure 24). The study at 0.5 years by Kumar et al.203 indicates that lower odds of BPAR are associated with TAC. This is in agreement with the pooled results at 1 year, although some heterogeneity is noted across studies (OR 0.59, 95% CI 0.37 to 0.94; I2 = 19.3%). The study reported by Hernández et al.130 at 2 years does not demonstrate a statistical difference between arms (OR 1.22, 95% CI 0.51 to 2.91).
TABLE 49
Biopsy-proven acute rejection for TAC + MMF vs. CSA + MMF

FIGURE 24
Forest plot: BPAR for TAC + MMF vs. CSA + MMF.
Severity of biopsy-proven acute rejection
Two studies51,130 report severity of BPAR separately at 1 and 2 years (Table 50). For year 1, results indicate that for people with BPAR, TAC + MMF and CSA + MMF have a similar proportion experiencing Banff III (TAC + MMF 7.8%; CSA + MMF 7.1%).51 The study by Hernández et al.130 indicates no clear difference for between arms for all three classifications.130
TABLE 50
Severity of BPAR at 1 year for TAC + MMF vs. CSA + MMF
Time to biopsy-proven acute rejection
Mean time to BPAR was reported by Ulsh et al.153 in favour of TAC (Table 51).
TABLE 51
Time to BPAR for TAC + MMF vs. CSA + MMF
Summary of results for TAC + MMF vs. CSA + MMF
- The effect estimate of five pooled studies51,102,103,130,203 at 1 year suggests that TAC + MMF is associated with higher odds of mortality (OR 1.62, 95% CI 0.77 to 3.44). However, although there is no evidence of heterogeneity across studies (I2 = 0.0%), the CIs are wide and cross ‘OR = 1’, indicating low precision and a lack of statistical significance. Results for 2 years and 5 years also demonstrate no statistically significant difference between treatments.
- Graft loss is reported for five studies.51,102,103,122,153 The OR for pooled results at 1 and 2 years (1.43 and 1.63, respectively) implies greater odds of graft loss for TAC + MMF; however, the CIs cross ‘OR = 1’, indicating no statistically significant difference between arms. The lack of difference remains at 5 years for the study reported by Kumar et al.203
- GRF is reported by three studies up to 3 years.51,103,130 Pooling of results for 1- and 2-year data demonstrated a statistically significant difference in GRF in favour of TAC (WMD 4.22 ml/minute/1.73 m2, 95% CI 1.23 to 7.20 ml/minute/1.73 m2; WMD 5.75 ml/minute/1.73 m2, 95% CI 2.76 to 8.74 ml/minute/1.73 m2, respectively).
- BPAR was reported by five studies,51,102,103,122,153 with four studies51,103,122,153 reporting at 1 year as being suitable for meta-analysis. The study at 0.5 years by Kumar et al.203 indicates that lower odds of BPAR are associated with TAC. This is in agreement with the pooled results at 1 year, although some heterogeneity is noted across studies (OR 0.59, 95% CI 0.37 to 0.94; I2 = 19.3%). Two studies51,130 report severity of BPAR separately at 1 year and 2 years with no clear difference in proportion of people with Banff III grading.
- Time to BPAR was reported by Ulsh et al.,153 with a difference in favour of TAC of 88.7 days (p = 0.0001).
TAC + MMF vs. TAC-PR + MMF
Four studies105,123,141,204 are reported investigating all outcomes other than time to BPAR and HRQoL for TAC (immediate release) + MMF vs. TAC-PR (prolonged release) + MMF.
Mortality
Four studies105,123,141,204 report on mortality: two studies report at 0.5 years105,123 and two studies report at 1 year141,204 (Table 52 and Figure 25). At each time point, one of the studies had no deaths in either arm and both ORs indicate no statistical difference (0.5 years, OR 0.65, 95% CI 0.23 to 1.84; 1 year, OR 0.78, 95% CI 0.31 to 2.01).
TABLE 52
Mortality for TAC + MMF vs. TAC-PR + MMF

FIGURE 25
Forest plot: mortality for TAC + MMF vs. TAC-PR + MMF.
Graft loss
Four studies105,123,141,204 report on graft loss: two studies report at 0.5 years105,123 and two studies report at 1 year.141,204 As illustrated by the forest plot (Table 53 and Figure 26), no clear benefit is seen for either immediate-release or TAC-PR with regard to graft loss at 6 months and 1 year. ORs for both are identical and < 1; however, CIs cross ‘OR = 1’, indicating no statistical difference between arms (OR 0.83, 95% CIs 0.30 to 2.30 and 0.47 to 1.47).
TABLE 53
Graft loss for TAC + MMF vs. TAC-PR + MMF

FIGURE 26
Forest plot: graft loss for TAC + MMF vs. TAC-PR + MMF.
Graft function
Graft function is reported by three studies:123,141,204 one study123 for 0.5 years and two studies141,204 for 1 year (Table 54 and Figure 27). Pooling of results at 1 year demonstrated no statistically significant difference in GRF (WMD 0.21 ml/minute/1.73 m2, 95% CI –2.10 to 2.53 ml/minute/1.73 m2); however, the single study by Albano et al.123 suggests immediate-release TAC to be more effective than TAC-PR for GRF (WMD 1.90 ml/minute/1.73 m2, 95% CI to 5.40 ml/minute/1.73 m2).
TABLE 54
Graft function for TAC + MMF vs. TAC-PR + MMF

FIGURE 27
Forest plot: GRF for TAC + MMF vs. TAC-PR + MMF.
Biopsy-proven acute rejection for TAC + MMF vs. TAC-PR + MMF
Three studies105,123,204 report BPAR at 0.5 years and two studies141,204 report at 1 year (Table 55 and Figure 28). Pooling of results at both time points shows no significant difference between arms (OR 1.37 95% CI 1.00 to 1.87; OR 1.03, 95% CI 0.48 to 2.17). Furthermore, moderate heterogeneity exists across studies (I2 = 34.8% and 44.4%).201
TABLE 55
Biopsy-proven acute rejection for TAC + MMF vs. TAC-PR + MMF

FIGURE 28
Forest plot: BPAR for TAC + MMF vs. TAC-PR + MMF.
Severity of biopsy-proven acute rejection
Two studies123,204 report severity of BPAR, both of which indicate that, for people with BPAR, the severity may be reduced with immediate TAC (Table 56).
TABLE 56
Severity of BPAR for TAC + MMF vs. TAC-PR + MMF
Summary for TAC + MMF vs. TAC-PR + MMF
- Four studies105,123,141,204 report on mortality: two studies report at 0.5 years123,204 and two studies report at 1 year.105,141 At each time point, one of the studies had no deaths in either arm and both ORs indicate no statistical difference (0.5 years, OR 0.65, 95% CI 0.23 to 1.84; 1 year, OR 0.78, 95% CI 0.31 to 2.01).
- Four studies105,123,141,204 report on graft loss: two studies report at 0.5 years105,123 and two studies report at 1 year.141,204 No clear benefit is seen for either immediate-release TAC or TAC-PR with regard to graft loss at 6 months and 1 year. ORs for both are identical and < 1; however, CIs cross ‘OR = 1’, indicating no statistical difference between arms (OR 0.83, 95% CI 0.30 to 2.30; and 95% CI 0.47 to 1.47). GRF is reported by three studies:123,141,205 one study for 0.5 years123 and two studies for 1 year.141,205 Pooling of results at 1 year demonstrated no statistically significant difference in GRF (WMD 0.21 ml/minute/1.73 m2, 95% CI –2.10 to 2.53 ml/minute/1.73 m2); however, the single study by Albano et al.123 suggests that TAC is more effective than TAC-PR for GRF (WMD 1.90 ml/minute/1.73 m2, 95% CI 1.70 to 2.10 ml/minute/1.73 m2).
MMF + TAC vs. MPS + TAC
As only one trial106 reported outcomes for this combination, results are presented in summary tables (Tables 57 and 58).
TABLE 57
Summary of outcomes for MMF + TAC vs. MPS + TAC
TABLE 58
Graft function for MMF + TAC vs. MPS + TAC
In contrast with other outcomes, GRF displays a significant difference in favour of MPS at 0.5 years and 1 year (0.5 years, MD –1.317; 1 year, MD –1.9019; p < 0.0001) (see Table 58). This effect is lost at later time points.
Overall, there appears to be no discernible difference between arms, as all CIs are wide and cross ‘OR = 1’. Time to BPAR is not reported.
Summary for MMF + CSA vs. MPS + TAC
Only one study106 was identified for this combination. No difference was identified between interventions, other than for GRF, where a statistically significant difference in favour of MPS at 0.5 years and 1 year (p < 0.0001) was noted. This effect is lost at later time points.
MMF + CSA vs. MPS + CSA
Only one trial using this combination is reported by Salvadori et al.;124 therefore, all outcomes are included in a summary table up to 1 year (Table 59). Overall, the OR indicates that MPS is associated with lower mortality (OR 4.12, 95% CI 0.46 to 37.14); however, the CIs are wide and the effect is not statistically significant. Graft loss initially has better odds for MPS at 0.5 years; however, this reverses at 1 year. Again, CIs imply no statistical significance. BPAR and severity of BPAR show no difference between interventions. GRF and time to BPAR are not reported.
TABLE 59
Summary of outcomes for MMF + CSA vs. MPS + CSA
Summary for MMF + CSA vs. MPS + CSA
- Only one trial reported by Salvadori et al.124 uses this combination. GRF and time to BPAR are not reported. All other results indicate no significant difference between MMF and MPS.
BEL + MMF vs. CSA + MMF
Three studies60,206,207 report on this combination of therapies. Time to BPAR and HRQoL are not reported
Mortality
Three studies60,125,206 report 1-year outcomes, with the Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial (BENEFIT)60 and the BENEFIT–Extended Criteria Donors (BENEFIT-EXT)142 providing data for up to 5 years. The ORs generally fall at < 1 for all time points, indicating that BEL has a lower association with mortality than CSA (Table 60 and Figure 29). However, the CIs indicate that this is not statistically significant.
TABLE 60
Mortality for BEL + MMF vs. CSA + MMF

FIGURE 29
Forest plot: mortality for BEL + MMF vs. CSA + MMF.
Graft loss
The OR for graft loss is also reported by three studies60,125,206 up to 5 years. Pooled results indicate that BEL may be preferable to CSA, although the results are not statistically significant (1 year, OR 0.74, 95% CI 0.42 to 1.31) (Table 61 and Figure 30). However, at 5 years, there may be more confidence that this effect is true (OR 0.40, 95% CI 0.19 to 0.87).
TABLE 61
Graft loss for BEL + MMF vs. CSA + MMF

FIGURE 30
Forest plot: graft loss for BEL + MMF vs. CSA + MMF.
Graft function
Graft function is reported by three studies60,125,206 up to 5 years (Table 62 and Figure 31). The results must be treated with caution because of substantial heterogeneity across studies, which may be caused by variations in methods of calculation and measurement of GRF (I2 = 73.6–91.2%). Pooling of results for 1- and 3-year data demonstrated a statistically significant difference for GRF in favour of BEL (WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 4.10 ml/minute/1.73 m2, and WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2, respectively).
TABLE 62
Graft function for BEL + MMF vs. CSA + MMF

FIGURE 31
Forest plot: GRF for BEL + MMF vs. CSA + MMF.
Biopsy-proven acute rejection
The results for BPAR indicate substantial heterogeneity for the 1-, 2- and 3-year time points (I2 = 58.7%, 38.4% and 62.2%, respectively) (Table 63).60,206,207 Overall, participants in the CSA arm appear to be less likely to experience BPAR at between 1 and 5 years, as opposed to those in the BEL arm (1 year, OR 1.53, 95% CI 0.78 to 3.02).
TABLE 63
Biopsy-proven acute rejection for BEL + MMF vs. CSA + MMF
Severity of biopsy-proven acute rejection
Three studies60,125,142 report severity of BPAR at 1 year (Table 64). Overall, there is no clear difference between arms in the proportion of people with BPAR experiencing Banff II or III classification.201,206
TABLE 64
Severity of BPAR for BEL + MMF vs. CSA + MMF
Summary for BEL + MMF vs. CSA + MMF
- The OR for graft loss up is also reported by three studies60,125,142 up to 4 years. Pooled results indicate that BEL may be preferable to CSA, although the results are not statistically significant (1 year, OR 0.74, 95% CI 0.42 to 1.31). However, at 5 years, there may be more confidence that this effect is true (OR 0.40, 95% CI 0.19 to 0.87).
- GRF is reported by three studies60,125,142 up to 5 years. The results must be treated with caution because of substantial heterogeneity across studies, which may be caused by variations in methods of calculation and measurement of GRF (I2 = 73.6–91.2%). Pooling of results for 1- and 3-year data demonstrated a statistically significant difference for GRF in favour of BEL (WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 14.10 ml/minute/1.73 m2 and WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2, respectively).
- In contrast with previous outcomes, results for BPAR are more clear for the three studies.60,125,142 However, there is substantial heterogeneity across studies at the 1-, 2- and 3-year time points (I2 = 58.7%, 38.4% and 62.2%, respectively).60,125,142 Overall, participants in the CSA arm appear to be less likely to experience BPAR at between 1 and 5 years, as opposed to those in the BEL arm (1 year, OR 1.53, 95% CI 0.78 to 3.02). Three studies60,125,142 report severity of BPAR at 1 year. Overall, there is no clear difference between arms in the proportion of people with BPAR experiencing Banff II or III classification.60,125,142
BEL + MMF vs. BEL + SRL vs. TAC + MMF
This combination is reported only in the Ferguson et al.,126 study therefore results are summarised in below (Table 65). Time to BPAR is not reported. Analysis indicates no statistical difference between arms for any outcome; however, the sample size is relatively low (n = 26 and n = 30).
TABLE 65
Summary of outcomes for BEL + MMF vs. BEL + SRL vs. TAC + MMF
EVL + CSA vs. MMF + CSA
Three RCTs131,143,150 investigating this combination of immunosuppressive therapies were identified. All outcomes other than time to BPAR were reported.
Mortality
Mortality is reported at 0.5,150 1131 and 3143 years (Table 66 and Figure 32). Results are pooled for the 1- and 2-year time points, where the OR is > 1, indicating a preference in favour of MMF; however, this is not statistically significant (OR 1.83, 95% CI 0.80 to 4.20; OR 1.06, 95% CI 0.60 to 1.85, respectively). This trend is reflected at 0.5 years and 3 years.
TABLE 66
Mortality for EVL + CSA vs. MMF + CSA
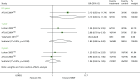
FIGURE 32
Forest plot: mortality for EVL + CSA vs. MMF + CSA.
Graft loss
Three RCTs131,143,150 report graft loss for this combination (Table 67 and Figure 33). There is considerable heterogeneity across studies for 1 and 3 years (I2 = 80.0% and 74.3%, respectively) therefore results must be treated with caution. The study reported by Lorber et al.,143 which favours MMF, appears to be in contrast with the ATLAS study;150 however, there is no statistically significant difference between arms for either trial.
TABLE 67
Graft loss for EVL + CSA vs. MMF + CSA

FIGURE 33
Forest plot: graft loss for EVL + CSA vs. MMF + CSA.
Graft function
Lorber et al.143 provide a median and range for GRF rather than a SD; therefore, results could not be pooled with the ATLAS study150 (Table 68). Overall, there is no significant difference in GRF between EVL + CSA and MMF + CSA (p = 0.1989 to 0.3703).
TABLE 68
Graft function for EVL + CSA vs. MMF + CSA
Biopsy-proven acute rejection
The pooled and unpooled ORs of < 1 for this outcome all suggest that EVL is associated with lower odds of BPAR; however, the CIs indicate a lack of statistical significance (Table 69 and Figure 34).131,143,150 There is no evidence of heterogeneity across studies.
TABLE 69
Biopsy-proven acute rejection for EVL + CSA vs. MMF + CSA

FIGURE 34
Forest plot: BPAR for EVL + CSA vs. MMF + CSA.
Severity of biopsy-proven acute rejection
Severity of BPAR is reported by only Takahashi et al.131 at 1 year (Table 70). No occurrences of Banff II or III classification were reported.
TABLE 70
Severity of BPAR for EVL vs. MMF
Summary for EVL + CSA vs. MMF + CSA
- Three RCTs131,143,150 report graft loss for this combination; however, there is significant heterogeneity across studies for 1 and 3 years (I2 = 80.0% and 74.3%, respectively). The study reported by Lorber et al.,143 which favours MMF, appears to be in contrast with the ATLAS study,150 which favours EVL; however, there is no statistical difference between arms for either trial.
- The pooled and unpooled ORs of < 1 for BPAR all suggest that EVL is associated with lower odds; however, the CIs indicate a lack of statistical significance.131,143,150 There is no evidence of heterogeneity across studies. Severity of BPAR is reported only by Takahashi et al.131 at 1 year, when no occurrences of Banff II or III classifications are reported.131
EVL + CSA vs. MPS + CSA
Three RCTs107,144,152 were identified reporting on this combination. All outcomes other than time to BPAR and HRQoL are reported.
Mortality
Pooled analysis of three studies107,144,152 at 1 year for mortality indicates no significant difference between EVL + CSA and MPS + CSA (OR 1.02, 95% CI 0.42 to 2.45; Table 71 and Figure 35). No heterogeneity was evident across studies.
TABLE 71
Mortality for EVL + CSA vs. MPS + CSA
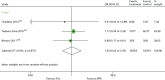
FIGURE 35
Forest plot: mortality for EVL + CSA vs. MPS + CSA.
Graft loss
The OR for graft loss is generated from three pooled studies,107,144,152 which indicates that EVL may be preferable in reducing graft loss; however, this result is not statistically significant (OR 0.65, 95% CI 0.15 to 2.87) (Table 72 and Figure 36). Furthermore, moderate heterogeneity is noted across studies.
TABLE 72
Graft loss for EVL + CSA vs. MPS + CSA

FIGURE 36
Forest plot: graft loss for EVL + CSA vs. MPS + CSA.
Graft function
Two studies107,144 report GRF; however, although results are pooled, the heterogeneity between them is extremely high (I2 = 91.2%) (Table 73 and Figure 37). As such, the evidence is unclear as to which treatment may be beneficial.
TABLE 73
Graft function for EVL + CSA vs. MPS + CSA

FIGURE 37
Forest plot: GRF for EVL + CSA vs. CSA + MPS.
Biopsy-proven acute rejection
Biopsy-proven acute rejection is reported by three studies107,144,152 at 1 year. Pooling of results indicates no statistically significant difference between EVL + CSA vs. MPS + CSA (OR 1.01, 95% CI 0.68 to 1.48) (Table 74 and Figure 38).
TABLE 74
BPAR for EVL + CSA vs. MPS + CSA

FIGURE 38
Forest plot: BPAR for EVL + CSA vs. MPS + CSA.
Severity of biopsy-proven acute rejection
The study reported by Tedesco-Silva et al.107 suggests that more people with BPAR receiving MPS experienced Banff II classification; however, there was no difference for Banff III (Table 75). There were no Banff II or III episodes reported in the EVL treatment for Chadban et al.,152 with only one episode among those receiving MPS treatment; however, the sample size is small.
TABLE 75
Severity of BPAR for EVL + CSA vs. MPS + CSA
Summary for EVL + CSA vs. MPS + CSA
- The study reported by Tedesco-Silva et al.107 suggests that more people with BPAR receiving MPS experienced Banff II grading; however, there was no difference for Banff III (see Table 78). There were no Banff II or III episodes reported in the EVL treatment for Chadban et al.,152 with only one episode among those receiving MPS treatment; however, the sample size is small.107
TABLE 78
Graft loss for SRL + CSA vs. MMF + CSA
EVL + MPS vs. CSA + MPS
Only the study reported by Mjörnstedt et al.133 investigated this combination of therapies. Therefore, outcomes are summarised in Table 76. Time to BPAR is not reported. Data are provided at 1 year, when there is no statistical difference between arms for mortality or graft loss. There is evidence to indicate greater odds of BPAR associated with EVL + MPS (OR 19.31, 95% CI 9.09 to 41.04). There is no significant difference in severity of BPAR.
TABLE 76
Summary of outcomes for EVL + MPS vs. CSA + MPS at 1 year
SRL + CSA vs. MMF + CSA
Three RCTs108,109,122 were identified for this combination of therapies. No time to BPAR or severity of BPAR was reported.
Mortality
Two studies109,122 were available for pooling at 1 year; however, one of the studies109 had no deaths in either arm (Table 77 and Figure 39). The ORs appear to indicate lower odds associated with mortality for SRL; however, this is not statistically significant (1 year, OR 0.49, 95% CI 0.04 to 5.59). The 2- and 5-year time points also show no statistically significant difference (2 years, OR 0.31, 95% CI 0.05 to 1.92; 5 years, OR 1.0, 95% CI 0.36 to 2.77).
TABLE 77
Mortality for SRL + CSA vs. MMF + CSA

FIGURE 39
Forest plot: mortality for SRL + CSA vs. MMF + CSA.
Graft loss
Three studies108,109,122 report on graft loss for SRL + CSA vs. MMF + CSA from 1 to 5 years (Table 78 and Figure 40).108,109,122 The ORs up to 4 years slightly favour MMF; however, there is no statistically significant effect overall. At 5 years, the OR becomes ‘1’, indicating no benefit for either treatment.

FIGURE 40
Forest plot: graft loss for SRL + CSA vs. MMF + CSA.
Graft function
Graft function is monitored by one study109 at 1 year (Table 79). No statistical difference is apparent between SRL and MMF (WMD 0.11 ml/minute/1.73 m2; p = 0.5708).
TABLE 79
Graft function for SRL + CSA vs. MMF + CSA
Biopsy-proven acute rejection
The study by Anil Kumar et al.122 reporting on BPAR at 1 year a similar percentage of events in both arms and therefore no difference between treatments (Table 80).122 At 2 years, Barsoum et al.108 report more favourable outcomes for SRL; however, this is not statistically significant (OR 0.65, 95% CI 0.22 to 1.87).
TABLE 80
Biopsy-proven acute rejection for SRL + CSA vs. MMF + CSA
Summary for SRL + CSA vs. MMF + CSA
- Two studies109,122 were available for pooling at 1 year; however, one of the studies109 had no deaths in either arm. The ORs appear to indicate lower odds associated with mortality for SRL; however, this is not statistically significant (1 year, OR 0.49, 95% CI 0.04 to 5.59). The 2- and 5-year time points also show no statistically significant difference (2 years, OR 0.31, 95% CI 0.05 to 1.92; 5 years, OR 1.0, 95% CI 0.36 to 2.77).
- GRF is monitored by one study109 at 1 year. No statistical difference is apparent between SRL and MMF (WMD 0.11 ml/minute/1.73 m2; p = 0.5708).
SRL + TAC vs. MMF + TAC
A total of eight RCTs94,110,112,114,122,145,155,180 were identified investigating SRL + TAC vs. MMF + TAC with all outcomes other than HRQoL reported.
Mortality
Eight RCTs94,110,112,114,122,145,155,180 report mortality from 0.08 years to 3 years (Table 81 and Figure 41). The ORs vary from < 1 at 0.08 years to > 1 at 3 years; however, the CIs are wide and cross ‘OR = 1’, indicating no statistical significance at any time point.
TABLE 81
Mortality for SRL + TAC vs. MMF + TAC

FIGURE 41
Forest plot: mortality for SRL + TAC vs. MMF + TAC.
Graft loss
Five RCTs112,114,122,145,180 were identified reporting graft loss (Table 82 and Figure 42). Four RCTs112,122,145,180 are pooled at 1 year, at which increased odds of graft loss are associated with SRL. However, the effect is not statistically significant (OR 1.43, 95% CI 0.44 to 4.66). There may also be moderate heterogeneity across studies following pooling (I2 = 38.8%). The study by Anil Kumar et al.122 provides follow-up to 5 years, with the OR of < 1 favouring SRL; however, the results are not statistically significant.
TABLE 82
Graft loss for SRL + TAC vs. MMF + TAC
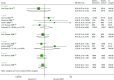
FIGURE 42
Forest plot: graft loss SRL + TAC vs. MMF + TAC.
Graft function
Three RCTs111,114,145 were identified reporting GRF; however, because of the different time points, only two RCTs111,114 could be pooled at 0.5 years (Tables 83 and 84; Figure 43). The results indicate no statistical difference between arms (WMD –1.875 ml/minute/1.73 m2, 95% CI –8.425 to 4.675 ml/minute/1.73 m2). Furthermore, substantial heterogeneity across studies is evident (I2 = 81.6%).
TABLE 83
Graft function for SRL + TAC vs. MMF + TAC (pooled results)
TABLE 84
Graft function for SRL + TAC vs. MMF + TAC (unpooled results)

FIGURE 43
Forest plot: GRF for SRL + TAC vs. MMF + TAC.
Biopsy-proven acute rejection
Biopsy-proven acute rejection is reported in four studies,112,122,145,180 with three studies112,122,145 pooled at 1 year (Table 85 and Figure 44). The ORs for 0.5 years and 1 year suggest that MMF + TAC has lower odds of BPAR; however, the effect is not statistically significant (1 year, OR 1.16, 95% CI 0.56 to 2.60). There is also a low level of heterogeneity (I2 = 27.8%).
TABLE 85
Biopsy-proven acute rejection for SRL + TAC vs. MMF + TAC

FIGURE 44
Forest plot: BPAR for SRL + TAC vs. MMF + TAC.
Severity of biopsy-proven acute rejection
Four studies94,112,114,155 report severity of BPAR (Table 86). No clear difference is apparent at either time point for Banff II or III classification between SRL and MMF.
TABLE 86
Severity of BPAR for SRL + TAC vs. MMF + TAC
Time to biopsy-proven acute rejection
Time to BPAR is reported by Sampaio et al.,112 which appears to favour MMF (Table 87).
TABLE 87
Time to BPAR for SRL + TAC vs. MMF + TAC
Summary for SRL + TAC vs. MMF + TAC
- Five RCTs were identified reporting graft loss.112,114,122,145,180 Four RCTs are pooled at 1 year where increased odds of graft loss are associated with SRL; however, the effect is not statistically significant (OR 1.43, 95% CI 0.44 to 4.66). There may also be moderate heterogeneity across studies following pooling (I2 = 38.8%). The study by Anil Kumar et al.122 provides follow-up to 5 years, with the OR of < 1 favouring SRL; however, the results are not statistically significant.
- Three RCTs111,114,145 were identified reporting GRF; however, because of the different time points, only two RCTs111,114 could be pooled at 0.5 years. The results indicate no statistical difference between arms (WMD –1.875 ml/minute/1.73 m2, 95% CI –8.425 to 4.675 ml/minute/1.73 m2). Furthermore, substantial heterogeneity across studies is evident (I2 = 81.6%).
- BPAR is reported in four studies,112,122,145,180 with three studies112,122,145 pooled at 1 year. The ORs for 0.5 years and 1 year suggest that MMF + TAC has lower odds of BPAR; however, the effect is not statistically significant (1 year, OR 1.16, 95% CI 0.56 to 2.60). There is also a low level of heterogeneity (I2 = 27.8%).
SRL + MMF vs. CSA + MMF
Ten studies91,115–118,127,134,146,147,149 were identified investigating SRL + MMF compared with CSA + MMF.
Mortality
Eight studies115–117,127,134,146,147,149 report on mortality, with seven pooled at 1 year (Table 88 and Figure 45). No statistically significant difference was evident at this time point (1 year, OR 0.98, 95% CI 0.28 to 3.42). Data are available up to 5 years; however, the effect is also not statistically significant (5 years, OR 1.15, 95% CI 0.42 to 3.13).115,209
TABLE 88
Mortality for SRL + MMF vs. CSA + MMF

FIGURE 45
Forest plot: mortality for SRL + MMF vs. CSA + MMF.
Graft loss
Eight studies115–117,127,134,146,147,149 report on graft loss from 0.5 years to 5 years (Table 89 and Figure 46). Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between SRL + MMF and CSA + MMF (1 year, OR 1.06, 95% CI 0.44 to 2.56). Flechner et al.127 and Büchler et al.134 report graft loss at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.57, 95% CI 0.05 to 7.25, I2 = 76.6%).
TABLE 89
Graft loss for SRL + MMF vs. CSA + MMF
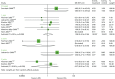
FIGURE 46
Forest plot: graft loss for SRL + MMF vs. CSA + MMF.
Graft function
Six studies117,118,127,134,146,149 report GRF (note, this includes Lebranchu et al.,67 with 68.9 ml/minute/1.73 m2 for SRL and 64.4 ml/minute for CSA; however, a SD is not provided). Pooled analysis for 0.5 years and 1 year suggests that improved GRF is associated with CSA, although this effect is not statistically significant (0.5 year, WMD 6.99 ml/minute/1.73 m2, 95% CI 0.45 to 13.53 ml/minute/1.73 m2; 1 year, WMD 9.41 ml/minute/1.73 m2, 95% CI –1.28 to 20.09 ml/minute/1.73 m2) (Table 90 and Figure 47). The individual studies for 2, 3, 4 and 5 years all have OR of < 1 and are statistically significant, therefore CSA appears beneficial in terms of GRF.
TABLE 90
Graft function for SRL + MMF vs. CSA + MMF

FIGURE 47
Forest plot: GRF for SRL + MMF vs. CSA + MMF.
Biopsy-proven acute rejection
Eight studies115–117,127,134,146,147,149 report on BPAR from 0.5 years to 5 years (Table 91 and Figure 48). Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between arms, although the OR falls in favour of CSA + MMF (1 year, OR 1.29, 95% CI 0.81 to 2.04). Flechner et al.127 and Büchler et al.134 report BPAR at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.77, 95% CI 0.37 to 1.63).
TABLE 91
Biopsy-proven acute rejection for SRL + MMF vs. CSA + MMF

FIGURE 48
Forest plot: BPAR for SRL + MMF vs. CSA + MMF.
Severity of biopsy-proven acute rejection
Severity of BPAR is reported by three studies116,127,134 at 1 year (Table 92). Flechner et al.127 also report results for 5 years. Sample sizes are relatively low, with similar proportions of people with BPAR experiencing Banff II and III classification.
TABLE 92
Severity of BPAR: SRL + MMF vs. CSA + MMF
Time to biopsy-proven acute rejection
Time to BPAR is reported by three studies127,134,146 (Table 93). A statistically significant difference is seen by Durrbach et al.146 (SRL 56 days, SD 57 days; CSA 94 days, SD 47 days; p = 0.0035).146 The studies reported by Büchler et al.134 and Flechner et al.127 show no statistical difference between treatments (p = 0.3858 and p = 0.982, respectively).
TABLE 93
Time to BPAR: SRL + MMF vs. CSA + MMF
Summary of results for SRL + MMF vs. CSA + MMF
- Eight studies115–117,127,134,146,147,149 report on graft loss from 0.5 years to 5 years. Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between SRL + MMF and CSA + MMF (1 year, OR 1.06, 95% CI 0.44 to 2.56). Flechner et al.127 and Büchler et al.134 report graft loss at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.57, 95% CI 0.05 to 7.25, I2 = 76.6%).
- Six studies117,118,127,134,146,149 report GRF (note, this includes Lebranchu et al.,149 with 68.9 ml/minute/1.73 m2 for SRL and 64.4 ml/minute/1.73 m2 for CSA; however, a SD is not provided). Pooled analysis for 0.5 years and 1 year suggests that improved GRF is associated with TAC, although this effect is not statistically significant (0.5 year, WMD 6.99 ml/minute/1.73 m2 95% CI 0.45 to 13.53 ml/minute/1.73 m2; 1 year, WMD 9.41 ml/minute/1.73 m2, 95% CI –1.28 to 20.09 ml/minute/1.73 m2). The individual studies for 2, 3, 4 and 5 years all have OR of < 1 and are statistically significant, therefore TAC appears to be beneficial in terms of GRF.
- Eight studies115–117,127,134,146,147,149 report on BPAR from 0.5 years to 5 years. Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between arms, although the OR falls in favour of CSA + MMF (1 year, OR 1.29, 95% CI 0.81 to 2.04). Flechner et al.127 and Büchler et al.134 report BPAR at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.77, 95% CI 0.37 to 1.63).
- Severity of BPAR is reported by three studies116,127,134 at 1 year. Sample sizes are relatively low, with similar proportions of people with BPAR experiencing Banff II and III classification. Time to BPAR is reported by three studies.127,134,146 A statistically significant difference is seen by Durrbach et al.146 (SRL 56 days, SD 57 days; CSA 94 days, SD 47 days; p = 0.0035). The studies reported by Büchler et al.134 and Flechner et al.127 show no statistical difference between treatments (p = 0.3858 and p = 0.982, respectively).
TAC + MMF vs. SRL + MMF
Four studies92,93,135,154 report outcomes for this combination of treatments. No time to BPAR or HRQoL is reported.
Mortality
Four studies92,93,135,154 are pooled with 1-year results for mortality; however, two of these studies93,135 had no deaths for either TAC + MMF or SRL + MMF (Table 94 and Figure 49). Furthermore, analysis suggests no statistically significant difference between TAC + MMF and SRL + MMF (OR 0.80, 95% CI 0.13 to 4.99). Heilman et al.135 also present results at 2 years (see Table 99). Again, results are not statistically significant (OR 2.10, 95% 0.19 to 23.83).
TABLE 94
Mortality for TAC + MMF vs. SRL + MMF

FIGURE 49
Forest plot: mortality for TAC + MMF vs. SRL + MMF.
TABLE 99
Summary of outcomes for TAC + MPS vs. SRL + MPS
Graft loss
Four studies92,93,135,154 are pooled with 1-year results for graft loss (Table 95 and Figure 50). Again, two of these studies93,135 had no graft loss in either arm. Although the OR implies that reduced graft loss is associated with TAC, this is not statistically significant (OR 0.68, 95% CI 0.18 to 2.58).
TABLE 95
Graft loss for TAC + MMF vs. SRL + MMF

FIGURE 50
Forest plot: graft loss for TAC + MMF vs. SRL + MMF.
Graft function
Two studies135,154 report GRF at 1 year and 2 years (Table 96 and Figure 51). The pooled ORs for both time points indicate no statistically significant difference between TAC + MMF and SRL + MMF (1 year, WMD –2.50 ml/minute/1.73 m2, 95% CI –6.85 to 1.85 ml/minute/1.73 m2).
TABLE 96
Graft function for TAC + MMF vs. SRL + MMF
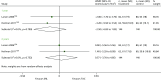
FIGURE 51
Forest plot: GRF for TAC + MMF vs. SRL + MMF.
Biopsy-proven acute rejection
Biopsy-proven acute rejection is reported by three studies92,93,135 (Table 97 and Figure 52). Pooled results indicate that there are lower odds of BPAR associated with TAC at 1 year (OR 0.32, 95% CI 0.12 to 0.87). There does not appear to be any evidence of heterogeneity across studies (I2 = 0.0%).
TABLE 97
Pooled results for BPAR – TAC + MMF vs. SRL + MMF

FIGURE 52
Forest plot: BPAR for TAC + MMF vs. SRL + MMF
Severity of biopsy-proven acute rejection
Only one study93 reports on severity of BPAR (Table 98). For Banff classification II, there is no difference at 1 year between TAC + MMF and SRL + MMF; however, the sample size is very small.
TABLE 98
Severity of BPAR for TAC + MMF vs. SRL + MMF
Summary of results for TAC + MMF vs. SRL + MMF
- Four studies92,93,135,154 are pooled with 1-year results for mortality; however, two of these studies had no deaths for either TAC + MMF or SRL + MMF. Furthermore, analysis suggests no significant difference between TAC + MMF and SRL + MMF (OR 0.80, 95% CI 0.13 to 4.99) Heilman et al.135 also present results at 2 years of mortality for TAC + MMF vs. SRL + MMF. Again, results are not statistically significant (OR 2.10, 95% 0.19 to 23.83).
- BPAR is reported by three studies.92,93,135 Pooled results indicate that there are lower odds of BPAR associated with TAC at 1 year (OR 0.32, 95% CI 0.12 to 0.87). There does not appear to be any evidence of heterogeneity across studies (I2 = 0.0%). Only one study93 reports on severity of BPAR. Banff classification I and II demonstrate no difference at 1 year between TAC + MMF and SRL + MMF; however, the sample size is very small.
TAC + MPS vs. SRL + MPS
The study by Silva et al.119 is the only one to report on this combination; therefore, a summary of outcomes at 2 years is presented in Table 99. The OR for BPAR appears to favour TAC (OR 0.63, 95% CI 0.3482 to 1.1397); however, this is not statistically significant. All other outcomes also show no statistically significant difference between arms.
TAC + SRL vs. MMF + SRL
Hamdy et al.120 is the only study to report on this combination; therefore, a summary of outcomes at 1–5 years is presented in Table 100. The OR for mortality at 3 years appears to favour MMF (OR 4.39, 95% CI 0.48 to 40.39); however, this is not statistically significant. All other outcomes also show no statistical difference between arms.
TABLE 100
Summary of outcomes for TAC + SRL vs. MMF + SRL
SRL + AZA vs. CSA + AZA
One trial148 reported investigating SRL + AZA vs. CSA + AZA, and a summary of outcomes at 0.5 years and 1 year is presented (Table 101). There is a statistically significant difference between both arms at 0.5 years and 1 year in favour of SRL + AZA (p < 0.0001) for GRF. There is no statistically significant difference between arms for other outcomes.
TABLE 101
Summary of outcomes for SRL + AZA vs. CSA + AZA
TAC + SRL vs. CSA + SRL
Two studies121,122 reported this combination, presenting outcomes at 1 year and 5 years. No severity or time to AR reported.
Mortality
At both 1 year and 5 years there is no statistically significant difference between TAC + SRL and CSA + SRL for mortality (Table 102).121,122 Notably, for Anil Kumar et al.122 there are no deaths in either arm at 1 year.
TABLE 102
Mortality for TAC + SRL vs. CSA + SRL
Graft loss
Two studies121,122 report graft loss, with pooled result at 1 year and individual results up to 5 years (Table 103 and Figure 53). Results are consistent across all time points for lower odds being associated with graft loss for TAC + SRL; however, the effect is not statistically significant (1 year, OR 0.68, 95% CI 0.16 to 2.90).
TABLE 103
Graft loss for TAC + SRL vs. CSA + SRL

FIGURE 53
Forest plot: graft loss for TAC + SRL vs. CSA + SRL.
Graft function
Chen et al.121 report GRF at 0.5 years and 1 year (Table 104), which appears to be statistically significantly greater for TAC + SRL at 0.5 years and 1 year (p < 0.0001 and p = 0.0004, respectively).
TABLE 104
Graft function for TAC + SRL vs. CSA + SRL
Biopsy-proven acute rejection
This is reported only by Anil Kumar et al.122 at 1 year (Table 105). The OR implies BPAR to be more likely for CSA + SRL; however, this is not statistically significant (OR 0.48, 95% CI 0.08 to 2.74).
TABLE 105
Biopsy-proven acute rejection for TAC + SRL vs. CSA + SRL
Summary of results for TAC + SRL vs. CSA + SRL
- Two studies121,122 report graft loss, with pooled result at 1 year and individual results up to 5 years. Results are consistent across all time points in showing that lower odds are associated with graft loss for TAC + SRL; however, the effect is not statistically significant (1 year, OR 0.68, 95% CI 0.16 to 2.90).
- Chen et al.121 report GRF at 0.5 years and 1 year, which appears to be statistically significantly greater for TAC + SRL at 0.5 years and 1 year (p < 0.0001 and p = 0.0004, respectively).
- BPAR is reported only by Anil Kumar et al.122 at 1 year. The OR implies BPAR to be more likely for CSA + SRL; however, this is not statistically significant (OR 0.48, 95% CI 0.08 to 2.74).
Induction therapy results
Network meta-analysis was performed for all induction studies reporting graft loss, mortality, BPAR and eGFR at 1-year follow-up. Figure 54 displays the network for included induction studies.
FIGURE 54
Network diagram for all included induction studies. Note: circles denote number of studies.
Graft loss
Ten RCTs71–74,87,95–98,137 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for graft loss (Figure 55).

FIGURE 55
Network diagram for induction studies reporting graft loss. Note: circles denote number of studies.
The DIC suggested little difference between the fit of the fixed- and random-effects models, with the fixed effects being the slightly better fit; thus, only the results of the fixed-effects model are shown in Table 106.
TABLE 106
Odds ratios for induction therapy from a fixed-effects model: posterior mean (95% CrI)
From these analyses there is little evidence to suggest that BAS and rATG are more effective than no induction/PBO in reducing graft loss, as the 95% CrIs include an OR of ‘1’. Furthermore, there is little evidence to suggest that rATG is more effective than BAS. Of the three treatments analysed in this network, rATG was estimated as having a 57% probability of being the most effective treatment, with BAS having a 38% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Mortality
Ten RCTs71–74,87,95–98,137 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for mortality (Figure 56).

FIGURE 56
Network diagram for induction studies reporting mortality. Note: circles denote number of studies.
The DIC suggested little difference between the fit of the fixed- and random-effects models, with the fixed effects being the slightly better fit, thus only the results of the fixed-effects model are shown in Table 106.
From these analyses there is little evidence to suggest that BAS and rATG are more effective than no induction/PBO in reducing mortality, as the 95% CrIs include an OR of ‘1’ (see Table 106), and there is little evidence to suggest that rATG is more effective than BAS. Of the three treatments analysed in this network, rATG was estimated as having a 54% probability of being the most effective treatment, with BAS having a 22% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Biopsy-proven acute rejection
Nine RCTs71–74,87,96–98,137 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for mortality (Figure 57).
FIGURE 57
Network diagram for induction studies reporting BPAR. Note: circles denote number of studies.
The DIC suggested little difference between the fit of the fixed- and random-effects models, with the fixed effects being the slightly better fit, and so only the results of the fixed-effects model are shown in Table 106.
From these analyses, evidence suggests that BAS and rATG are more effective than no induction/PBO in reducing BPAR and that rATG is more effective than BAS. Of the three treatments analysed in this network, rATG was estimated as having a 96% probability of being the most effective treatment, with BAS having a 3% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Graft function
Five RCTs71–73,87,97 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for GRF (Figure 58).

FIGURE 58
Network diagram for induction studies reporting GRF. Note: circles denote number of studies.
The DIC suggested very little difference between the fit of the fixed- and random-effects models. For comparison with the above outcomes, the results of the fixed-effects model are shown in Table 107.
TABLE 107
Mean effects for induction therapy for the outcome GRF from a fixed-effects model: posterior mean (95% CrI)
There is no evidence to suggest that BAS or rATG is more effective than PBO/no induction, and no evidence to suggest that one treatment is more effective than the other. BAS has a 89% probability of being the most effective treatment, whereas rATG has a 5% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Maintenance therapy results
Network meta-analysis was performed for all maintenance studies reporting graft loss, mortality, BPAR and eGFR at 1-year follow-up. Figure 59 displays the network for included induction studies.
FIGURE 59
Network diagram for all included maintenance studies reporting graft loss. Note: circles denote number of studies.
Data on 13 treatments from 49 studies51,59,76,80,82–84,86,88–90,92,93,100,102–104,107–112,115,117,118,121,122,125–127,129,131,133–136,138,142–145,147,149–152,155,210 were potentially includable in the NMA (Figure 60). However, 11 studies had zero events in all treatment arms, so would not contribute information to the NMA; therefore, they were excluded from the NMA. Owing to the exclusion of these studies, the treatment EVL + MPS could not be included in the network. Therefore, data from 40 studies51,59,76,80,82–84,86,88–90,92,100,103,104,107,108,110–112,117,118,122,125–127,129,134,136,138,142–145,147,149–152,155 (including five three-arm studies51,104,126,152,155 and one four-arm study122) on the effectiveness of 12 treatments to reduce graft loss informed the NMA. Thirteen82,90,100,104,112,126,127,138,144,145,147,149,152 of the 40 studies had at least one treatment arm with no graft loss events; therefore, 0.5 was added to each cell.

FIGURE 60
Network diagram for maintenance studies reporting graft loss. Note: circles denote number of studies.
The DIC indicated that the random-effects model was a slightly better fit to the data than the fixed-effects model (154.4 vs. 157.5), and so results from only the random-effects models are presented here. The results of the fixed-effects models are given in Appendix 6. The probabilities that each treatment was the most effective in reducing graft loss compared with all other treatments are shown in Table 108.
TABLE 108
Probability that each treatment is the most effective treatment for reducing graft loss
Although the results suggest that EVL has a 60% probability of being the most effective treatment for reducing graft loss compared with all other treatments (with SRL + AZA having a 29% probability), there is little evidence to suggest that treatment with EVL reduces graft loss compared with other treatments. The posterior median ORs for EVL compared with all of the other treatments are < 1, indicating a reduction in the odds of having a graft loss; however, the upper 95% CrIs limits are > 1, suggesting that EVL could increase the odds of a graft loss compared with all other treatments (Table 109). In fact, there is little evidence from the NMA to suggest that any treatment is more effective at reducing graft loss than any other treatment.
TABLE 109
Odds ratios (intervention vs. comparator treatment) for the outcome graft loss from a random-effects NMA: posterior median (95% CrI)
There is no evidence to suggest that this network is affected by inconsistencies between the direct and indirect evidence (see Appendix 6). The DICs were very similar between the consistency and inconsistency models (154.4 vs. 153.7) and the 95% CrIs based on the direct evidence overlapped those based on the direct and indirect evidence.
Mortality
Data on 13 treatments from 52 studies51,59,76,78,80,83,84,86,88–90,92,93,100,102–104,107–112,115–118,120–122,125–127,129–131,133–136,138,142,145,147,149–151,155,210 were potentially includable in the NMA (Figure 61). However, 10 trials93,102,104,109,115,117,121,131,135,149 had zero events in all arms and were excluded from the NMA, resulting in 42 trials51,59,76,78,80,83,84,86,88–90,92,100,103,107,108,110–112,116,118,120,122,125–127,129,130,133,134,136,138,142–145,147,150–152,155,210 contributing to the NMA (including four three-arm trials51,104,122,152,155 and one four-arm trial122). Twelve59,76,78,90,92,100,120,126,127,136,145,152 of the 42 included trials had zero events in at least one treatment arm and so 0.5 was added to all cells in those trials.

FIGURE 61
Network diagram for maintenance studies reporting mortality. Note: circles denote number of studies.
Although the DIC indicated that the fixed-effects model was a slightly better fit to the data than the random-effects model (137.7 vs. 139.5), the random-effects results are presented here and used in the economic model for consistency, as the remaining maintenance treatment analyses indicated the random-effects model to be the best-fitting model. The results of the fixed-effects models are given in Appendix 6. The probabilities that each treatment was the most effective in reducing graft loss compared with all other treatments are shown in Table 110.
TABLE 110
Probability that each treatment is the most effective treatment for reducing mortality
The regimens SRL + AZA (34%), EVL (30%) and BEL + SRL (27%) were estimated to have the greatest probabilities of being the most effective treatments to reduce mortality compared with all others, with the remaining treatments having a very low probability of being the best treatment. This reflects the findings presented below (see Table 120), which show that SRL + AZA, EVL and BEL + SRL are consistently estimated to have posterior median ORs of < 1 compared with all treatments, but, as the upper 95% CrI limits are > 1, there is the possibility that these treatments could increase mortality compared with other treatments.
TABLE 120
Infections: induction therapies
The NMA suggests that BEL + MMF is more effective than TAC + MMF and SRL + MMF at reducing mortality. However, there is a great deal of uncertainty associated with many of the results presented (Table 111), especially for BEL + SRL.
TABLE 111
Odds ratios (intervention vs. comparator treatment) for the outcome mortality from a random-effects NMA: posterior median (95% CrI)
There is no evidence to suggest that this network is affected by inconsistencies between the direct and indirect evidence (see Appendix 6). The DICs were slightly lower for the consistency model than for the inconsistency model (139.5 vs. 143.9) and the 95% CrIs that were based on the direct evidence overlapped those based on the direct and indirect evidence.
Biopsy-proven acute rejection
Thirteen treatments and 42 studies51,59,76,81–83,86,88–90,92,93,100,103,107,110,112,115–118,120,121,125–127,129,131,133–136,142,144,145,147,149,150,152,210 (including three three-arm studies51,126,152 and one four-arm study122) contribute to this NMA (Figure 62).
FIGURE 62
Network diagram for maintenance studies reporting BPAR. Note: circles denote number of studies.
The DIC for the random-effects models was lower than that for the fixed-effects model (156.3 vs. 170.8) and so the random-effects model results are reported here (see Appendix 6 for fixed-effects results). The probabilities that each treatment was the most effective in reducing graft loss compared with all other treatments are shown in Table 112.
TABLE 112
Probability that each treatment is the most effective treatment for reducing BPAR
The regimen BEL + SRL has the highest probability (58%) of being the most effective treatment compared with all other treatments for reducing BPAR; however, there is no evidence that BEL + SRL is any more effective than the other treatments (Table 113). CSA + AZA has a 0% probability of being the best treatment and there is evidence to suggest that many treatments are more effective than CSA + AZA (see Table 112). The results from the NMA also indicate that MMF + CSA, TAC + MMF and SRL + TAC are all more effective than EVL + MPS at reducing BPAR. However, as with the other NMAs for maintenance therapy, there is a great deal of uncertainty associated with the estimated ORs. Therefore, apart from CSA + AZA and EVL + MPS performing poorly in some comparisons, it is difficult to say that any one treatment is more effective than another, as the 95% CrIs are so wide.
TABLE 113
Odds ratios (intervention vs. comparator treatment) for the outcome BPAR from a random-effects NMA: posterior median (95% CrI)
There is no evidence to suggest that this network is affected by evidence inconsistencies (see Appendix 6). The DIC was slightly lower for the consistency model than for the inconsistency model (156.3 vs. 159.7) and the 95% CrIs that were based on the direct evidence overlapped those based on the direct and indirect evidence.
Graft function
Twelve treatments and 35 studies51,59,60,76,82,84,102–104,107,109,115,117,118,120,121,125,126,129–131,133–136,138,142,144,145,147,149,150,152,155 (including four three-arm studies51,104,126,155) contribute to this NMA (Figure 63).

FIGURE 63
Network diagram for maintenance studies reporting BPAR. Note: circles denote number of studies.
The DIC was lower for the random-effects model than for the fixed-effects model (147.8 vs. 323.7), suggesting a better fit to the data for the random-effects model. Therefore, the random-effects model results are reported (see Appendix 6 for fixed-effects model results). The treatment with the highest probability of being the most effective (Table 114) is BEL + SRL (44% probability), with SRL + AZA having a 28% probability. The results in Table 115 suggest that a number of treatments (TAC + AZA, TAC + MMF, BEL + MMF and SRL + AZA) are more effective than CSA + AZA, and also that TAC + AZA, TAC + MMF and BEL + MMF are more effective than SRL + TAC. However, because of the limited direct evidence informing many of the comparisons, the 95% CrIs are very wide for a number of comparisons, limiting conclusions to be made on the effectiveness of one treatment over another.
TABLE 114
Probability that each treatment is the most effective treatment for GRF
TABLE 115
Mean differences (intervention vs. comparator treatment) for the outcome ‘GRF’ from a random-effects NMA: posterior median (95% CrI)
For the random-effects model, there was little evidence of inconsistency within the network (see Appendix 6).
Summary for network meta-analysis
Induction therapy
- There is no evidence to suggest that BAS or rATG is more effective than PBO/no induction, or each other, in reducing the odds of graft loss, mortality or CRC-GFR.
- rATG and BAS are both estimated to be more effective than PBO/no induction at reducing BPAR, but the evidence does not suggest a difference between the two treatments.
- Evidence suggests that although no treatment effect is seen for rATG, BAS is estimated to be more effective than PBO/no induction for increasing CRC-GFR.
Maintenance therapy
None of the maintenance regimens performed consistently well on all four outcomes. An overview of probability ranking on the four outcomes is presented (Table 116). However, because the analyses included 12 or 13 treatment regimens for each of the four outcomes, the results should be treated with great caution.211 In addition, differences between treatments in probability of being best of < 90% cannot be given much credence.211
TABLE 116
Probability that each treatment is the most effective treatment for mortality, reducing graft loss, BPAR and GRF
In all NMAs for maintenance therapy there is a great deal of heterogeneity:
- There is no evidence to suggest that one treatment is any more effective at reducing the odds of graft loss than any other treatment.
- There is evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments are estimated to be any more effective at reducing mortality than any other treatment.
- MMF + CSA, TAC + MMF and SRL + TAC are estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR. In addition, TAC + AZA and EVL + CSA are also estimated to be more effective than CSA + AZA at reducing the odds of BPAR. However, apart from CSA + AZA and EVL + MPS performing poorly in some comparisons, it is difficult to say that any one treatment is more effective than another as the 95% CrIs are very wide.
- Similarly, a number of treatments, TAC + AZA, TAC + MMF and BEL + MMF, are estimated to be more effective than CSA + AZA and MMF + CSA at increasing GRF. In addition, SRL + AZA is estimated to be more effective than CSA + AZA at increasing GRF. However, because of a lack of direct evidence, the 95% CrIs are wide for a number of comparisons. As a result, conclusions on the effectiveness of one treatment over another are limited.
Adverse events
Adverse events for each study are presented below. We conducted numerous comparisons and meta-analyses of the adverse effects of treatment reported in included RCTs at 1 year, as other time points had insufficient data for pooling. All of the meta-analyses (and associated forest plots) can be found in Appendix 7, rather than the main body of the report; however, the results are summarised as follows:
Induction therapy
All 13 induction studies71–74,87,95–98,123,128,137,148 reported some AE data. The time of follow-up varied from 6 months to 7 years in the individual studies (Table 117). Most studies reported a 1-year follow-up, although the AEs reported varied across the studies. The following AEs are summarised below: NODAT, PTLD, malignancy (including PTLD), any infections and CMV.
TABLE 117
Adverse events overview: induction therapies
New-onset diabetes mellitus
Seven studies87,95–97,123,128,148 reported NODAT events and their frequencies are shown in Table 118. The studies that reported NODAT events showed frequencies ranging from 0 to 5 out of 58 (9%). None of the comparisons suggests a statistically significant difference.
TABLE 118
New-onset diabetes mellitus: induction regimens
Malignancy and post-transplant lymphoproliferative disorder
Ten studies71–73,87,95,97,123,128,137,148 reported malignancy, including PTLD. The frequency of these events can be seen in Table 119. Frequencies ranged from 0 to 3/168 (2%). No statistically significant differences between treatments were noted.
TABLE 119
Malignancy and PTLD: induction regimens
Infections
Ten studies reported71–74,95,97,98,123,137,148 infections related to the induction therapies (Table 120). Frequencies ranged from 0 to 129 out of 173 (75%). At 6 months and 1 year, a statistically significant difference in favour of BAS is indicated.
Cytomegalovirus
Thirteen studies71–74,87,95–98,123,128,137,148 reported CMV events in induction therapies (Table 121). Frequencies ranged from 0 to 49 out of 151 (32%), with a statistically significant difference noted for BAS vs. rATG (three studies). For Lebranchu et al.87 and Mourad et al.98 a reduced occurrence of CMV is seen for the BAS arm, whereas for the study reported by Brennan et al.,137 fewer occurrences are seen for rATG.
TABLE 121
Cytomegalovirus: induction regimens
Maintenance therapy
Most of the 75 maintenance studies (Table 122) reported some AE data. The time of follow-up varied from 6 months to 10 years. Most studies reported 1-year follow-up, although the AE reported varied across the studies. The following AEs are summarised below: NODAT, PTLD, malignancy (including PTLD), any infections and CMV. All AEs are tabulated and narratively described in the sections below.
TABLE 122
Adverse events overview: maintenance therapies
New-onset diabetes mellitus
Only one study148 out of 13 found statistically significant difference for TAC + AZA vs. CSA + AZA at the 6-month time point in favour of CSA (Table 123). Vincenti et al.125 found CSA + MMF to have a statistically significant difference to BEL + MMF, but, again, only at 6 months. There is a statistically significant increase in NODAT for SRL high + TAC at 6 months when compared with SRL low + TAC and MMF + TAC.94 Two other studies51,122 show an increase in NODAT: Grinyo et al.51 for MMF + low TAC and Anil Kumar et al.122 for TAC + MMF.
TABLE 123
New-onset diabetes mellitus: maintenance therapies
Malignancy and post-transplant lymphoproliferative disorder
For all combinations reporting malignancy and PTLD, no statistically significant difference was seen between arms (Table 124).
TABLE 124
Malignancy and PTLD: maintenance regimens
Infections
Maintenance therapy studies that reported infection rates gave frequencies of 9 out of 237 (4%) to 85 out of 85 (100%; Table 125). Despite the relatively common occurrence of infections, only one study150 displayed a statistically significant difference between arms in favour of SRL low + TAC, as opposed to SRL high + TAC and MMF + TAC.
TABLE 125
Infections: maintenance regimens
Cytomegalovirus
Studies that reported the frequencies of CMV showed that this ranged from 0 to 7 out of 27 (26%) (Table 126).
TABLE 126
Cytomegalovirus: maintenance regimens
The CSA + MMF arm of the following trials displayed a statistically significant difference, in terms of increased episodes of CMV: Sadek et al.,86 Vítko et al.,94 Takahashi et al.,131 Büchler et al.,134 Kreise et al.,116 Tedesco-Silva et al.107 and Grinyo et al.51 Krämer et al.58 reported a statistically significant difference for TAC-PR + MMF vs. TAC + MMF and Van Gurp et al.114 found increased events for TAC + MMF as opposed to SRL + TAC.
Summary of clinical effectiveness
Summary of pairwise comparisons
Overall, we found that, despite the volume of evidence, there is little impact on effectiveness conclusions from the head-to-head comparisons, particularly for graft loss and mortality. However, this may be a reflection of the lack of long-term data, as very few studies reported all outcomes beyond 1 year, and also the frequently substantial level of heterogeneity across studies. Furthermore, the quality of trials was variable and, as a result of reporting omissions, it was difficult to make a general assessment regarding quality.
Induction
- We found no evidence to suggest BAS or rATG are more effective than PBO, no induction or each other in reducing the odds of mortality. Similarly, for graft loss, we found no evidence of a statistically significant difference for BAS or rATG vs. PBO, no induction or each other.
- For the head-to-head comparisons, we found evidence to suggest that rATG and BAS are more effective than PBO or no induction at reducing BPAR (rATG at 1 year, OR 0.41, 95% CI 0.24 to 0.52 BAS at 1 year, OR 0.53, 95% CI 0.40 to 0.70). However, there is no statistically significant difference between BAS and rATG.
- Time to BPAR is reported only for rATG vs. no induction and BAS vs. rATG. The one study96 for rATG vs. no induction found that more participants experienced BPAR at 7–10 days with no induction than with rATG (seven participants for rATG vs. 30 participants for no induction). There was no statistically significant difference between interventions for BAS vs. rATG.
Maintenance
- We found no evidence that any maintenance therapies were preferable to others in terms of mortality.
- For graft loss outcomes reported by maintenance studies, we found evidence that at 5 years BEL + MMF may be superior to CSA + MMF (OR 0.40, 95% CI 0.19 to 0.87, I2 = 0.0%). The 0.5-year time point has only two studies and a substantial level of heterogeneity (I2 = 72.2%); therefore, the OR of 0.58 and 95% CI 0.09 to 3.59, which indicates that MMF is more effective at reducing graft loss, must be treated with caution.201 The results for 1 year suggest no difference between arms (OR 0.76, 95% CI 0.38 to 1.50). The Merville et al.138 study appears to show more of an effect in favour of MMF; however, the population is much smaller than that for the Tricontinental study89 and the Sadek et al.86 study. Weimer et al.104 found no evidence of graft loss in either arm.
- Several treatments showed a beneficial effect with regard to reducing BPAR, although this varied across time points. For all the following combinations, the arm containing TAC displayed lower odds associated with BPAR:
- TAC + AZA vs. CSA + AZA (0.5 years, OR 0.50, 95% CI 0.32 to 0.79; I2 = 50.1%; 1 year, OR 0.50, 95% CI 0.39 to 0.64; I2 = 8.1%; 4 years, OR 0.38, 95% CI 0.25 to 0.57)
- TAC + MMF vs. CSA + AZA (0.5 years, OR 0.64, 95% CI 0.41 to 0.98; 1 year, OR 0.35, 95% CI 0.15 to 0.82)
- TAC + MMF vs. CSA + MMF (1 year, OR 0.59, 95% CI 0.37 to 0.94, I2 = 19.3%)
- TAC + MMF vs. SRL + MMF (1 year, OR 0.32, 95% CI 0.12 to 0.87, I2 = 0.0%)
- TAC + SRL vs. TAC + MMF (0.5 years, OR 0.65, 95% CI 0.44 to 0.96).
- For CSA + MMF vs. CSA + AZA, at 0.5 years and 1 year, there is statistically significant evidence to suggest that MMF is more effective (0.5 years, OR 0.50, 95% CI 0.35 to 0.72, I2 = 35.1%).
- TAC is also associated with lower odds of reduced GRF for:
- TAC + MMF vs. CSA + MMF (3 years, WMD 4.60 ml/minute/1.73 m2, 95% CI 1.35 to 7.85 ml/minute/1.73 m2)
- TAC + MMF vs. TAC-PR + MMF (0.5 years, WMD 1.90 ml/minute/1.73 m2, 95% CI 1.70 to 2.10 ml/minute/1.73 m2)
- TAC + SRL vs. CSA + SRL (0.5 years, MD 6.35 ml/minute/1.73 m2, p < 0.0001; 1 year, MD 5.25, p = 0.0004).
- For MMF + TAC vs. MPS + TAC, MPS at 1 year and 3 years is more effective (1 year, MD 1.9 ml/minute/1.73 m2, p < 0.0001; 3 years MD 0.5 ml/minute/1.73 m2, p = 0.0016). BEL appears more effective at 1 year and 3 years for BEL + MMF vs. CSA + MMF (1 year, WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 14.10 ml/minute/1.73 m2; I2 = 73.6%; 3 years, WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2; I2 = 89.5%); however, heterogeneity across studies is substantial. Where there are two comparisons involving SRL and CSA, the regimen including MMF suggests CSA to be more beneficial up to 5 years (5 years, WMD 9.10 ml/minute/1.73 m2, 95% CI 1.68 to 16.52 ml/minute/1.73 m2), yet, in contrast, the regimen including AZA suggests SRL to be more effective (1 year, MD 10.8 ml/minute/1.73 m2, p < 0.0001).
- Time to BPAR is generally poorly reported and therefore it is challenging to form a conclusion. Again, TAC + AZA vs. CSA + AZA shows conflicting results for two studies; however, the statistically significant result suggests that BPAR is achieved more quickly for participants receiving TAC rather than CSA (MD 24 days; p = 0.0033). This is also true for TAC + MMF vs. CSA + MMF (MD 46.7 days; p < 0.0001). When SRL + TAC and MMF + TAC are compared, a reduced time to BPAR is seen for MMF (MD 48.6 days; p = 0.0017). For SRL + MMF vs. CSA + MMF, one146 of three studies127,134,146 demonstrates a statistically significant difference in favour of CSA (MD 38 days; p = 0.0035); however, the other two studies127,134 show no difference.
- For TAC + AZA vs. CSA + AZA, there may be lower odds of the more severe BPAR for the arm containing TAC. Similarly, for TAC + MMF vs. TAC-PR + MMF, TAC has a lower proportion of people experiencing the more severe BPAR of Banff III classification.
Summary for network meta-analysis
Induction therapy
- There is no evidence to suggest BAS or rATG are more effective than PBO/no induction or each other in reducing the odds of graft loss or mortality, which is in agreement with the pairwise comparisons.
- rATG and BAS are both estimated to be more effective than PBO/no induction, with rATG being more effective than BAS at reducing BPAR.
- Evidence suggests that although no treatment effect is seen for rATG, BAS is estimated to be more effective than PBO/no induction for increasing CRC-GFR.
Maintenance therapy
- For all NMAs for maintenance therapy there is a great deal of heterogeneity.
- There is no evidence to suggest that one treatment is any more effective at reducing the odds of graft loss than any other treatment.
- There is evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments are estimated to be any more effective at reducing mortality than any other treatment.
- A number of treatments are estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR, and CSA + AZA and SRL + TAC at increasing GFR, but no other treatments are estimated to be any more effective at reducing the odds of BPAR or increasing GFR than any other treatment.
Comparison between clinical effectiveness analyses
Induction
Network meta-analysis and pairwise comparisons were in agreement for all comparable outcomes other than GRF, for which NMA suggested that BAS may be more effective than PBO/no induction.
Maintenance
- Pairwise comparisons found no evidence that any maintenance therapies were preferable to others in terms of mortality; however, NMA found evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF.
- Following NMA, there is no evidence to suggest that one treatment is any more effective at reducing the odds of graft loss than any other treatment. For pairwise comparisons, there is some evidence that BEL + MMF may be superior to CSA + MMF; CSA + MMF may be superior to CSA + AZA; and TAC + AZA may be superior to CSA + AZA.
- A number of treatments were estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR by the NMA, but no treatments found to be any more effective than any other. As for the pairwise comparisons, the arm containing TAC displayed lower odds associated with BPAR for TAC + AZA vs. CSA + AZA; TAC + MMF vs. CSA + AZA;TAC + MMF vs. CSA + MMF and TAC + MMF vs. SRL + MMF.
- The NMA found evidence that CSA + AZA and SRL + TAC were effective at increasing GFR, but no other treatments were estimated to be any more effective than any other treatment. Although the pairwise comparison found that TAC was generally associated with lower odds of reduced GRF for TAC + MMF vs. CSA + MMF; TAC + MMF vs. TAC-PR + MMF; TAC + SRL vs. CSA + SRL. For MMF + TAC vs. MPS + TAC, MPS was more effective.
Current assessment (Technology Assessment 85)
Relevant to this review, the current assessment (TA85) found that BAS, TAC and MMF consistently reduced the incidence of short-term (1-year) AR compared with conventional immunosuppressive therapy. The independent use of BAS, TAC and MMF was associated with a similar absolute reduction in 1-year acute rejection rate (ARR) (approximately 15%).
The trials did not assess how the improvement in trials, the impact of the newer immunosuppressants on long-term graft loss and patient survival remain uncertain.
The absence of both long-term outcome and quality of life from trial data makes assessment of the clinical effectiveness challenging.
Ongoing studies
Searches of ClinicalTrials.gov and Controlled Trials were conducted (see Appendix 1 for the search strategy used). All searches were carried out in January 2015. A total of 256 trials were considered to be relevant to this review and were investigated further. Sixty-nine studies were identified as ongoing (active not recruiting, n = 16; not yet recruiting, n = 7) or recruiting (n = 46). In 26 trials the current status was recorded as ‘unknown’. Twenty-three trials had terminated, two had been suspended and three had been withdrawn; of these, five had results available. Finally, 133 studies were completed. A summary of the trials is provided in Table 127. The search of ongoing studies did not identify any additional RCTs for inclusion in PenTAG systematic review; 18 studies were already considered in PenTAG review. An overview of these trials is provided in Appendix 8.
TABLE 127
Summary of studies
Critique of company submissions’ search strategies
Submissions from four companies were presented, summarising evidence on the effectiveness of immunosuppressive therapies in renal transplantation: Sandoz, Astellas, Bristol-Myers Squibb and Novartis.
Sandoz
The company’s literature search is primarily focused on finding studies that report on Adoport®, Sandoz’s licensed version of TAC. The searches presented by Sandoz are transparent, replicable and consistent with the aims of the company’s submission, which is a systematic review of Adoport with no economic model.
Sandoz’s literature searches have been conducted in a range of bibliographic databases, including MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and NHS Economic Evaluation Database (NHS EED). These searches have been supplemented with an unreported search of Sandoz’s internal databases.
We believe these searches to be adequate but we are unable to exclude the possibility of reporting bias. The search strategies are geared to locate studies that include the brand name (Adoport) or drug name (TAC) and company name (Sandoz). It is feasible that a title/abstract might merely mention the drug name without a brand or company stated and, if such a study existed, this would be missed by the company’s literature searches. The nature of RCT reporting makes this unlikely for trial data but, for AEs or economic literature, it is a possibility. However, as the manufacturer made an unreported search of its own databases it is unlikely it would have missed one of its own trials.
Sandoz’s submission summarised the evidence on Adoport and compared Adoport with Prograft®, the Astellas-licensed version of TAC. It identified 26 papers: one RCT (reported in two papers) and 24 non-randomised studies (non-RCTs). The RCT was a pharmacokinetics study and had no clinical effectiveness data. None of the included studies is considered in PenTAG systematic review (Table 128).
TABLE 128
Sandoz’s submission: included studies
In summary, the results of Sandoz’s submission are not comparable with the results of the current HTA review.
Astellas
The literature searches have been conducted in the key bibliographic databases, MEDLINE, EMBASE, The Cochrane Library and Cochrane NHS EED.
The literature searches used minimal free-text search terms without the use of truncation or controlled indexing, and selected synonyms were used for the interventions/comparators. This reflects poor sensitivity and, combined with the fact that searching has been conducted on only the abstracts of potentially includable studies, it is possible that some studies may have been missed.
The submission set out to compare the efficacy and safety of TAC (Prograf) therapy with the efficacy and safety of current alternative treatments [TAC-PR (Advagraf), CSA, SRL and BEL] in addition to EVL, as primary immunosuppressive therapies in people undergoing renal transplantation.
Thirty-eight RCTs were identified: 19 studies comparing TAC and CSA regimens, 10 studies comparing SRL and TAC regimens [CNI avoidance (six studies), CNI avoidance and steroids withdrawal (one study), CNI minimisation (three studies)], three trials comparing TAC-PR and TAC regimens, two studies reporting on BEL and six studies reporting on EVL. Two studies239,240 included information for two comparisons. No head-to-head studies comparing TAC with BEL, and TAC with EVL, were identified (Table 129). Two separate NMAs were performed: one comparing TAC with EVL, and another comparing TAC with BEL.
TABLE 129
Astellas’ submission: included studies
In summary, Astellas’ results suggest no significant differences between TAC and EVL regimens, and less BPAR in BEL than in TAC. In the head-to-head comparisons, no differences between TAC and TAC-PR were identified. In addition, more AR episodes were identified in CSA than in TAC and in SRL than in TAC.
In comparison, the PenTAG NMA found evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments were estimated to be any more effective at reducing mortality than any other treatment. In addition, BEL + MMF are estimated to be more effective than CSA + AZA and MMF + CSA at increasing GRF. The head-to-head comparisons suggested that the clinical effectiveness of TAC-PR and TAC are similar, with TAC having a lower proportion of people experiencing the more severe BPAR of Banff III classification (OR 0.11, 95% CI 0.01 to 0.87; I2 = 0.0%). We also found some benefits to using TAC regimens compared with CSA regimens. For a full summary of head-to-head comparisons see Summary of pairwise comparisons, above.
Bristol-Myers Squibb
The literature searching used for this submission is not sufficient to provide a systematic and transparent review of BEL. The literature searching takes the following structure: (terms for TAC) AND (a methodological search filter to limit to RCTs). The literature search does not include any search terms for BEL, the intervention under submission by the company, or CSA.
In practice, this means that the searches will pick up studies of BEL only if BEL is in comparison with TAC. The company states that BEL has not been compared with TAC in head-to-head RCTs, noting that, in the case of BENEFIT59 and BENEFIT-EXT,142 CSA was the main licensed treatment used in clinical practice. This statement further confuses the rationale for using TAC as the named intervention in the literature search for this submission. It is therefore likely that includable trials have been missed (Table 130).
TABLE 130
Bristol-Myers Squibb’s submission: included studies (RCTs)
In summary, because of the issues with the literature searches in Bristol-Myers Squibb’s submission, Bristol-Myers Squibb’s conclusions are not comparable with the results of the current HTA review (see Table 130).
Novartis
The company’s literature search for this submission is systematic, robust and transparent. The company has searched all of the required databases and made an exhaustive attempt to locate published and unpublished studies. The submission compared the efficacy and safety of MPS and EVL, as primary immunosuppressive therapies in people undergoing renal transplantation. A total of seven RCTs, three open-label extension studies of RCTs, as well as three non-RCTs with MPS regimen were identified in the systematic review. A total of 14 studies (25 publications and two unpublished clinical study reports) with EVL regimen were identified in the systematic review; eight RCTs, five prospective studies and one observational study (Table 131).
TABLE 131
Novartis’ submission: included studies
In summary, Novartis’ results suggests that MMF and MPS are comparable. Similar conclusions were made in the current HTA review in head-to-head studies. In addition, the submission suggested the use of EVL in early CNI minimisation. The NMA results of the current HTA review did not suggest that EVL regimens were better in reducing mortality or graft loss and improving GRF than all other treatments. However, the EVL + MPS regimen was estimated to be less effective than the MMF + CSA regimen in reducing the odds of BPAR. In addition, the EVL + CSA regimen was estimated to be more effective than the CSA + AZA regimen in reducing the odds of BPAR. However, apart from the CSA + AZA and EVL + MPS regimens performing poorly in some comparisons, it is difficult to say that any one treatment is more effective than another, as the 95% CIs are very wide.
- Assessment of clinical effectiveness - Immunosuppressive therapy for kidney tran...Assessment of clinical effectiveness - Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model
- Methods - Interventions designed to improve therapeutic communications between b...Methods - Interventions designed to improve therapeutic communications between black and minority ethnic people and professionals working in psychiatric services: a systematic review of the evidence for their effectiveness
- Assessment of cost-effectiveness - A systematic review and economic evaluation o...Assessment of cost-effectiveness - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
- Meta-analysis of diagnostic tests for acute kidney injury - The future for diagn...Meta-analysis of diagnostic tests for acute kidney injury - The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation
- Assessment of clinical effectiveness - A systematic review and economic evaluati...Assessment of clinical effectiveness - A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD
Your browsing activity is empty.
Activity recording is turned off.
See more...