Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Reeves BC, Scott LJ, Taylor J, et al. The Effectiveness, cost-effectiveness and acceptability of Community versus Hospital Eye Service follow-up for patients with neovascular age-related macular degeneration with quiescent disease (ECHoES): a virtual randomised balanced incomplete block trial. Southampton (UK): NIHR Journals Library; 2016 Oct. (Health Technology Assessment, No. 20.80.)

The Effectiveness, cost-effectiveness and acceptability of Community versus Hospital Eye Service follow-up for patients with neovascular age-related macular degeneration with quiescent disease (ECHoES): a virtual randomised balanced incomplete block trial.
Show detailsResource use and unit costs
Tables 16–22 present the main results of the analysis of resource use and costs. In summary, Tables 16 to 19 present resource-use results, with Table 20 providing information on most of the unit costs which would be added to the resource-use information, and staff unit cost information is presented in the next table (Table 21). The resource-use and unit cost information are combined and presented in Table 22, which reports the cost of a monitoring review performed by community optometrists.
TABLE 16
Optometrist capital (equipment and building) resources
TABLE 22
Cost of a monitoring review performed by community optometrists
TABLE 19
Time spent by ophthalmologists on revisiting webinars and consulting other resources
TABLE 20
The ECHoES trial unit costs (2013/14 prices unless otherwise stated)
TABLE 21
Salaries of staff employed in optometrist practices
More specifically, Table 16 shows the mean numbers of equipment items which the community optometrists stated that they already had in their practices when they completed the online resource questionnaire. Although most practices had a CF camera, fewer than half had a projector which includes an ETDRS (Early Treatment of Diabetic Retinopathy Study) chart or a retro-illuminated light box.
In terms of floor space, the respondents said that the average floor space was 173 m2 and the average number of rooms in the practice was 3.5 (SD 2.20 rooms). Of the 39 community optometrists who replied to the question about the need to make modifications to their premises in order to assess nAMD patients, just over 50% said that they would need to do so (mean 0.54, SD 0.505).
In terms of staffing resources, Table 17 presents a breakdown of activities for the monitoring review, the staff members who would perform the various tasks and the average predicted time for the tasks. The optometrists said that they would always take a patient history, carry out a clinical examination and make the final assessment, and that they would be largely responsible for other activities such as undertaking OCTs; pre-registration optometrists and other support staff could help in some of these other activities. The optometrists stated that they expected to book appointments for patients only rarely, as this activity was mainly done by clerical/administrative staff.
TABLE 17
Staff performing each task and average duration of each task
Tables 18 and 19 present information on the number of times that optometrists and ophthalmologists revisited the webinars and consulted other resources. As already described (see Chapter 3, Participants’ views on ECHoES trial training), the ophthalmologists were less likely to revisit the webinars or seek out other sources of information, which might be predicted given that they have more experience than optometrists in caring for nAMD patients.
TABLE 18
Time spent by optometrists on revisiting webinars and consulting other resources
The medical retina experts spent 30 hours preparing and delivering the webinar training for the ECHoES trial (i.e. 15 hours for preparing the webinars, 5 hours for agreeing on the content of webinars and 10 hours for delivering the webinars to the participants).
Table 20 provides a summary of the unit costs which were attached to the resource-use information above.
In terms of unit cost information on salaries for staff working in community optometrist practice, Table 21 provides a breakdown of the salary bands for different types of staff.
Table 22 shows the combination of resource-use and unit cost information which produces the cost for each of the cost categories. The table also shows the sum of these categories, which leads to the total average cost per optometrist monitoring review equal to £51.82 per review (SD £8.153 per review). This sum compares to the average cost of £75.60 (SD £44.31) for ophthalmologists performing a monitory review, as costed in the IVAN trial. The cost of purchasing and setting up equipment and facilities accounted for £2 per patient, with staff labour accounting for much of the rest of the remaining cost of ophthalmologist-based monitoring reviews.
Cost-effectiveness of monitoring by optometrists compared with ophthalmologists
The care pathway cost table (see Table 23) shows the cost and effect information combined, highlighting the impact that incorrect assessments could have on costs. The pathway includes the cost of a monitoring consultation itself and also downstream costs (e.g. ranibizumab injections and follow-up visits based on the care cost pathway decision tree).
TABLE 23
Care pathways costs: base-case analysis
Table 23 shows that, of the vignettes that experts rated as reactivated, the optometrists made more correct decisions than the ophthalmologists (39.43% compared with 36.51%) and were less likely to misclassify reactivated lesions as suspicious or quiescent. When optometrists correctly judge the lesion as reactivated, the patient needs to be referred for another monitoring review at the hospital eye clinic before actually receiving treatment (anti-VEGF injection). There were 795 vignettes assessed in this category for the optometrists (39.43%) and only 736 (36.51%) for the ophthalmologists. There are two points to note here. First, if the optometrist is correct in his or her diagnosis in the model of care delineated in this study, the additional cost for an ophthalmologist-led monitoring review will represent an unnecessary cost. However, if the optometrist is incorrect then the further ophthalmologist-led monitoring review will represent a cost saving because in our model of care we assume that the ophthalmologists are able to detect the mistake and, therefore, the patient will not undergo the unnecessary treatment. Second, and inversely to the optometrist case, when an ophthalmologist says that a patient requires treatment there is not a second monitoring review; the patient simply continues to have their treatment, thereby directly incurring costs. This represents a cost saving with respect to the optometrist care model if the ophthalmologist’s diagnosis is correct (cost of a second monitoring review avoided), but will instead represent an additional unnecessary cost in the event that the patient undergoes unnecessary treatment. Of the vignettes that experts rated as suspicious, 10 (0.50%) were incorrectly classed as reactivated observations in this category for the optometrists, compared with only one (0.05%) in the ophthalmology group. In this case, the second monitoring review in the optometrists’ pathway is actually helpful, as it can prevent unnecessary treatment. However, if we assume that this ophthalmologist would go on to provide treatment when the patient did not need it, this would cost £877 compared with only £118 for an optometrist making an incorrect decision here (this figure is the cost of optometrist review, plus the cost of an ophthalmologist review).
For the truly quiescent vignettes that the health professional incorrectly assesses as reactivated, the ophthalmologists go ahead with treatment, whereas the optometrists will refer a patient to the HES where the mistake will be identified before treatment is given. However, although the cost consequences of incorrectly rating quiescent eyes as reactivated are much smaller for optometrists than for ophthalmologists, optometrists are three times more likely to make this error. For cases where participants correctly rate the vignette as suspicious, only the routine monitoring review is considered here, as both optometrists and ophthalmologists will recommend a subsequent routine check, so options will be cheaper for optometrists than ophthalmologists in this case, reflecting the differential in cost between the two professionals. A similar pathway is implemented in ‘suspicious’ cases incorrectly rated as ‘quiescent’.
Table 24 presents the base-case analysis of the cost-effectiveness of optometrists compared with ophthalmologists in performing monitoring reviews. The cost of a monitoring review is based on an average of the patient cost pathways in the previous table (unweighted according to the most likely pathways). The table shows that the mean care pathway cost for each assessment is quite similar between groups, at £410.78 for optometrists and £397.33 for ophthalmologists, producing an incremental cost difference of £13.45 (95% CI £17.96 to £44.85). The higher cost for optometrists than for ophthalmologists may reflect the fact that optometrists were more likely to incorrectly classify vignettes as reactivated, thereby incurring higher unnecessary costs for the health service.
TABLE 24
Base-case analysis of cost-effectiveness of monitoring review performed by optometrists versus cost of a monitoring review performed by ophthalmologists
In terms of effectiveness information, the proportion of correct treatment decisions is also quite similar, with around 85% of optometrists and ophthalmologists making correct decisions; ophthalmologists made marginally more correct decisions, but this difference was not statistically significant. With higher mean costs (albeit not statistically significant) and a lower proportion of correct treatment decisions (albeit not statistically significant) differences between the groups, Figure 10 shows that the strategy of optometrists performing monitoring reviews is dominated by ophthalmologist-led reviews, with optometrist-led monitoring reviews being more costly and less effective. However, the differences are extremely small: optometrist-led reviews increase the total costs by only £13 per review (3% of the total cost of the care pathway) and result in only one more incorrect decision per 101 monitoring reviews conducted. Furthermore, there is substantial uncertainty around this finding. The CEAC shown in Figure 11 shows that the probability that it is cost-effective for optometrists to perform monitoring reviews is below 30% regardless of how much we are willing to pay per correct decision. In fact, if the NHS is willing to pay less than £600 per correct decision, which is likely to be the case, the probability that it is cost-effective to conduct monitoring by community optometrists is 14% for willingness to pay equal to £200 and 8% for willingness to pay equal to £600.
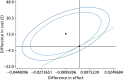
FIGURE 10
Optometrists vs. ophthalmologists cost-effectiveness plane.

FIGURE 11
Cost-effectiveness acceptability curve. Reproduced open access from Violato et al. under the terms of the Creative Commons Attribution (CC BY 4.0) licence.
Thus, it appears that there is no willingness-to-pay level for which we can be 95% confident that the two ways of performing a monitoring review differ in value; that is, they differ in terms of cost-effectiveness.
The CEAC in Figure 11 shows the probability that the optometrists are cost-effective compared with ophthalmologists for a range of willingness-to-pay thresholds. If a decision-maker is willing to pay £200 for an extra correct retreatment decision, then the probability of optometrists being cost-effective is around 14%. The probability that optometrists are cost-effective changes little across a broad range of willingness-to-pay thresholds, indicating that this probability is invariant to the willingness-to-pay threshold.
Figure 10 presents the cost-effectiveness plane and graphs the point estimate of the ICER and two confidence ellipses, with the outer ellipse representing the 95% CI and the inner being the 85% confidence ellipse for our base-case analysis. The figure shows that the 95% CI is not definable and there is no willingness-to-pay threshold for which we can be 95% confident that the optometrist-led and the ophthalmologist-led monitoring reviews differ in value from each other. The widest definable Fieller interval is 85% and the green line shows the tangent to the 85% confidence ellipse.
Sensitivity analyses
One-way sensitivity analyses investigated the impact of varying the way of delivering treatment for lesions assessed as reactivated, which is one of the main cost drivers in our analysis. In the base-case analysis it was assumed that treatment for an active lesion consisted of one ranibizumab injection given during an injection consultation. In three of our sensitivity analyses, one ranibizumab injection was replaced with alternative treatments to reflect new emerging practices across eye hospitals (sensitivity analyses 1 and 2) and to make a comparison with a much cheaper drug assessed in the IVAN trial (sensitivity analysis 3):
- Sensitivity analysis 1: all patients in whom treatment for a reactivated lesion was initiated were assumed to receive a course of three injections of ranibizumab at three injection consultations, with no additional monitoring reviews. The ‘mandatory’ three injections, at monthly intervals, matched the discontinuous treatment regimen administered in the IVAN trial (although in the IVAN trial monitoring continued thereafter).
- Sensitivity analysis 2: treatment was assumed to be given in the form of one aflibercept injection during an injection consultation.
- Sensitivity analysis 3: treatment consisted of one bevacizumab injection given during an injection consultation.
- Sensitivity analysis 4: only considered the cost of a monitoring review rather than considering the cost of the whole pathway.
Sensitivity analysis 1 (three ranibizumab injections and consultations) increases the costs of lesion care at the eye hospital because more treatment and consultations are required and, once combined with data on the consequences of retreatment decisions in our care pathways model, optometrists remained dominated, although there remained very little difference in costs or effects between optometrists and ophthalmologists. Akin to our base-case analysis, there was also no acceptable willingness-to-pay threshold for which we can be 95% confident that the two alternative ways of performing a monitoring review differ in value. A similar result was found for the sensitivity analysis that used aflibercept rather than ranibizumab for treatment, which was predictable given that aflibercept is more expensive than ranibizumab. When switching to the much cheaper bevacizumab (in place of ranibizumab), with a reduced cost of treatment, again very little difference was found in costs and effects difference across the two groups, with optometrist-led reviews again being more costly and less effective than ophthalmologists-led monitoring reviews. However, the fourth sensitivity analysis, which considered only the cost of the monitoring review, rather than the total care pathway information, found that community optometrist-led care cost £23.70 less per consultation than ophthalmologist-led care (the difference between the crude consultation costs, p < 0.001). As a result, optometrist-led care was less effective and significantly less costly than ophthalmologist-led care. Ophthalmologist-led care cost an additional £2389 per additional correct treatment decision compared with optometrist-led care. Although the maximum the NHS is willing to pay for a correct retreatment decision is unknown, it is unlikely to be this high (since this figure is higher than the cost of simply treating all patients without assessing whether or not the eye is quiescent). At ceiling ratios of £600 or lower, we can be > 95% confident that optometrists are a cost-effective option compared with ophthalmologists in this fourth sensitivity analysis. This final sensitivity analysis highlights how important it was to have built a simple decision model to explore the consequences after the initial monitoring review and not just use the information from the initial review. However, it also suggests that the conclusions may be sensitive to the assumptions within the decision tree. The economic evaluation section in Appendix 3 presents the results of the sensitivity analysis in respect to the cost care pathways, cost-effectiveness analysis and the respective cost-effectiveness planes for the four sensitivity analyses.
Budget impact
Data on the prevalence and incidence of nAMD (Table 25) were used to calculate that around 219,000 patients currently attend VEGF clinics in England in any given month, of which 52,000 (19%) have bilateral disease (Table 26). In our budget impact calculations, we assumed that patients would be referred from the HES to community optometrists for monitoring if they did not meet the IVAN retreatment criteria 1 month after finishing a course of anti-VEGF treatment. We assumed that patients with bilateral disease would not be referred from the HES to community optometrists for monitoring if they met retreatment criteria in either eye in the same month; the probability of meeting retreatment criteria was assumed to be independent in the two eyes.
TABLE 25
Data inputs for budget impact calculations
TABLE 26
Results of budget impact calculations
In the IVAN trial, 18% of study eyes in the discontinuous treatment arms completed a 3-month cycle of treatment each month, of which 38% still met retreatment criteria at the next visit. The probability of meeting retreatment criteria and the duration of quiescence were constant over the trial period; therefore, no distinction was made between the first and subsequent years of treatment. Applying these figures to the national patient numbers and allowing for patients with bilateral disease suggests that around 21,949 of the total of 219,514 patients currently attending clinics may be eligible for referral to community optometry review each month (see Table 26), assuming that such referrals would occur in all patients who are quiescent according to the IVAN trial criteria 1 month after their last anti-VEGF injection. In the IVAN trial, quiescence lasted a median of 61 days; allowing for this duration and extrapolating monthly figures to 12 months suggests that 535,548 monitoring reviews could be done by community optometrists in England (see Table 26) each year.
Applying the results of the ECHoES trial costing analysis suggests that the initial monitoring consultation is £23.70 cheaper if performed by community optometrists rather than by hospital ophthalmologist-led teams. Scaling this up across the 535,548 visits that could be transferred to community optometrists suggests that initial savings of £12.7M could be made for the NHS in England. However, this figure takes no account of the increased numbers of second monitoring reviews or additional intravitreal injections that result from optometrist judgements. If we allow for the costs accrued from the entire pathway (see Table 24), optometrist care is £13.45 more costly and therefore referring patients to community optometry review will cost an additional £7.2M across England.
- Results: health economics (objective 4) - The Effectiveness, cost-effectiveness ...Results: health economics (objective 4) - The Effectiveness, cost-effectiveness and acceptability of Community versus Hospital Eye Service follow-up for patients with neovascular age-related macular degeneration with quiescent disease (ECHoES): a virtual randomised balanced incomplete block trial
- Clinical effectiveness results - Chair-based yoga programme for older adults wit...Clinical effectiveness results - Chair-based yoga programme for older adults with multimorbidity: RCT with embedded economic and process evaluations
- Treatment of extravasation injuries in infants and young children: a scoping rev...Treatment of extravasation injuries in infants and young children: a scoping review and survey
- A pragmatic randomised controlled trial of the effectiveness and cost-effectiven...A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of ‘PhysioDirect’ telephone assessment and advice services for physiotherapy
- Acta1 actin alpha 1, skeletal muscle [Mus musculus]Acta1 actin alpha 1, skeletal muscle [Mus musculus]Gene ID:11459Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...