Transition from response to infliximab to loss of response in primary responders
There was insufficient published information to model an ADA test-based treatment strategy. The model therefore addresses patients responding to IFX maintenance therapy (the transition probabilities used are summarised in Table 35). It should be emphasised that there were no prospective or other test-directed management studies describing outcomes for IFX responders followed from maintenance treatment through to treatments subsequent to LOR to maintenance. Therefore, by necessity, model structure for the intervention arm is based on the algorithms used in the two identified RCTs describing test-based patient management, specifically the TAXIT trial73 for responders and Steenholdt et al.123 for patients with LOR to maintenance IFX (see Chapter 3, Objective B: description of algorithms prescribing patient management following test outcomes for drug and/or anti-drug antibody levels); we aimed to use as many data from these RCTs as possible to populate the model. Unfortunately the control arm in the TAXIT trial does not provide information for the model’s standard care arm (a no-test management strategy) because all patients in the TAXIT trial were dose-optimised according to test results prior to randomisation; consequently, the model structure for the standard care arm is based on expert clinical advice and alternative studies were examined for model input.
Standard care arm: loss of response to infliximab maintenance
For the standard care arm, three studies that reported reasonable quality data for time to LOR or to cessation of IFX treatment for patients on maintenance treatment with IFX were identified.82,128,159 Reconstructed Kaplan–Meier plots with candidate parametric models are shown in Figure 60. For the Juillerat et al.159 study, several models provided reasonable fit to 130 cycles.
These three studies generate fairly different transition probabilities. Because of its size, the availability of observed data to 130 cycles (model time horizon), and the inclusion of only CD patients, the Juillerat et al. study159 was selected for model inputs. In Juillerat et al.,159 21% of patients received dose escalation, but the time to escalation was not reported. However, Ma et al.161 have reported the time to LOR requiring dose escalation for patients with CD on IFX maintenance therapy; Weibull and Gompertz models provided best fits to the Ma et al.161 data.
Figure 61 shows both Juillerat et al.159 and Ma et al.161 data with Weibull parametric models. Transition probabilities generated by these Weibull models were used for economic model input. These allow estimates of the percentage of time over 130 cycles spent in each of the following conditions: (1) untreated with IFX, (2) in standard dose treatment with IFX and (3) in escalated dose treatment with IFX. The resulting percentages were 35.6%, 24.0% and 40.4%, respectively.

FIGURE 61
Reconstructed Kaplan–Meier plots and exponential fits for time to cessation of IFX treatment and time to LOR requiring dose escalation of IFX by 4-week cycle (studies of Juillerat et al. and Ma et al.).
Standard care arm treatment after loss of response to infliximab
On failure of response to IFX maintenance (with or without dose escalation) it is assumed patients are switched to ADA induction therapy followed by maintenance on ADA for those responding to induction. We classify those that fail induction as patients who have lost response during the first cycle of treatment. We have taken this from the Gauging Adalimumab Efficacy in Infliximab Nonresponders (GAIN) RCT,162 which investigated ADA for patients who had failed IFX. Thereafter, the transition probability for LOR to ADA was derived from the study by Karmiris et al.48 of 152 CD responders receiving ADA followed up prospectively (Figure 62). Exponential and Weibull distributions provided a reasonable fit to reconstructed IPD. Combined data from Sandborn et al.162 for induction failure on ADA (after IFX failure) and Karmiris et al.48 for failure after successful induction with ADA, provided a transition probability of 0.058553 per cycle that was used in the model.
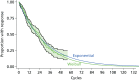
FIGURE 62
Reconstructed Kaplan–Meier and Weibull model for time to LOR for patients with CD on maintenance therapy with ADA by 4-week cycle.
After failure of ADA we have assumed patients remain in a LOR state until such time that they receive surgery. This assumption was necessitated by lack of data and was based on advice of clinical experts. The transition to surgery was based on a large Canadian study.163
Time to surgery
No data were found for time to surgery for patients who experience LOR or a failure to regain response after a treatment switch aimed to reinstate a response. We identified three studies163,183,184 that provided time from diagnosis to surgery for recent cohorts of patients with CD (i.e. coincident with the era of anti-TNF-α therapies for CD). Vester-Anderson et al.183 reported surgical relapse rates of 6%, 18% and 23% at 1, 5 and 7 years (91 cycles) after diagnosis, respectively; similarly a UK study175 that included 137 patients observed approximately 24% primary surgery 5 years after diagnosis (Figure 63) and a larger Canadian study163 included > 1000 patients and also data for recurrent surgery (see Figure 63). Figure 63 shows the time to primary surgery was similar in the UK and Canadian studies. As a result of its size and because it provided data for both primary and recurrent surgery, the Canadian study was used in the economic modelling for both primary and recurrent surgery.

FIGURE 63
Time from diagnosis of CD (a) to primary surgery in the UK; (b) to primary surgery in Canada; and (c) time from first surgery to second surgery in Canada.
Crohn’s disease patients in the TAXIT trial73 and Steenholdt et al.123,124 management studies varied considerably in the time from diagnosis to study entry and also in if they had experienced previous surgery (e.g. TAXIT trial73 patients, on average, were diagnosed 13.7 years prior to entry and 70% had received previous surgery; in Steenholdt et al.123,124 patients were diagnosed, on average, 9 years before entry). Surgery was not a primary or secondary outcome measure in these studies, but each reported that one patient received surgery (1/69 by week 20 in Steenholdt et al.123,124 and 1/251 by week 52 in the TAXIT trial73). It appears that during the short follow-up periods observed the use of surgery was a relatively rare event. The Weibull and Gompertz parametric models (see Figure 63) of time to surgery generated probabilities of progressing to surgery that varied considerably according to time from diagnosis; the economic model was not based on newly diagnosed patients, and data for other health states used in modelling were not based on newly diagnosed patients; in the absence of more appropriate data transition probability to surgery we used an exponential (constant hazard) fit to the Canadian data so as to capture an approximate average transition probability (see Table 37). It is recognised that these selections are somewhat arbitrary and that modelling extends beyond the observed data.
Maintenance of surgery-induced remission
The scant evidence about maintenance of surgically induced remission in CD was reviewed by Gordon et al.164 in a Cochrane systematic review. It should be noted that the authors’ rated the included studies to be at high risk of bias for these outcomes. At 2 years across three studies there was no difference in risk of clinical relapse between patients receiving purine analogues and those receiving 5-ASA (fixed-effects pooled RR 1.01, 95% CI 0.81 to 1.24). The total events were 146 among 265 patients. Assuming a constant hazard the estimated transition probability to post-surgical clinical relapse is 0.023971 per cycle (95% CI 0.025398 to 0.035624 per cycle). In the economic model, this was taken to apply for both therapies (5-ASA and purine analogues). Relative to purine analogues, the review data suggest that patients receiving no therapy (placebo group in two studies) were at 1.35 (95% CI 1.06 to 1.72) greater risk of clinical relapse. Assuming a constant hazard provided an estimated transition probability of 0.050961 per cycle (95% CI 0.033248 to 0.108412 per cycle); this was used in the model for the group given no therapy. One study185 included in the Gordon et al.164 review found a RR for clinical relapse of 0.5 for IFX versus purine analogues; this study observed only three events among 22 patients giving, on assumption of constant hazard, a transition probability to clinical relapse for IFX-treated patients of 0.0119855 per cycle.
In view of the considerable uncertainty necessarily associated with this estimate of response loss with IFX, and the lack of information on timing of events, we looked for alternative data. Baert et al.77 reported time-to-event data for reintroduction of IFX following at least 15 months after LOR despite dose optimisation. During the ≥ 15-month IFX holiday some patients received surgery. Time to LOR after IFX reintroduction is shown in Figure 64 together with the exponential fit used to estimate transition probabilities for the economic model. Owing to a lack of data, we have assumed the same transition probabilities for patients receiving anti-TNF-α in combination with immunosuppressants to be the same as that for IFX alone.
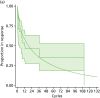
FIGURE 64
Reconstructed Kaplan–Meier plot and Weibull fit for time to LOR after reintroduction of IFX after surgery by 4-week cycle (based on data from Baert et al.). (a) Weibull; and (b) exponential.
Intervention arm: loss of response to test-directed infliximab maintenance
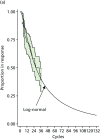
Only two management studies of IFX responders were found and one of these, Vaughn et al.,128 was a retrospective study at considerable risk of selection bias such that the large reported advantage for the poorly defined test-based strategy lacks face validity (Figure 65). The TAXIT73 randomised controlled management study of responders to IFX maintenance did not report time to LOR. ‘Durable remission’ among the TAXIT trial IBD patients at week 52 post randomisation (13 cycles) was reported to be almost the same for test algorithm strategy patients who were dose escalated, or who received no dose adjustment, or whose dose was reduced (28.6%, 26.4% and 25%, respectively). On this basis we have assumed that LOR was also unlikely to differ significantly between these groups. The p-value for the comparison of test-based dosing with clinically based dosing was 0.88. Of patients with CD in the TAXIT trial intervention arm, 79.77% were in clinical remission at randomisation and 62.6% in clinical and biological remission at week 52. There was no time-to-event data for clinical remission; however, if a constant hazard is assumed for loss of remission the resulting transition probability is 0.018477165 per cycle (Figure 66). This represents a very severe test for LOR because patients without clinical remission are likely to be retained in anti-TNF-α treatment because of a partial response. The retrospective management study of Vaughn et al.128 reported vastly superior performance for 39 IBD patients receiving a test algorithm strategy relative to 68 patients given a clinically based dosing strategy; when time to treatment cessation for these 39 patients was fitted with an exponential distribution a transition probability of only 0.003928414 per cycle is generated (see Figure 66). These transition probabilities are very different and it is doubtful that either generates an appropriate input for the economic model.

FIGURE 65
Log-normal models for retention in IFX maintenance therapy for IFX responders (based on Vaughn et al. and used in sensitivity analysis).
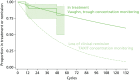
FIGURE 66
Time to event for responders receiving a test algorithm strategy. Time to clinical remission in the TAXIT trial and retention in treatment in Vaughn et al. by 4-week cycle.
In the TAXIT study before dose optimisation, 131 out of 178 (73.59%) patients with CD were in clinical remission; after dose optimisation with a test-directed dose adjustments, 138 out of 173 (79.77%) were in remission (five patients with CD could not be optimised to target trough level). According to ITT analysis, this represents a 3.9% improvement. With continued test-directed dosing post randomisation, 62.6% of patients with CD were in remission (clinical and biological) at 52 weeks, whereas 54.9% were in remission with clinically based post-randomisation dosing, implying a small advantage for the testing strategy (approximately 7.7%) (p = 0.353 for comparison between groups). These small differences (3.9% and 7.7%) can be explained by the play of chance and are obviously associated with considerable uncertainty. We found no other evidence of clinical benefit from a test algorithm-based strategy. In the absence of other evidence demonstrating an advantage for a test algorithm-based strategy the model uses the same probability for LOR to IFX as used for the standard care arm (fit to Juillerat et al.159 data).
Intervention arm: regain of response with test-directed treatments following response loss to maintenance infliximab
The treatments for patients with LOR to maintenance IFX were informed by the management study of Steenholdt et al.123 [Chapter 3, Objective C2: studies relating test results to clinical state of patients (correlation studies)]. Patients enrolled in this study had failed IFX maintenance in which patients received ‘regular infusions of 5 mg/kg’. It is recognised that this regimen does not exactly correspond to the dose being received by patients during the 52 weeks of the TAXIT trial,73 in which dose was variously adjusted to bring trough IFX to a target range. In Steenholdt et al.123 patients received concurrent testing at the time of IFX failure and subsequent treatment followed an algorithm based on test results and was aimed at regaining response.
Concurrent testing identified four groups of intervention patients in the following proportions: (1) IFX negative and antibodies positive (n = 5; 15.15%); (2) IFX negative and antibodies negative (n = 1; 3.03%); (3) IFX positive and antibodies negative (n = 26; 78.79%); and (4) IFX positive and antibodies positive (n = 1; 3.03%). The study reported the proportion who regained a response by 12 weeks, but time-to-event data were not reported. We have assumed that those who had not regained response by week 12 have lost response at a rapid rate over three cycles and remained in the non-response state (until surgery was implemented), and those who were in a response state at week 12 then proceeded to lose response at a given rate dependent on their algorithm-directed treatment regimen. The number of patients in all groups except group 3 was small, and so outcomes are associated with great uncertainty. We have assumed that the single group 4 individual (positive test results for both IFX and antibodies to IFX) had the test results confirmed and was subsumed according to the treatment algorithm into group 3, which then accounts for 27 out of 33 (81.8%) of intervention patients. Unfortunately, the various treatments used for the group 3 patients were insufficiently prescribed to be usable (e.g. surgery ‘should be considered’).
For intervention group 1 patients (15.15% of IFX failures), the algorithm-prescribed treatment was a switch to maintenance therapy with ADA: at 12 weeks 2 out of 5 had regained response. This is a poor response rate, but is based on only five patients and is uncertain. We have therefore used the same transition probabilities for these patients as for ADA-treated patients in the standard care arm (based on the GAIN RCT and on the study by Karmiris et al.48 described in Figure 62).
The single group 2 patient (3.03% of intervention patients) received IFX intensification and failed to regain response by week 12. However, all control arm patients in the trial also received IFX intensification and at 12 weeks 19 out of 36 had regained response; which, when combined with the single group 2 patient, provides an estimate of 19 out of 37 (51.3%) in response at week 12. We assume the last patients move to non-response at constant hazard over the first three cycles (12 weeks) providing a transition probability of 0.19948 per cycle. However, using this transition probability would considerably disadvantage the model intervention arm relative to the control arm, and is based on a single time point estimate for a small group of patients. Therefore, the rate of loss of regained response for group 2 was assumed to be the same as that for dose-escalated IFX patients described by Ma et al.,161 which is based on 6 years of time-to-event data (see Figure 61 for the model based on data from Ma et al.161).
In groups 3 and 4 (81.81% of IFX failures), 16 out of 27 had regained a response at 12 weeks and 11 out of 27 were in a state of non-response. We assume that the latter group lost response at constant hazard over the 12 weeks providing a transition probability of 0.16004 per cycle. As the treatment for group 3 patients was not prescribed, other than that it lacked anti-TNF-α, we have assumed that after cycle 4 (12 weeks) LOR occurs at constant hazard based on the Rutgeerts et al. RCT165 placebo arm (background therapies including purine analogues, steroids, methotrexate and 5-ASA), in which about half of patients had previously received previous anti-TNF-α therapy. This suggested a transition probability of 0.08617343 per cycle.
In the Steenholdt et al. study123 about half of group 3 patients likely received IFX in contradiction to the specified treatment according to the algorithm.
Of these, 12 patients continued IFX (nine patients from group 3 and one patient from group 4). The applied IFX regimen was (all received 5 mg/kg):
- IFX q8 regimen (two infusions during the trial, i.e. weeks 0 and 8) – n = 5
- IFX q4 regimen (four infusions during the trial, i.e. weeks 0, 4, 8 and 12) – n = 2
- IFX q4 regimen but not throughout the entire trial (three infusions during the trial) – n = 1
- IFX q4 regimen but not throughout the entire trial (two infusions during the trial) – n = 2
- IFX q4 regimen but not throughout the entire trial (one infusions during the trial) – n = 2.
The remaining two patients had been switched to ADL because of misinterpretation of test results (see Figure 2). Both patients were in group 3.
The applied ADL regimen was:
- ADL induction (160 mg–80 mg–40 mg) and followed by 40 mg every other week.
This indicates the various treatments received by the 14 patients in the intervention arm who did not receive algorithm-directed treatments. In view of these difficulties it is difficult to discern how treatment received relates to response observed at cycle 3.
The patients with LOR from all groups remain on palliative care in a LOR state until surgery. It is possible that some of these patients (and also those patients with LOR after ADA in the standard care arm), at some time may be reintroduced to IFX (or possibly ADA) prior to surgery and may regain response; however, lack of evidence precluded modelling this. We have assumed that after surgery various treatments are administered in attempts to maintain post-surgical remission and that these are the same as for the standard care arm.
Publication Details
Copyright
Included under terms of UK Non-commercial Government License.
Publisher
NIHR Journals Library, Southampton (UK)
NLM Citation
Freeman K, Connock M, Auguste P, et al. Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling. Southampton (UK): NIHR Journals Library; 2016 Nov. (Health Technology Assessment, No. 20.83.) Appendix 17, Transition probabilities derived from published studies.