Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Freeman K, Connock M, Auguste P, et al. Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling. Southampton (UK): NIHR Journals Library; 2016 Nov. (Health Technology Assessment, No. 20.83.)
![Cover of Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling](/corehtml/pmc/pmcgifs/bookshelf/thumbs/th-ukhta2083-lrg.png)
Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling.
Show detailsOverview
Anti-tumour necrosis factor alphas (TNF-αs), including infliximab (IFX) (Remicade®, Merck Sharp & Dohme Ltd, Kenilworth, NJ, USA) and adalimumab (ADA) (Humira®, AbbVie Inc., North Chicago, IL, USA), are given to patients with inflammatory bowel disease (IBD), including Crohn’s disease (CD), as a second- or third-line therapy. Response to anti-TNF-α treatment varies among patients treated for inflammatory chronic conditions. Although some patients stay in response over a long period of time, some are weaned off the drug because it is no longer needed and others may lose response at some stage during treatment. Loss of response (LOR) can occur for various reasons, the most common being (1) formation of antibodies against the drug, which neutralise the drug’s action, rendering it ineffective; and (2) ongoing illness as a result of inflammation that is not meditated by TNF-α. It has been proposed that measurement of serum levels of anti-TNF-α and its antibodies can aid the management of patients with chronic diseases on anti-TNF-α drugs.
Measurement of anti-TNF-α levels and its antibodies can be carried out concurrently (concurrent testing strategy) or antibody testing can be carried out conditional on the absence of measurable drug levels (reflex testing strategy).
The linked evidence approach that was adopted in this review is a methodology to handle shortcomings in the evidence for medical test evaluations.1 The idea is to link evidence from other relevant research to the expected benefits of the test in question when direct evidence from the test and its effects on patient outcomes is absent. The decision-analytic model for the cost-effectiveness analysis is informed by systematically identified indirect evidence to predict the impact of the test under evaluation on patient outcome. The validity of this approach is dependent on the similarity of the populations, tests and outcomes across the linkages.1
This report contains reference to confidential information provided as part of the National Institute for Health and Care Excellence (NICE) appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Descriptions of the health problem: Crohn’s disease
Crohn’s disease is a chronic, fluctuating, episodic, inflammatory condition of the digestive tract; it is uncommon and is currently estimated to affect about 115,000 people in the UK,2 with about 3000 new cases diagnosed each year.3 The aetiology of CD is still largely unknown but environmental, genetic and immunological factors are believed to play a role, as are previous infections and smoking.4
Aetiology and pathology
Crohn’s disease can affect adults, adolescents or children. CD manifests mainly during late adolescence or early adulthood. The first onset most commonly occurs between the ages of 16 and 30 years, with a second peak between the ages of 60 and 80 years. Women are slightly more frequently affected than men, but in children it is seen more often in boys than in girls. CD is most common in white people in westernised countries and has its highest prevalence among Jewish people of European descent.4
Crohn’s disease follows a pattern of acute disease (relapse) interspersed with periods of remission (lack of symptoms). CD causes inflammation of the lining of the digestive tract, which, depending on the individual, occurs at any location from the mouth to the rectum, but most commonly affects the end of the small intestine (terminal ileum; 35%) or the connection between the small intestine and large intestine (ileocaecal region; 40%).5 Within individuals the disease location is fairly constant.
Fistulising CD describes the condition in patients who have developed complications in the form of abnormal connections between the bowel and other organs known as fistulae. Fistulae develop in between 17% and 43% of people with CD.6 Active luminal CD describes the condition in patients who have inflammation in the tube of the intestine.
The main symptoms of CD depend on the location of disease. They include abdominal pain, chronic or nocturnal diarrhoea, anal lesions, rectal bleeding, weight loss and swelling of the abdomen with tenderness. Complications include strictures, perforations, abdominal obstructions and development of fistulae. Extraintestinal symptoms related to intestinal inflammation include inflammation of the joints, skin, liver and the eyes.7 CD in children is often noticed because of growth failure.8 Symptoms range in severity and the assessment of severity is used to classify CD into mild, moderate or severe disease according to disease activity scales. A response is defined as a reduction in symptoms.
Measurement of disease activity
Crohn’s disease can be difficult to diagnose because symptoms overlap with those of other gastrointestinal disorders, such as ulcerative colitis (UC) and irritable bowel syndrome. Investigations to aid diagnosis include taking the patient’s medical history, physical examination, blood and stool tests and, finally, endoscopy to confirm diagnosis. As the treatment for CD depends on the location and severity of disease, an assessment of disease activity once disease is confirmed is important. However, disease activity is difficult to assess, and a global measure which includes clinical, endoscopic, biochemical and pathological features to define the heterogeneous disease pattern of CD is not available.9 This means that there is no ‘reference standard’ for the assessment of disease severity, which has important implications for this assessment. For example, there is no standardised definition for when remission has been achieved. The two most commonly used measures of disease activity are the Crohn’s Disease Activity Index (CDAI) and the Harvey–Bradshaw Index (HBI) (a simplified version of the CDAI), which are based on the patient’s history, physical features and laboratory data. A paediatric CDAI that emphasises the less subjective laboratory parameters has been developed.10 Additional measures include the Perianal Disease Activity Index (PDAI), the Inflammatory Bowel Disease Questionnaire (IBDQ) and the Crohn’s Disease Endoscopic Index of Severity.5 However, these tools have been primarily developed for clinical trials rather than clinical practice. In clinical practice, the use of endoscopy to assess mucosal healing as an indicator of response and remission is becoming increasingly important, and the potential of objective laboratory markers, such as C-reactive protein (CRP) and faecal calprotectin (FC), for the assessment of disease activity, risk of complications and prediction of relapse, and for monitoring the effect of therapy has been recognised.11
The variables measured by the CDAI measures include number of liquid stools, abdominal pain, general well-being, extraintestinal complications, use of anti-diarrhoeal drugs, abdominal mass, haematocrit and body weight.12 These are weighted according to their ability to predict disease activity, leading to an individual score ranging from 0 to 600. The CDAI has been criticised for giving too much weight to relatively subjective items;12 however, more objective measures, such as mucosal healing on endoscopy, are not infallible either because of the patchy distribution of inflammation in CD. Samples taken for examination may not necessarily be representative of the whole bowel.2
Although the CDAI uses a symptom diary of the patient over 7 days for the assessment, the HBI uses only a 1-day diary entry for assessment. Furthermore, the HBI does not take into consideration body weight, haematocrit and use of drugs for diarrhoea for the measurement of disease activity. HBI scores range from 0 to 20.13
In the absence of standardised definitions in which scores correspond to the different disease severity stages, this review adopts the definitions from the NICE guidance technology appraisal 187:6
- Remission is defined as a CDAI score of < 150 points.
- Moderate to severe disease is defined as a CDAI score of > 220 points.
- Severe disease is defined as a CDAI score of > 300 points.
Response (i.e. relief of symptoms) has often been defined as a reduction in the CDAI score of at least 70 points from baseline.14
Severe active CD was defined for the purpose of the indication of IFX or ADA treatment as:
Very poor general health and one or more symptoms such as weight loss, fever, severe abdominal pain and usually frequent (3–4 or more) diarrhoeal stools daily. People with severe active Crohn’s disease may or may not develop new fistulae or have extra-intestinal manifestations of the disease. This clinical definition normally, but not exclusively, corresponds to a Crohn’s Disease Activity Index (CDAI) score of 300 or more, or a Harvey–Bradshaw score of 8 to 9 or above.
NICE (2010) technology appraisal number 187. Infliximab (Review) and Adalimumab for the Treatment of Crohn’s Disease. London: NICE. Available from www.nice.org.uk/guidance/TA187.6 Reproduced with permission. This information is accurate at time of publication
Furthermore, the Practice Parameter Committee of the American College of Gastroenterology has produced definitions of disease severity.7 These are as follows.
Mild to moderate disease
Mild–moderate disease applies to ambulatory patients able to tolerate oral alimentation without manifestations of dehydration, toxicity (high fevers, rigors, prostration), abdominal tenderness, painful mass, obstruction, or > 10% weight loss.
Hanauer and Sandborn7
Moderate to severe disease
Moderate–severe disease applies to patients who have failed to respond to treatment for mild–moderate disease or those with more prominent symptoms of fever, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting (without obstructive findings), or significant anaemia.
Hanauer and Sandborn7
Severe to fulminant disease
Severe–fulminant disease refers to patients with persisting symptoms despite the introduction of steroids as outpatients, or individuals presenting with high fever, persistent vomiting, evidence of intestinal obstruction, rebound tenderness, cachexia, or evidence of an abscess.
Hanauer and Sandborn7
Remission
‘Remission’ refers to patients who are asymptomatic or without inflammatory sequelae and includes patients who have responded to acute medical intervention or have undergone surgical resection without gross evidence of residual disease. Patients requiring steroids to maintain well-being are considered to be ‘steroid-dependent’ and are usually not considered to be ‘in remission’.
Hanauer and Sandborn. Reprinted by permission of Nature Publishing Group from Macmillan Publishers Ltd: The American Journal of Gastroenterology, Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. Am J Gastroenterol 2001;96:635–43,7 copyright 2001
Management and care pathway
The treatment of CD is complex; in general, it aims at (1) reducing symptoms through induction and maintenance of remission, (2) minimising drug-related toxicity and (3) reducing the risk of surgery.15 The management options for CD include drug therapy [e.g. glucocorticosteroids, 5-aminosalicylic acid (5-ASA), antibiotics, immunosuppressants, TNF-α inhibitors], enteral nutrition, smoking cessation and, in severe or chronic active disease, surgery. The choice of treatment among the available drugs is influenced by patient age, site and activity of disease, previous drug tolerance and response to treatment, and the presence of extraintestinal manifestations.16,17 Enteral nutrition is widely used as a first-line treatment to facilitate growth and development in children and young people. Adjuvant therapy commonly coexists and includes management of extraintestinal manifestations, antibiotics, corticosteroids or immunosuppressant therapy. Between 50% and 80% of people with CD require surgery because of complications such as strictures causing symptoms of obstruction, fistula formation, perforation or failure of medical therapy.2
Once remission has been achieved, maintenance therapy can be considered following assessment of the course and extent of CD, effectiveness and tolerance of previous treatments, presence of biological or endoscopic signs of inflammation, and potential for complications.15
Induction of remission according to the National Institute for Health and Care Excellence Clinical Guideline number 1522
Usually, at first presentation, patients with active CD are recommended to receive monotherapy treatment with conventional steroid therapy (i.e. glucocorticoids including prednisolone, methylprednisolone or intravenous hydrocortisone), which is aimed at inducing remission as a first-line treatment. Alternatively, treatment with budesonide, 5-ASA or enteral nutrition may be offered for patients who do not choose to take or who are intolerant of glucocorticosteroid therapy.
The addition of an immunosuppressant (azathioprine, mercaptopurine or methotrexate) to a conventional glucocorticosteroid or budesonide is recommended as an add-on therapy for inducing remission in patients who have active CD and who have experienced two or more inflammatory exacerbations in a 12-month period, or in whom glucocorticosteroid doses cannot be tapered. As advised in the current online version of the British National Formulary (BNF)18 or BNF for Children,19 the effects of azathioprine, mercaptopurine and methotrexate, as well as levels of neutropenia (in patients on azathioprine or mercaptopurine), should be monitored.15
In adults and children aged 6–17 years with severe active CD who fail to respond to the first line of treatment with conventional therapy (e.g. immunosuppressants or corticosteroids), or who are intolerant to or who have contraindications to conventional therapy, anti-TNF-α agents (IFX and ADA) are recommended as treatment options within their licensed indications. The administration of anti-TNF-α agents is recommended until 12 months after the start of treatment or until treatment failure (including the need for surgery), whichever occurs first. Reassessment and monitoring of disease activity (at least every 12 months) is advised to ascertain the clinical appropriateness of ongoing treatment. Usually, treatment is initiated with the less expensive drug (i.e. IFX), considering drug administration costs, dose and product price per dose. The use of anti-TNF-α drugs for the treatment of CD is covered in the 2010 NICE technology appraisal guidance 187 [IFX (review) and ADA for the treatment of CD], which is summarised in NICE guidelines.6
Surgery should be considered early in the course of the disease for patients whose disease is limited to the distal ileum or for children and young people who have growth impairment despite optimal medical treatment and/or who have refractory disease.2
Maintenance of remission according to the National Institute for Health and Care Excellence clinical guideline 1522
Patients with CD in remission can be managed with or without maintenance treatment. The options for maintenance (including treatment or no treatment) need to be discussed with patients and parents or carers. The discussion should include risk of relapse and the potential side effects of drug treatments. Patients who decide not to use maintenance treatment should agree follow-up plans (e.g. frequency and duration of visits) and should receive information on markers and symptoms of relapse (e.g. unintended weight loss, abdominal pain, diarrhoea or general ill-health) to ensure that they keep their disease appropriately under review with their health-care professionals.
Patients with CD in remission who choose to receive maintenance therapy may be offered a single drug such as azathioprine or mercaptopurine if remission has been induced using a conventional glucocorticosteroid or budesonide. Methotrexate can be offered if remission was induced by methotrexate or to patients who are not able to tolerate, or who have contraindications to, azathioprine or mercaptopurine. Treatment with 5-ASA can be used to maintain remission after surgery.
If remission has been achieved with anti-TNF-α medication, then maintenance with anti-TNF-α with or without an immunosuppressant can be used. Continuation of treatment with IFX or ADA during remission is advised only if there is evidence of ongoing active disease assessed by clinical symptoms, biological markers and endoscopy, if necessary. The balance between harms and benefits of ongoing treatment should be taken into account. The guideline states that patients who relapse after anti-TNF-α treatment may start it again.15
National Institute for Health and Care Excellence guidelines
The NICE guideline technology appraisal 1876 describes when IFX or ADA should be used to treat patients with severe active or fistulising CD in the NHS in England and Wales.
Infliximab
The guideline states:
Infliximab has a UK marketing authorisation for the treatment of:
NICE (2010) technology appraisal number 187. Infliximab (Review) and Adalimumab for the Treatment of Crohn’s Disease. London: NICE. Available from www.nice.org.uk/guidance/TA187.6 Reproduced with permission. This information is accurate at time of publication
Administration of IFX should follow this pattern:
. . . 5-mg/kg intravenous infusion over a 2-hour period followed by another 5-mg/kg infusion 2 weeks after the first. If a person’s disease does not respond after two doses, no additional treatment with infliximab should be given. In people whose disease responds, infliximab regimens include maintenance treatment (another 5-mg/kg infusion at 6 weeks after the initial dose, followed by infusions every 8 weeks) or re-administration, otherwise known as episodic treatment (an infusion of 5-mg/kg if signs and symptoms of the disease recur).
NICE (2010) technology appraisal number 187. Infliximab (Review) and Adalimumab for the Treatment of Crohn’s Disease. London: NICE. Available from www.nice.org.uk/guidance/TA187.6 Reproduced with permission. This information is accurate at time of publication
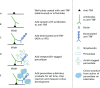
For fistulising disease, the first three doses at weeks 0, 2 and 6 are considered as induction therapy and additional IFX therapy should not be given if the first three doses have not induced a response. The patient pathway for people responding to IFX induction therapy and moving onto maintenance therapy is given in Figure 1.

FIGURE 1
Patient pathway of patients with CD on IFX therapy.
Adalimumab
Adalimumab can be used to treat severe active CD in adults whose disease has not responded to treatment with an immunosuppressant and/or corticosteroid, or who are intolerant to or have contraindications to such therapies.
Administration of ADA should follow this pattern:
The adalimumab induction treatment dose regimen for adults with severe Crohn’s disease is 80 mg via subcutaneous injection, followed by 40 mg 2 weeks later. After induction treatment the recommended dose is 40 mg every other week. This can be increased to 40 mg every week in people whose disease shows a decrease in response to treatment.
NICE (2010) technology appraisal number 187. Infliximab (Review) and Adalimumab for the Treatment of Crohn’s Disease. London: NICE. Available from www.nice.org.uk/guidance/TA187.6 Reproduced with permission. This information is accurate at time of publication
Anti-tumour necrosis factor alpha agents
Crohn’s disease is associated with elevated levels of the immune regulatory protein, TNF-α. The reasons for this elevation in CD are still largely unknown. TNF-α is a small cell signalling protein (cytokine) involved in inflammatory responses primarily by influencing regulation of various effector cells of the immune system. TNF-α has been shown to have a role in several inflammatory diseases including CD, UC, rheumatoid arthritis and ankylosing spondylitis. Anti-TNF-α agents bind to cell surface TNF-α and free TNF-α, and block their activity. Blocking of TNF-α with anti-TNF-α drugs has been shown to be successful for some patients with inflammatory diseases, including CD. Anti-TNF-α agents recommended by NICE for the treatment of CD are IFX and ADA. These monoclonal antibodies are introduced into the human body to bind and block TNF-α. They are classed as monoclonal antibodies because they are derived from genetically engineered immune cells, which are all daughters of a single parent cell, so that in culture they generate and secrete antibodies that are all of identical structure to and affinity for TNF-α.15
Infliximab
Infliximab is a chimeric (mouse–human) monoclonal antibody. It is said to be chimeric because the genetic code determining its amino acid sequences is partly derived from the mouse genome and partly from the human genome. IFX belongs to the immunoglobulin gamma type 1 (IgG1) group of antibody molecules (Figure 2). It should be borne in mind that IgG1 molecules are globular (not linear as in the diagram) and that they are glycoproteins, which have carbohydrate chains attached (not shown in Figure 2). As IFX is generated from cultured mouse cells, the carbohydrate part of the molecule corresponds to that of mouse rather than human glycoproteins.15
Infliximab is composed of human IgG1 heavy-chain constant regions and human kappa light-chain constant regions (together representing 70% of the genetic make-up of the molecule), plus mouse-derived heavy- and light-chain variable regions (30% of the genetic make-up, 4/12 domains), which carry the binding sites with high affinity and specificity to TNF-α (see Figure 2). IFX was the first anti-TNF-α agent that was approved and licensed for treating severe active CD and active fistulising CD in adults and children aged > 6 years. It is administered intravenously over 1–2 hours.
Side effects of IFX include:
- allergic reaction to the infusion (or IFX) apparent by:
- hives (red, raised, itchy patches of skin) or other skin rashes
- difficulty swallowing or breathing
- pains in the chest or muscle, or joint pain, fever or chills
- swelling of the face or hands
- headaches or a sore throat.
- serious viral or bacterial infections including tuberculosis, especially in people aged > 65 years
- skin reactions including psoriasis (red scaly patches), rashes, skin lesions, ulcers and hives, and swollen face and lips
- worsening of heart problems
- increased risk of cancer or lymphoma
- liver inflammation.
Many of the side effects are reversible if the drug is stopped.15
Adalimumab
Adalimumab is a purely human IgG1 monoclonal antibody. ADA is a more recent anti-TNF-α therapy that was approved for treating CD in adults only. It is administered as a subcutaneous injection by a doctor or nurse, or can be self-injected by the patient or a family member.15
Side effects of ADA include:
- reactions to the injection including pain, swelling, redness, bruising and itching
- allergic reaction to ADA including:
- rashes or hives
- swollen face, hands and feet
- trouble breathing.
- greater susceptibility to infections such as colds, influenza, pneumonia, sepsis and tuberculosis
- skin reactions including psoriasis (scaly patches), eczema, other skin rashes and ulcers
- skin cancer, lymphoma or leukaemia
- damage to nerves (demyelination)
- lupus.
Many of the side effects are reversible if the drug is stopped.15
Significance to the NHS and current service cost
The aim of successful therapies in CD is to prolong remission and to minimise relapse. Patients’ quality of life (QoL) fluctuates through time and, unsurprisingly, has been found to be better during remission. Studies using various disease-specific health-related QoL measures (such as McMaster’s IBDQ, IBDQ-36, short IBDQ, Rating Form of IBD Patient Concerns, Cleveland Clinic questionnaire and Gastrointestinal Quality of Life Index) show a clear correlation between health-related QoL and symptoms. These measures in patients with CD have allowed utility estimates to be developed for patients in various clinical states.20 QoL has been found to be somewhat worse in CD than in UC, and substantially worse in relapse than in healthy matched individuals. It has also been found to be similar or worse to that experienced in many other medical conditions.21 Gastroenterologists tend to rely on global clinical judgement, which tends to be less reproducible than QoL assessment tools, but is, of course, simpler for decision-making in everyday clinical practice.9
Patients with CD can be cared for in primary or secondary care depending on symptom severity. Although general practitioners manage patients in remission or with mild symptoms, patients with more severe active disease are managed in secondary care. These are patients who are likely to be steroid dependent, on immunosuppressants or anti-TNF-αs, or requiring surgery. It has been estimated that about 50% of patients with CD experience at least one flare per year. Of these, 20% of patients will require hospitalisation.22 Disease flares have been found to be associated with a two- to threefold increase in hospitalisation and a 20-fold increase in cost compared with managing patients in remission.23 Audit data show that anti-TNF-α agents are potentially cost-saving by successfully maintaining patients in remission and reducing hospital admissions. Cost reductions of £138 per patient at 6 months24 and £2750 per patient at 12 months (excluding IFX costs) have been demonstrated in a before-and-after study of IFX therapy.25 However, in that study both non-responders (£3608) as well as responders (£1656) incurred a considerably higher annual cost than the mean annual costs of long-term care in CD of £631/£762 (UK) and £838/£796 (Europe) estimated in another study using decision modelling.26 Using 2008 prevalence figures, the total annual cost to the NHS for the approximate 60,000 patients with CD was estimated at £38M. Updating this figure with more recent prevalence data (115,000), but still using 2008 prices, would double that cost to £73M. This, however, might be a modest estimate when considering the wide range of measured 6-month costs for individual patients with CD (£73–£33,254).23 The main drivers of costs were hospital admission, surgery and anti-TNF-α treatment.26 In the 2003 Health Technology Assessment (HTA) publication by Clark et al.,3 the average cost of a single 5-mg/kg IFX infusion for a 70-kg patient was reported to be £1804.80. No comparable data for ADA are available.
The significance of CD to the NHS is increased by the fact that the prevalence of CD is increasing and the disease affects many people at a young age. The lifetime care costs for patients with CD are now comparable to the costs of caring for patients with other major chronic diseases, such as diabetes mellitus and cancer.22 This argues not only clinically but also economically for interventions that keep patients in remission and out of hospital. This review focuses on whether or not monitoring anti-TNF-α agents and their antibodies with enzyme-linked immunosorbent assays (ELISAs) could potentially contribute to this aim.
Rationale for measuring anti-tumour necrosis factor alpha drug and anti-drug antibody levels
Responders and non-responders definitions and incidence rates
Similar to other treatment regimens for CD, anti-TNF-α treatment aims to induce remission (defined as < 150 points on CDAI and no draining fistulae), in which case it is described as induction therapy, and to prevent relapse (maintenance therapy). However, failure to induce a response and LOR to anti-TNF-α are common problems in clinical practice. The lack of consensus regarding clear definitions for response and remission result in inconsistencies in the reported incidence rates of non-response and LOR covered in this section.
Primary non-response
Patients not achieving at least a 70-point reduction on the CDAI during induction therapy are classed as primary non-responders. Incidence rates of primary non-response vary greatly depending on the clinical outcome measured (response/remission) and on the time point that the assessment of response is undertaken. For example, A Crohn’s disease Clinical trial Evaluating infliximab in a New long-term Treatment regimen (ACCENT) I, a randomised controlled trial (RCT) that investigated the benefit of maintenance IFX therapy in 573 active CD patients, assessed response after a single IFX infusion at 2 weeks.27 The ACCENT II study was a post hoc analysis to determine the efficacy and safety of IFX therapy in patients with fistulising CD in which patients were assessed at 10 weeks.28 The Crohn’s trial of the fully Human Antibody adalimumab for Remission Maintenance RCT assessed response to ADA at 4 weeks to evaluate the drug’s efficacy and safety in the maintenance of response and remission in 854 patients with moderate to severe CD.29 However, Ben-Horin and Chowers30 stated that in clinical practice non-response should not be assessed before 8–12 weeks, as remission might still be induced at this time. It is therefore not surprising that a review of incidence rates found that non-response ranged from 20% to 40% in clinical trials and from 10% to 20% in ‘real-life’ series.30 In contrast, lack of remission at week 4 in patients with luminal CD was reported to be as high as 67% for IFX and 64% for ADA.31,32 The true magnitude of the rate of primary non-response is therefore difficult to determine.
Factors associated with non-response are believed to include:30,33,34
- severity of disease
- duration of disease
- smoking
- drug elimination
- drug binding
- anti-drug antibodies
- alternative non-TNF-α-mediated disease pathways
- concomitant treatment with immunosuppressants
- prior failure of other anti-TNF-α.
Loss of response
Patients with an initial response to anti-TNF-α treatment can lose response at any time during induction or maintenance therapy despite intensification of treatment (i.e. increase in dose or decrease in dosing interval). Again, lack of a clear definition, assessment at different time points, different outcome measures and different drug doses mean that reported incidence rates of secondary LOR vary considerably across studies. The true extent of this problem is largely unknown. Gisbert and Panes,35 in their review, reported a range of LOR to IFX of 11–48% (mean 37%) for varying lengths of follow-up, and de Boer et al.33 reported a range of 21–46% for LOR to ADA. For this reason the incidence of LOR is better expressed as the annual risk of LOR per patient-year (13% for IFX35 and 20.3% for ADA36). LOR to ADA and IFX did not differ significantly in a retrospective study of 375 patients; however, patients treated with ADA required more dose optimisation intervention than patients on IFX.37
The following factors are believed to prevent LOR:14
- pre-medication with steroids
- concomitant immunosuppressants
- maintenance therapy as opposed to episodic treatment.
Mechanisms of LOR to anti-TNF-α agents are still unclear. The next section describes some of the possible mechanisms in more detail.
Anti-drug antibodies
Anti-drug antibodies can be elicited by IFX or ADA during therapy as a response by the human immune system to these foreign proteins; this is termed immunogenicity of anti-TNF-α agents. These anti-drug antibodies bind to the anti-TNF-α agent and neutralise its action. If sufficient amounts of antibodies are present, the individual loses response to the drug treatment. During scheduled maintenance therapy, the incidence of anti-drug antibodies is 5–18%27,38,39 and 3–17%39 for IFX and ADA, respectively. The similar rates for IFX and ADA might initially appear counterintuitive as ADA is a fully human recombinant protein, whereas IFX is partly human and partly mouse protein and, therefore, ‘more’ foreign. However, ADA, similar to IFX, is a foreign protein that will prompt a response when coming into contact with the immune system. This indicates that the degree of ‘human-ness’ is not the main determinant of immunogenicity (i.e. formation of antibodies to a foreign protein).39
Levels of antibodies have been found to be higher during episodic treatment, at 36–61%,39–41 than levels found during maintenance therapy. This indicates that other factors may influence immunogenicity. The true incidence of antibodies in anti-TNF-α-treated patients is, therefore, unknown. The ability to mount an immune response and measurement of that response depends on a number of factors, including the method of measuring antibody levels and age, and also depends on concomitant treatment with immunosuppressants.14,27,40–44 For that reason, concomitant immunosuppressants might be given to patients to prevent or reduce the formation of antibodies. This effect was not observed in one study for ADA,14 and Vermeire et al.45 reported that increasing anti-TNF-α above antibody-binding capacity might have similar effects to immunosuppressants, by neutralising free antibodies. Vande Casteele et al.46 made a similar observation for transient antibodies (antibodies detectable for a short period during a series of follow-up test assays conducted during a course of infusions), while sustained antibodies did not disappear after dose optimisation and were associated with LOR.
The clinical importance of antibodies can be presented as:39
- positive/negative/inconclusive
- high/low
- above/below a threshold in arbitrary units or in µg/ml
- drug concentration
- clinical effect (duration of response, need for dose intensification or switch drug)
- impact on safety (infusion reactions).
The clinical relevance of antibodies has been debated. However, numerous studies report the correlation between presence of antibodies with low or absent drug levels and consequent response.27,38,40,43,45,47,48 This can be explained by antibodies binding to the epitope of anti-TNF-α and neutralising the drug (i.e. making it unable to bind to TNF-α and inhibiting the working mechanism of anti-TNF-α) or by forming immune complexes with the drug (non-neutralising antibodies), which are subsequently cleared from the circulation (reducing the drug’s bioavailability).49
Although the importance of the neutralising antibodies has been universally acknowledged,14,34,49,50 reviewers seem to disagree in their conclusion about the importance of non-neutralising antibodies.14,34 Over 90% of antibodies to IFX and ADA are neutralising.51 In a meta-analysis, Garces et al.49 estimated that detectable antibodies can decrease response to anti-TNF-α by as much as 80%.
An interesting additional observation was made by Steenholdt et al.,52 who showed that immunoglobulin gamma (IgG) antibodies reacting with the fragment antigen-binding portion (see Figure 2) of IFX exist in IFX-naive IBD patients prior to treatment. The presence of pre-existing antibodies affected response and safety of IFX treatment in patients with CD, and the study concluded that the clinical utility of measuring pre-treatment antibodies should be assessed.
Drug levels
Although anti-drug antibodies have been shown to reduce anti-TNF-α drug levels, there are other known mechanisms that affect drug levels. These include dose and dosing interval, body mass index, sex, serum albumin levels (serum albumin transports drugs and can affect the half-life of drugs), concomitant immunosuppressants, severity of inflammation, mode of administration (intravenous vs. subcutaneous) and drug half-life.14,44
As a consequence, drug levels vary considerably between patients and within individuals over time.34 Following administration of the anti-TNF-α agent, circulating drug concentration will be at its peak level; the concentration just before the next round of treatment is classed as ‘trough level’. The optimal time of testing drug levels within this cycle has been debated44 and it is largely unknown what the optimal drug levels would be at the different time points. Although a threshold trough level is thought to be needed for effectiveness, it is also known that supratherapeutic levels can cause infections and other adverse events.14
Anti-tumour necrosis factor alpha and antibody level monitoring in Crohn’s disease
One of the key studies to demonstrate and quantify the link between drug and anti-drug antibody levels, immunosuppressant therapy and response in patients with CD on anti-TNF-α agents was the study by Baert et al.43
Baert et al.43 was an early, and influential, study of the development of anti-drug antibodies to IFX in patients with CD; this study stimulated numerous subsequent investigations. The study enrolled 125 consecutive patients (38 with fistulising and 87 with luminal disease) who received 5 mg/kg of IFX at 0, 2 and 6 weeks. Responders (89/125, 71%) were retreated with this regimen if they required restart of IFX therapy according to clinical judgement. Mean treatment period was 10 months and median follow-up was 36 months. Anti-drug antibodies and IFX serum levels were measured before and at 4, 8 and 12 weeks after each infusion, using ELISA (PROMETHEUS® ELISA, Prometheus Laboratories Inc., San Diego, CA, USA). After five infusions, 76 out of 125 (61%) patients were classified as positive for anti-drug antibodies.
When a level of 8 µg of anti-drug antibodies/ml serum was selected, it was found that concentrations of anti-drug antibodies were > 8 µg/ml in 24 out of 56 (43%) patients taking immunosuppressants, compared with 52 out of 69 (75%) not taking immune suppressants. The relative risk (RR) of anti-drug antibodies concentration > 8 µg/ml in patients taking compared with those not taking suppressive therapy was 2.40 [95% confidence interval (CI) 1.65 to 3.66; p < 0.001]. Infusion reactions had occurred in 27% of patients by the fifth infusion. The median anti-drug antibody level in patients experiencing infusion reactions was higher than in those with no reactions (p < 0.001). Reactions were significantly more common in patients not taking immunosuppressant therapy than in those who were taking it. When time to next infusion was taken as a measure of response duration, it was found that response duration was reduced in those with anti-drug antibodies levels > 8 µg/ml relative to those with levels < 8 µg/ml (median 35 days vs. 71 days; p < 0.001).
The level of IFX at 4 weeks after an infusion was correlated with the level of anti-drug antibodies prior to the infusion (R2 = 0.34; p < 0.001) and was positively correlated with duration of response. IFX level and anti-drug antibodies level were independent variables influencing response duration. Logistic regression indicated that the only variable that influenced a 4-week level of IFX > 12 µg/ml was the use of immunosuppressant therapy. IFX level was higher in those without an infusion reaction than those with one.
In summary, the results of this study suggest that production of anti-drug antibodies is common during IFX therapy; anti-drug antibodies are associated with reduced IFX levels; duration of response is reduced by the presence of anti-drug antibodies; and production of anti-drug antibodies may be reduced when concomitant immunosuppressant therapy is employed.
Further evidence steadily accumulated from retrospective analyses of multiple clinical trials and case series,38,53–55 and the observation that detectable trough levels of drug are associated with greater clinical efficacy is now well established.44
This accumulating evidence has formed the basis of investigations into drug and anti-drug antibody monitoring in anti-TNF-α-treated patients with CD.
Without monitoring, the options for a clinician if the anti-TNF-α agent fails are to wait and see, to intensify drug treatment, to switch drug within its class or to switch to a different class of drugs.34 Measuring drug and anti-drug antibodies, however, could enable clinicians and patients to make informed choices on management. A number of studies have investigated the clinical utility of measuring drug and anti-drug antibody levels in sera by translating the clinical management decision following a test outcome into a treatment algorithm stipulating the management pathways for patients with a specific test outcome in clinical practice.56–59
Drug and anti-drug antibody monitoring could be undertaken in good responders (i.e. those responding to an initial induction course of anti-TNF-α treatment), as well as in patients with LOR (i.e. those initially responding to anti-TNF-α treatment but losing this response over time). The use of these technologies provides a clinician with potentially useful information that may guide an individual patient’s future treatment. Such information may aid in anticipating the LOR in responders or allow drug optimisation, whereas for non-responders such analyses may help in estimating the likelihood of various candidate reasons for LOR. In non-responders with low levels of drug and high levels of anti-drug antibodies, for example, the loss or lack of response may be surmised to be because of rapid clearance of the drug as a result of the action of anti-drug antibodies. On the other hand, a low level of anti-TNF-α in the absence of anti-drug antibodies may be suggestive of non-immune mechanisms of rapid drug clearance, whereas high levels of drug in the absence of antibodies in non-responders may be suggestive of a pathology for the condition independent of TNF-α in a particular patient. Algorithms for future treatment based on anti-TNF-α and anti-drug antibody estimates have been published.56–59
In theory, the application of the tests in conjunction with an appropriate algorithm for treatment based on test results may:
- improve QoL and other outcomes (e.g. faster healing of flare-ups, reduced abdominal pain and associated diarrhoea)
- optimise the treatment plan (facilitate adoption of the most suitable future treatment for individual patients; this might involve a switch to an alternative anti-TNF-α or a biologic with an alternative mechanism of action)
- minimise the risk of drug overdose and associated adverse events
- allow earlier de-escalation of therapy, leading to a reduction in the overall drug used
- help to reduce the amount of drugs used inappropriately, unnecessary hospital visits, risk of surgery and associated costs.15
Description of technology under assessment
Intervention technologies
Various assay procedures for anti-TNF-α agents and for anti-drug antibodies have been developed in the belief that the levels of circulating anti-TNF-α and of anti-drug antibodies can provide information useful to clinicians in indicating potential reasons for treatment failure, and for dosage or treatment adjustment.
Commercially available ELISA kits (the LISA-TRACKER® ELISA kits, Theradiag, Marne La Vallee, France, or Alpha Laboratories, Heriot, UK; the TNF-α-Blocker ELISA kits, Immundiagnostik AG, Bensheim, Germany; and the Promonitor® ELISA kits, Proteomika, Progenika Biopharma, Bizkaia, Spain) are the intervention technologies designed to measure IFX and ADA levels, and their antibodies and are investigated in this review.
These are all particular examples of solid-phase ELISAs. They estimate the following molecules in patient blood sera:
- IFX
- ADA
- anti-IFX antibodies
- anti-ADA antibodies.
Details of the ELISA kits available from these companies are summarised in Appendix 1.
Other ELISAs commercially available for measuring these molecules in sera include the SHIKARI® ELISA kits (Matriks Biotechnology Co. Ltd, Ankara, Turkey). These are not included as index tests in the NICE scope.
In the UK a number of non-commercial kits are also available, but these are not the focus of this report.
Other methodologies based on alternative principles of detection and measurement include (1) radioimmunoassays (RIAs) (liquid-phase assays using the radioisotope 125I to label TNF); (2) cell reporter assays based on genetically engineered cells incubated in culture medium; and (3) mobility shift assays (liquid-phase assays using size-exclusion high-performance liquid chromatography with fluorescent dye detection). The differences in these assays may have an effect on their individual performance, and describing and contrasting them will help the reader to understand the abilities and limitations of the assays.
Enzyme-linked immunosorbent assays for infliximab and adalimumab
For details of the ELISA kits, refer to Appendix 1. All three specified ELISA methods employ similar principles in which, typically, microtitre plates with 96 wells coated with reagent receive the patient serum samples or various standards and calibrators. Reagents are added with wash steps between additions. The final step involves quantifying the amount of a peroxidase label in the titre well, this amount being proportional to the amount of anti-TNF-α or anti-drug antibody in the patient’s sample or in the calibrator standard.15
The amount of peroxidase present in the well is quantified using a timed incubation with excess substrates [hydrogen peroxide + 3,3’,5,5’-tetramethylbenzidine (3,3’,5,5’-tetramethylbiphenyl-4,4’-diamine)]. Peroxidase catalyses the following reaction:
The incubation is stopped after an appropriate time by the addition of acid and the accumulated chromogen quantified by measuring optical density with a spectrophotometer.
The reagents used for coating the microtitre plate wells and the reagents used in subsequent steps of the assay procedure differ in detail according to manufacturer. The LISA-TRACKER assays for IFX and for ADA are illustrated in Figure 3.
Serum samples from patients may contain soluble TNF-α receptors; these could compete with anti-TNF-α for the immobilised TNF-α on the microtitre well plate and may potentially interfere with the assay. The assay quantifies free anti-TNF-α. Samples may contain anti-TNF-α bound to antibodies to anti-TNF-α, especially in patients who have lost a response to treatment. These anti-TNF-α–antibody complexes will be washed away at the first wash step, leaving only free anti-TNF-α bound to immobilised TNF-α. The amount of anti-TNF-α lost at the wash step is likely to vary between patients and is unknown; the practical implications of this are uncertain.15
TNF-α-Blocker and Promonitor assays for anti-TNF-α drugs differ from the LISA-TRACKER assay in that the well coat is not TNF-α, but rather a reagent (antibody or antibody fragment) able to bind specifically to the TNF-α binding site of IFX or of ADA that is added to the microtitre well in the patient’s sample (or calibrator). After washing, the second reagent is a peroxidase-labelled antibody able to bind the fragment crystallising (Fc) region of the anti-TNF-α antibody (Figure 4). Thus, fewer steps and a single reagent are used to detect well-bound anti-TNF-α drug. Table 1 summarises the information describing the mechanisms underlying these assays.
TABLE 1
Summary of ELISAs to be considered in this review for detection of IFX and ADA
Enzyme-linked immunosorbent assays for anti-drug antibodies
The LISA-TRACKER assays for antibodies to IFX and to ADA are illustrated in Figure 5.
This assay quantitatively estimates only free antibodies to anti-TNF-α. Therefore, anti-drug antibodies bound to the drug are lost at the first wash. The amount of bound anti-drug antibody is likely to vary between patients and is unknown. Whether anti-drug antibodies directed at non-idiotypic regions of the drugs (e.g. glycoprotein moieties, variable non-idiotypic mouse regions of IFX, etc.) are detectable or present in samples appears to be insufficiently investigated to date and is therefore uncertain. However, in vitro tests indicate that about 90% of anti-drug antibodies bind to the TNF-binding region of anti-TNF-α drugs.51 These, and the other anti-drug antibodies, may hasten clearance of drug from the circulation as well as neutralising its binding capacity.
Tumour necrosis factor alpha and Promonitor assays differ from LISA-TRACKER assays in employing a single reagent for detecting well-bound anti-drug antibodies rather than two (biotinylated IFX or biotinylated ADA, plus avidin-conjugated peroxidase). Table 2 summarises the information describing the mechanisms underlying these assays.15
TABLE 2
Summary of ELISAs to be considered in this review for detection of antibodies to IFX and ADA
Brief overview of identified assay methods
There are no gold standard assays for anti-TNF-α agents or for antibodies to anti-TNF-α agents that might provide a robust basis for comparisons between the performances of different assays. According to US Medical Insurance assessments ‘candidate’ assays have been insufficiently investigated to establish any as a gold standard and, according to Steenholdt,60 the evidence is incomplete on how these different assays may compare in practice.14,61–64
There appear to be four types of assay which differ fundamentally from each other. These are as follows.
- ELISAs: solid-phase assays. These are available as commercial kits and several in-house methods are mentioned in the literature. Generally, the ELISAs quantitatively measure only ‘free’ anti-TNF-α and ‘free’ anti-drug antibodies, and it is acknowledged that the level of the unmeasured ‘bound’ anti-TNF-α and of ‘bound’ anti-drug antibody may vary considerably between patients. Thus, for some patient samples there is an unknown and unmeasured amount of anti-TNF-α and of anti-drug antibody present, in addition to the measured ‘free’ levels. In theory, this represents a potential deficiency in ELISAs, although whether or not this is serious in practice is difficult to gauge, especially in the absence of an established gold standard. This deficiency appears to have been one stimulus for the development of methods based on alternative principles. It is possible, however, that the relative convenience and cheapness of ELISAs means that this inability to measure total anti-TNF-α and total anti-drug antibody is supportable in practice.
- RIAs: liquid-phase assays. These are provided as a total service rather than as purchasable kits. They measure total anti-TNF-α and total anti-drug antibody (probably as long as the anti-drug antibody light chain is λ class). These RIAs use 125I-labelled human TNF-α and 125I-labelled anti-TNF-α. These are commercially available or may be relatively easily constructed from commercially available materials; however, in the absence of purchasable assay kits, it is unlikely that any hospital laboratory would set up such assays for routine use. In these assays the patient’s sample is mixed with a solution containing a fixed amount of 125I-labelled TNF-α or 125I-labelled anti-TNF-α further antibody (e.g. rabbit anti-human immunoglobulin λ-chain), which promotes the formation of immune complexes that are pelleted by centrifugation. The 125I in the pellet is quantified in a gamma counter. Potential disadvantages include: (1) radiolabelled reagents do not keep indefinitely (125I decays with a half-life of 59 days); (2) the laboratory needs to be equipped for handling hazardous (radioactive) materials; (3) some staff training may be necessary; and (4) the laboratory requires a gamma counter (preferably automated for high throughput). These factors obviously have cost implications for setting up RIAs.
- Cell reporter assays: these assays utilise genetically engineered cells that respond to the presence of anti-TNF-α agents by synthesising light-generating enzymes. The enzymes are allowed to accumulate during an incubation period and are then supplied with appropriate substrates resulting in light emission measured with a luminometer. Samples with anti-TNF-α will lead to light emission and samples with antibodies to anti-TNF-α will quench light emission (for further information see Appendix 2).
- Mobility shift assays: the mobility shift assay depends on detecting the shift in mobility of fluorescent probes when bound to either anti-TNF-α or anti-drug antibodies (for further information, see Appendix 2).
Timing and use of assays
The anti-TNF-α and anti-drug antibody assays are most frequently administered just before the next administration of the anti-TNF-α agent. This is said to allow measurement of a ‘trough’ level of anti-TNF-α and has been adopted to minimise effects from the presence of anti-TNF-α–anti-drug antibody immune complexes in samples. For patients whose response to therapy has waned, the results of the tests are frequently dichotomised using a cut-off assay result. Thus, on the basis of anti-TNF-α assays patients are classified as having therapeutic levels of anti-TNF-α or subtherapeutic levels, and on the basis of anti-drug antibody assay results they are classified as having clinically significant levels of anti-drug antibodies or insignificant levels. Such classifications yield four categories of patient for whom different explanations of failed response are possible. Algorithms have been developed prescribing treatment pathways and/or further diagnostic tests (e.g. colonoscopy) based on such classification.15
Current usage of assays in the NHS
Current practice for monitoring TNF-α inhibitor antibody and drug levels in the UK is patchy because of the lack of agreed consensus and evidence for its cost-effectiveness. In-house tests are performed in a few laboratories in England. However, demand is low, analyses are often undertaken in batches and it can be weeks (in some cases) before a clinician receives a result on which to act.
Although some centres have local monitoring protocols in conjunction with their link laboratory, there is, as yet, no agreed algorithm for clinicians to refer to which allows for the translation of the results of the tests into coherent plans for patient management according to test outcome.
However, recent emerging evidence to support anecdotal practice that such monitoring could be useful in managing patients with TNF-α inhibitors, has encouraged a cautious increase in uptake.
It is expected that therapeutic monitoring of TNF-α inhibitors might be useful in a number of clinical scenarios in the treatment of CD in the NHS, including for primary and LOR to anti-TNF-α therapy and in the optimisation of dosages for those who are already responding.
- Introduction - Clinical effectiveness and cost-effectiveness of use of therapeut...Introduction - Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling
- Modelling progression of kidney disease in type 2 diabetes - Optimal strategies ...Modelling progression of kidney disease in type 2 diabetes - Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness
- Qualitative process evaluation - Patient-reported outcome measures for monitorin...Qualitative process evaluation - Patient-reported outcome measures for monitoring primary care patients with depression: the PROMDEP cluster RCT and economic evaluation
- List of abbreviations - Feasibility of a RCT of techniques for managing an impac...List of abbreviations - Feasibility of a RCT of techniques for managing an impacted fetal head during emergency caesarean section: the MIDAS scoping study
- Discussion and conclusions - Optimal strategies for identifying kidney disease i...Discussion and conclusions - Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness
Your browsing activity is empty.
Activity recording is turned off.
See more...