Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Brown S, Tiernan J, Biggs K, et al. The HubBLe Trial: haemorrhoidal artery ligation (HAL) versus rubber band ligation (RBL) for symptomatic second- and third-degree haemorrhoids: a multicentre randomised controlled trial and health-economic evaluation. Southampton (UK): NIHR Journals Library; 2016 Nov. (Health Technology Assessment, No. 20.88.)

The HubBLe Trial: haemorrhoidal artery ligation (HAL) versus rubber band ligation (RBL) for symptomatic second- and third-degree haemorrhoids: a multicentre randomised controlled trial and health-economic evaluation.
Show detailsRecruitment and participant flow
Participants who were randomly assigned, received intended treatment and were analysed for the primary outcome
Recruitment to the trial is represented in Figure 3. A total of 372 participants were randomly assigned to receive RBL or HAL; 187 patients were allocated to receive RBL, and 185 were allocated to receive HAL. Two of these participants (both randomised to RBL) were removed from the trial completely, as they were ineligible at the time of consent; therefore, a total of 370 participants were entered into the trial. In total, 969 patients were screened for entry into the trial and the reasons for non-recruitment are shown in Table 5.

FIGURE 3
Recruitment graph.
TABLE 5
Reasons for non-recruitment to the trial for eligible patients
Patient preference was the main reason for non-consent by patients, with 128 patients opting for RBL and 70 patients opting for HAL.
Losses and exclusions after randomisation
A total of 340 participants received treatment as part of the trial and the participant flow is shown in Figure 4; reasons for withdrawal during the trial are provided in Table 6. Primary outcome data were collected for 337 participants (161 HAL and 176 RBL): 256 patient questionnaires were returned at 12 months; 236 GP forms were returned at 12 months, and 337 consultant forms were completed at 12 months. There were 183 participants where all 3 of the 12 months forms were completed/returned.

FIGURE 4
Flow chart summarising the numbers of each type of questionnaire completed throughout the trial.
TABLE 6
Reasons for participant withdrawal during the trial
Dates defining the periods of recruitment and follow-up
The first site to open to recruitment was STH NHS Foundation Trust on 9 September 2012, and recruitment finished at all sites on 6 May 2014. Follow-up was due to end 1 year after the end of recruitment but to allow for the delay in receiving the trial treatment we extended this period and the follow-up was completed at sites on 28 August 2015.
Table 7 shows the duration between randomisation and treatment (waiting time) by treatment group for the trial. These data do not include individuals who withdrew prior to treatment; 24 participants withdrew prior to the procedure in the HAL group, compared with six (eligible) participants in the RBL group. In the RBL group, 114 of 179 (63.4%) participants received treatment on the same day as they were randomised (0 days), whereas none of the HAL group participants was treated on the same day. Although the maximum waiting time is greater for RBL, the mean and median shows that waiting times are higher for HAL.
TABLE 7
Duration between randomisation and treatment in the trial (waiting time)
Baseline data
The characteristics of the participants included in the final analysis is shown in Table 8. The groups appear balanced at baseline in regards to gender, age, BMI, the grade of haemorrhoids and previous treatment for haemorrhoids.
TABLE 8
Baseline demographical data by randomised group
The majority of the procedures were conducted by consultant or equivalent (clinician accredited for independent practice, including a clinical nurse specialist), or supervised by a consultant (87% for RBL and 96% for HAL). There were a few cases that were carried out by a trainee doctor. However, these trainees met our pre-existing competencies.
Numbers analysed
The ITT population, in which patients were analysed by their original assigned groups, included all 161 participants in the HAL arm and 176 in the RBL arm for whom recurrence data were available from either the patient, clinician or GP. Of these, the patient-completed questionnaire was returned for 125 and 131 participants in the HAL and RBL groups, respectively. The number of participants included in each analysis is provided for each measure in this section.
Outcomes and estimation
Recurrence (primary outcome)
The overall proportion of participants with a recurrence at 12 months was 49% in the RBL arm compared with 30% in the HAL arm (absolute difference 19.6%; adjusted OR 2.23, 95% CI 1.42 to 3.51; p = 0.0005). The breakdown of recurrences, overall and by criterion, is presented in Table 9. Self-reported recurrence rates were almost identical, with 29% of respondents in both arms stating that they believed that their haemorrhoids had either improved or been cured (adjusted OR for self-reported recurrence = 1.06, 95% CI 0.60 to 1.85; p = 0.85). The increased recurrence associated with RBL is mainly attributable to the high rate of additional procedures undertaken following the initial procedure [33%, compared with 14% in the HAL group (adjusted OR for further procedure = 2.86, 95% CI 1.65 to 4.93; p < 0.001)]. A further three (2%) participants in the RBL arm were considered to have symptoms that were consistent with recurrent haemorrhoids following blind review. In two cases the participants were recorded as possibly requiring further treatment at their 6-week visit but were subsequently lost to follow-up; a third patient had been hospitalised twice for excessive bleeding, but had not undergone treatment.
TABLE 9
Recurrence at 1 year
Sensitivity analyses were undertaken in which alternative covariates (EQ-5D-5L at randomisation or preoperative, BMI at randomisation, and grade of haemorrhoids at randomisation) were adjusted for; doing so yielded reasonable consistency in the ORs (range 2.05–2.81), all of which remained statistically significant. The PP analysis again provided a similar OR (2.21, 95% CI 1.38 to 3.54; p = 0.001).
Of the baseline covariates assessed, none had a statistically significant association with recurrence. Recurrences were more common, however, among participants who were male, had grade III haemorrhoids, had undergone previous treatment and had higher symptom scores.
Among the 80 participants who required a further intervention, the majority of participants underwent a single procedure. In most cases this was RBL, as described in Table 9, although some variation was noted across centres as described in Table 10: as the primary interest is to document second-line treatment, the treatment groups here refer to treatment, as received, as opposed to ‘as randomised’, which was reported previously. As RBL is a brief procedure with (relatively) minimal inconvenience to the patient, it could be argued that a second-line RBL is not itself indicative of a recurrence because the initial haemorrhoids remain incompletely treated. Consequently, an additional (and unplanned) analysis investigated the extent to which recurrence differed if follow-up RBL were not considered a recurrence. In total, 45 participants (31 in the RBL arm, 11 in HAL) underwent RBL as follow-up procedure. Of the 31 patients who underwent repeat RBL, six considered their haemorrhoids to be unchanged or worse at 1 year; three underwent further procedures; and one was considered a recurrence based on blind review. In the HAL arm, 8 of the 11 participants who were undergoing subsequent RBL considered their haemorrhoids to be unchanged or worse at 1 year and two participants underwent further procedures. Thus, if subsequent RBL were not considered a recurrence, the number with recurrent haemorrhoids is 66 (37.5%) in the RBL arm and 44 (27.3%) in the HAL arm (adjusted OR 1.53, 95% CI 0.96 to 2.44; p = 0.071). If a single HAL is compared with multiple RBLs the number with recurrent haemorrhoids is 66 (37.5%) in the RBL arm and 48 (30%) in the HAL arm (adjusted OR 1.35, 95% CI 0.85–2.15; p = 0.20).
TABLE 10
Type of second procedure by treating centre
Persistent significant symptoms at 6 weeks
At 6 weeks, data were available for 150 participants in the RBL arm and 143 in the HAL arm: 43 (24%) participants in the RBL arm reported their haemorrhoids as being unchanged or worse compared with 12 (7%) in the HAL arm; additionally, one participant in each arm had subsequently undergone RBL, thus resulting in the overall number of patients with persistent symptoms being 44 (29%) compared with 13 (9%) (adjusted OR 4.35, 95% CI 2.19 to 8.65; p < 0.001).
Haemorrhoid symptom severity score
The haemorrhoid symptom severity scores are summarised in Table 11 and displayed graphically in Figure 5: higher scores indicate more severe symptoms. At 6 weeks, haemorrhoids symptom severity scores were higher in the RBL group, indicating that short-term symptoms were less pronounced following HAL. The mean (SD) scores were 4.0 (3.5) in the RBL group and 3.0 (3.1) in the HAL group, with an adjusted mean difference of 1.0 (95% CI 0.3 to 1.8; p = 0.010). No difference was apparent at 1 year, with the mean (SD) being 3.6 (3.2) for RBL and 3.6 (3.3) for HAL (adjusted difference = 0.0, 95% CI –0.8 to 0.8; p = 0.98).
TABLE 11
Participants with haemorrhoid symptom severity scores

FIGURE 5
Haemorrhoid symptom severity scores. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.
A further (post hoc) analysis looked at the proportion of participants whose symptom score was either ‘0’ or ‘1’, as this corresponds to the definition used by Nyström et al.42 These numbers are shown in Table 12. The proportions are consistent with the previous analysis, with a greater proportion of participants in the HAL arm reporting either a score of 0 or 1 at 6 weeks.
TABLE 12
Percentage of participants with haemorrhoid symptom severity scores of ‘0’ or ‘1‘
European Quality of Life-5 Dimensions (5-level version)
The EQ-5D-5L health tariff is summarised in Table 13 and Figure 6: higher figures indicate a better health state. Prior to procedure, the mean health utility was around 0.9 in both groups but declined at days 1 and 7 in the HAL group. For RBL the mean (SD) at 1 day was 0.84 (0.19) and at 7 days 0.92 (0.15); in other words, health state was reduced for the first day but had reverted back at 1 week. By contrast, the mean health state for HAL had not returned to baseline values by 7 days, with the mean (SD) being 0.76 (0.22) and 0.83 (0.18) at 1 and 7 days, respectively. The adjusted mean differences were 0.08 (95% CI 0.04 to 0.13; p < 0.001) at 1 day and 0.08 (95% CI 0.05 to 0.12; p < 0.001) at 7 days. The two arms were nearly similar with no statistical differences (and above baseline values) at all time points from day 21 onwards.
TABLE 13
The EQ-5D-5L health utility
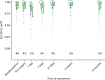
FIGURE 6
The EQ-5D-5L. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.
The EQ-5D-5L inventory was also used to derive the health state used in QALYs that are reported within the health-economic analysis in the following section.
Vaizey faecal incontinence score
Vaizey faecal incontinence scores are provided in Table 14 and Figure 7; higher scores indicate more severe incontinence. No between–group differences were noted in the Vaizey faecal incontinence scores. An improvement of around 1 unit was noted in both arms at 6 weeks, with a difference between arms of –0.1 (95% CI –1.3 to 1.0; p = 0.86). The improvement was maintained at 1 year, with a difference of –0.5 (95% CI –1.8 to 0.7; p = 0.38). A summary of these findings is presented in Table 14 and Figure 7.
TABLE 14
Vaizey faecal incontinence scores
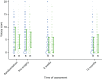
FIGURE 7
Vaizey faecal incontinence score. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.
Pain
Haemorrhoidal pain was assessed by asking the patient to rate his/her pain related to haemorrhoids as of today and over the last week. Pain today was asked at baseline and again at days 1, 7 and 21, and, finally, at 6 weeks. Pain over the last week was asked at days 7 and 21, and, finally, at 6 weeks. Both of these are summarised in Tables 15 and 16 and Figures 8 and 9; a score of ‘0’ = ’no pain’, whereas a score of ‘10’ = ’worst imaginable pain’. The change in pain as recorded by VAS is shown in Table 17. Pain was increased in the HAL group at one and seven days after the procedure, but the groups were similar at day 21 and at 6 weeks.
TABLE 15
Level of pain relating to haemorrhoids: pain experienced today
TABLE 16
Level of pain relating to haemorrhoids: pain experienced over last week

FIGURE 8
Visual analogue scale pain scores: pain today.
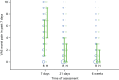
FIGURE 9
Visual analogue scale: pain over the last 7 days. Note that the area of data points is proportional to the number of participants with that value. Vertical lines represent median and IQR.
TABLE 17
Change in VAS pain from baseline
Clinical appearance of haemorrhoids at proctoscopy
Proctoscopy was conducted at the 6-week visit only if the patient reported continuing symptoms; proctoscopy was conducted more often in the RBL group. The data in Table 18 show that, in the majority of patients in whom proctoscopy was conducted, the grade had reduced at 6 weeks, with a one-grade increase observed in only four patients in the RBL group and none in the HAL group.
TABLE 18
Grade of haemorrhoids at 6 weeks and change from baseline
All important harms or unintended effects in each group
A total of 15 individuals reported SAEs. One patient (in the RBL arm) experienced several episodes of bleeding following the procedure: further investigations revealed that the patient had a rectal tumour. This SAE was classified as pre-existing and is not included in Table 19. Of the remaining 14 SAEs, 12 followed HAL and two followed RBL. One of the participants had been randomised to the RBL arm but was switched over to HAL, but the summaries in Table 19 are based on the treatment actually received and include all treated participants. All 14 events were expected.
TABLE 19
Serious adverse events by classification
Other complications, which were not classified as ‘serious’, included fissures, which occurred in 3 out of 143 (2%) of the HAL group and 0 out of 150 in the RBL group. There were no cases of fistula formation or anal stenosis in either group. At 6 weeks, 10 out of 143 (7%) of the HAL group and 10 out of 150 (7%) of the RBL group had evidence of anal skin tags.
Post hoc analyses
Some post hoc analyses have been discussed in previous sections [see Recurrence (primary outcome) regarding repeat RBL and recurrence, and see Haemorrhoid Symptom Severity Score’ regarding Nyström’s use of ‘0’ or ‘1’ on the symptoms scale being a signal of cure].
Pre-randomisation questionnaire
As previously mentioned, the baseline was originally measured prior to procedure as opposed to at randomisation. Following a recommendation from the DMEC, the protocol was subsequently amended to allow collection at both time points. The rationale for so doing was that self-reported severity may differ once the allocated treatment is known, thereby introducing an expectation bias. Specifically, participants randomised to the more intensive HAL surgery may rationalise their planned treatment by reporting higher levels of pain and discomfort than those randomised to receive RBL. Furthermore, it is possible that symptoms may change over time, which would also cause imbalance in pre-treatment values, as waiting time for HAL was longer than for RBL.
The agreement between pre-randomisation and pre-treatment values were therefore investigated among patients for whom data were available at both time points. The following are presented for each self-reported measure:
- The Bland–Altman plot for agreement within each treatment arm, together with the overall mean difference and 95% limits of agreement (also known as a 95% reference interval). The plot is the difference between pre-treatment and pre-randomisation (i.e. the change between the two) plotted against the average of the two. The 95% limits of agreement (also known as reference limits) for the difference were derived based on a normal distribution and quantify how closely the pre-randomisation and pre-treatment value agree. The average is plotted on the horizontal axis, which allows assessment of whether or not (dis)agreement varies according to severity.
- The difference between pre-treatment and pre-randomisation values against time elapsed between the two. The reference limits were the same as those produced for the Bland–Altman plot. Plotting the difference against elapsed time between the two measures allows an assessment of whether or not (dis)agreement increases with the time elapsed between the two measurements.
The agreement is displayed visually for each method in Figures 10–13. The difference and 95% limits of agreement are also summarised in Table 20, along with the difference between the two means and the ratio of their variances.

FIGURE 13
Agreement of VAS pain scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
TABLE 20
Agreement between self-completed measures of symptoms, incontinence, EQ-5D-5L and pain pre-randomisation and pre-treatment
Overall, the extent of agreement was generally similar regardless of the severity. For the VAS pain score, there was some indication that the pre-randomisation and pre-procedure time points were in closer agreement in the HAL arm than the RBL arm, as demonstrated by the ratio of the two variances (2.63; F-test p < 0.001). The same was also true for incontinence, as estimated by the Vaizey tool, where the difference between randomisation and procedure again tended to be greater in the HAL arm than the RBL arm (ratio of variances 3.14; p < 0.001). On the other hand, the two values differed less in the HAL arm for the EQ-5D ‘thermometer’ health state (ratio of variances 0.48; p = 0.004), although this may reflect a handful of participants with outlying values. There was no indication of any systematic change (i.e. no consistent deterioration or spontaneous resolution between randomisation and procedure) on any measure.
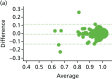
FIGURE 11
Agreement of EQ-5D-5L scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
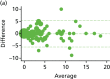
FIGURE 12
Agreement of Vaizey scores at randomisation and pre-procedure. Bland–Altman graphs are given for (a) HAL and (b) RBL. Difference between the measures in relation to elapsed time between the two is given for (c) HAL and (d) RBL.
Haemorrhoidal artery ligation device
Among participants undergoing HAL, the preferred surgical device appeared to be determined by site rather than participant characteristics. Five sites (n = 73) used the AMI HALO device on all participants, with the remaining 12 sites using the THD device in 87 cases and the HALO device in two. There was no significant difference in outcomes between the types of device, although fewer recurrences were seen in those in the THD arm (26% vs. 35%; adjusted OR 0.65, 95% CI 0.33 to 1.29; p = 0.22). Symptoms and pain were also similar for the two procedures, data are shown in Table 21. The Vaizey faecal incontinence score was higher for THD than HALO at 6 weeks (mean scores 5.2 vs. 3.2) and 1 year (means 5.6 vs. 3.5), but also prior to procedure (means scores 6.3 vs. 4.8). As a consequence, the magnitude of the difference at 6 weeks (mean difference = 2.0, 95% CI 0.5 to 3.6; p = 0.01) was greater than the change from baseline (mean difference incorporating an adjustment for baseline = 1.3, 95% CI –0.3 to 2.8; p = 0.11).
TABLE 21
Outcomes for the two HAL devices used in the trial
- Results - The HubBLe Trial: haemorrhoidal artery ligation (HAL) versus rubber ba...Results - The HubBLe Trial: haemorrhoidal artery ligation (HAL) versus rubber band ligation (RBL) for symptomatic second- and third-degree haemorrhoids: a multicentre randomised controlled trial and health-economic evaluation
- maturase K, partial (chloroplast) [Olea paniculata]maturase K, partial (chloroplast) [Olea paniculata]gi|475051451|gb|AGI56277.1|Protein
- Clinical and economic evaluation of laparoscopic surgery compared with medical m...Clinical and economic evaluation of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease: 5-year follow-up of multicentre randomised trial (the REFLUX trial)
- Offer of a bandage versus rigid immobilisation in 4- to 15-year-olds with distal...Offer of a bandage versus rigid immobilisation in 4- to 15-year-olds with distal radius torus fractures: the FORCE equivalence RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...
