Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Brabyn S, Araya R, Barkham M, et al. The second Randomised Evaluation of the Effectiveness, cost-effectiveness and Acceptability of Computerised Therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? A randomised controlled trial. Southampton (UK): NIHR Journals Library; 2016 Nov. (Health Technology Assessment, No. 20.89.)

The second Randomised Evaluation of the Effectiveness, cost-effectiveness and Acceptability of Computerised Therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? A randomised controlled trial.
Show detailsTrial design
This study was as a multisite, pragmatic, open, two-arm, parallel-group randomised controlled trial. Participants were recruited from primary care through direct referral by their GP or a postal invitation from GP records. Participants were individually randomised to one of two arms:
- minimally supported cCBT
- telephone-facilitated cCBT.
Participants in both arms were given access to a free-to-use cCBT program (MoodGYM), an accompanying booklet, and a Freephone number for technical support, and continued with usual GP care. Participants in the telephone-facilitated cCBT arm were additionally allocated a telephone support worker (TSW) who provided a programme of weekly telephone calls. The programme is described in more detail in Intervention.
Participants were followed up over the course of 12 months, with data collected at 4 and 12 months post randomisation.
Approval
Ethics approval was granted by Bradford Research Ethics Committee (10/H1302/95) on 20 December 2010 and from relevant research and development committees. The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) ISRCTN55310481.
Trial sites
The study was conducted in four UK sites with well-established networks of practices from which to recruit. These were the Universities of York, Bristol, Sheffield and Manchester.
Participants
The study population included patients in primary care with depression or low mood as determined by a score of ≥ 10 on the Patient Health Questionnaire-9 (PHQ-9).13 This cut-off point is known to detect clinical depression (major depression) in a UK primary care population with a sensitivity of 91.7% and a specificity of 78.3%.
The participants were recruited from a mix of rural and urban GP practices in and around Bristol, Avon, Somerset, Gloucestershire, Manchester, Sheffield and South Yorkshire, York, Humberside and East Yorkshire, Durham, Tyneside and Northumberland.
Inclusion criteria
Participants who met the following criteria were eligible to enter the study:
- aged ≥ 18 years
- not currently in receipt of cCBT or specialist psychological therapy
- score of ≥ 10 overall (indicating moderate, moderately severe or severe depression) and < 3 for item 9 (measuring suicidal thoughts)14 on the PHQ-9 depression severity measure.
Both incident and prevalent cases were included. In line with the pragmatic nature of this trial reflecting usual GP care, patients were eligible to participate whether or not they were in receipt of antidepressant medication, and those with comorbid physical illness or non-psychotic functional disorders were not excluded.
Exclusion criteria
We excluded potential participants who
- were actively suicidal as identified by the GP or as reported by item 9 on the PHQ-9
- had been bereaved within the last year
- had given birth within the last year
- had a diagnosis of psychotic depression
- had a primary diagnosis of alcohol or drug abuse
- were not able to read and write in English.
Participant recruitment
All recruiting researchers were given training in all aspects of the trial including trial recruitment, informed consent, adverse event reporting procedures, and risk assessment and reporting procedures. Each researcher was also given a study reference manual with full instructions on all the standard procedures. Participating GPs were provided with a GP manual with details of the trial processes, a GP information leaflet, adverse event reporting guidance, information about the interventions and contact details for the trial team.
Direct referral
Practices taking part in this study were provided with patient information packs containing a cover letter, patient information sheet, copy of the consent form and a prepaid envelope addressed to the local researcher, to give to patients with depression who were receptive to participating in the trial. The GP or representative could complete and fax a referral form and patient permission-to-contact form to the study researcher who, following a consideration period of at least 2 days, then approached the patient to discuss the study in more detail and confirm eligibility and continuing interest. The study design and approvals allowed that other health-care professionals attached to practices, such as nurses or primary care mental health workers, could refer patients to the study in the same way, but in the event participants were only referred by GPs.
Participation identification from general practitioner records
General practitioner practices were also asked to conduct a search of their records to identify patients presenting with depression or low mood, and screen for potentially eligible participants. Patient information packs supplied by the research team were sent from the GP practice inviting interested patients to return a completed permission-to-contact form to the research team. A member of the study team then made contact to confirm eligibility and discuss the study in further detail.
Screening for eligibility
After receiving permission-to-contact forms, the researcher contacted the potential participant to discuss the study, answer any questions and then confirm that the patient was still interested and eligible. To assess eligibility the potential participant was asked to confirm that he or she still met the inclusion criteria, in particular those that may not have been known to the GP, such as bereavement and drug and alcohol problems. The researcher also ensured that the participant understood what participation entailed, had access to the internet and wanted to take part in the trial. Participants were then asked whether they preferred the baseline assessment to be conducted over the telephone or at a face-to-face interview.
Consenting participants
Potential participants who preferred to have baseline data collected by telephone were asked to return the signed consent form in the prepaid envelope in the information pack. Those who preferred a face-to-face interview completed the consent process at the first meeting.
All potential participants were given a full explanation of the study and the opportunity to ask any questions or discuss concerns. Researchers emphasised that participants could withdraw consent at any point and would not have to give any explanation nor would their joining the study or leaving it at any point affect their GP care. Participants were also reminded that by consenting they agreed to their GPs being informed of their participation. Written informed consent was then taken, both participant and researcher signing and dating the consent forms and each keeping a copy.
Baseline assessment
Having consented to join the trial, participants completed a series of baseline questionnaires providing biographical, health status, health state utility and service use data. The participant was then randomly allocated according to the process outlined below (Figure 1).
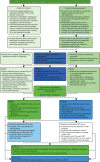
FIGURE 1
Flow of recruitment to the trial. HCP, health-care professional.
Randomisation
Participants were randomly allocated in a ratio of 1 : 1 to one of two treatment conditions: (1) minimally supported cCBT or (2) telephone-facilitated cCBT. Both conditions included log-in details for a free-to-use cCBT program (MoodGYM), a booklet accompanying the program, usual GP care, and access to a free helpline for troubleshooting and general help with the program. Simple randomisation was performed using a computer-generated random number sequence. The REEACT-2 trial researchers telephoned a secure randomisation line at the York Trials Unit and were given the participant’s allocation and log-in details for the MoodGYM program. By default, randomisation was done at the face-to-face interview and the participant informed immediately and given a copy of the MoodGYM booklet. If the interview finished after the randomisation line closed, the participant was informed the next day. The researcher then informed the trial manager who allocated TSWs to participants in the supported cCBT arm.
Sample size
The REEACT-2 trial was powered on the basis of an ability to detect a between-group difference in depression severity. We sought to recruit 350 patients with depression – 175 participants per arm. The REEACT-2 trial was designed to have sufficient power to detect a Cohen’s d effect size of 0.30 with 80% power (one-sided 5% significance level) allowing for loss to follow-up of 20%, in line with our empirically based estimates from the REEACT trial. The final sample size for the two arms was 369 participants.
Intervention
In line with the template for intervention description and replication (TIDier)15 guidelines the intervention is described below using the prescribed checklist headings.
Rationale
This trial built on preliminary findings from the REEACT trial (ISRCTN91947481). In the REEACT trial, we found that participants were not always keen to engage with the cCBT programs, and they expressed a desire to receive a greater level of support. Participants were happy to receive this support over the telephone, but felt that this should reflect and emphasise the practical elements of CBT. One way to encourage engagement with self-help technologies is to offer complementary low-intensity support by telephone.16 The REEACT-2 study was designed to examine if a programme of structured telephone facilitation would enhance engagement with the MoodGYM program and whether or not enhanced engagement would lead to better outcomes. The telephone-facilitation programme was designed to be capable of delivery by appropriately trained and supervised individuals who are not necessarily people with a professional background in mental health. The support programme was designed to be delivered after a brief structured training programme and with reference to a treatment manual.
Materials
MoodGYM is a free-to-use, internet-based, interactive CBT program for depression, developed and copyrighted at the Australian National University Centre for Mental Health Research. The online program is accompanied by a booklet with exercises and quizzes, and consists of five interactive modules released sequentially and lasting approximately 30–45 minutes and a sixth session that is predominantly consolidation and revision. Study participants were asked to aim to complete one session each week. The program provides patients with CBT techniques to overcome patterns of unhelpful thinking using cartoon characters to represent habits of thought.17
Procedures
Experimental intervention: weekly supportive/facilitative telephone calls plus cCBT (telephone-facilitated cCBT).
Participants in the experimental group received regular (ideally weekly) telephone calls from a trained worker to offer support, guidance and encouragement.
The telephone facilitation programme comprised eight telephone calls to be completed alongside the cCBT program within the 12–14 weeks between the first contact from the TSW and the 4-month follow-up time point. The purpose of the first and longest session (30–40 minutes) was to introduce the participant to the principles of CBT and the MoodGYM program and booklet, explain the process and help the participant identify difficulties and goals, and feel confident about engaging with the intervention.
The following six sessions were between 10 and 20 minutes long and were intended to provide motivation and to help participants identify any barriers to engagement and to the achievement of their goal(s). The final session helped participants to consolidate what they had learned and discussed their next steps and, if appropriate, how they might use the program in the future. The telephone-facilitation programme was delivered according to a manual developed by Professor Karina Lovell in conjunction with the REEACT-2 trial team.
Comparator intervention: minimally supported computerised cognitive behaviour therapy
All patients in the control group were registered as users of MoodGYM and given a unique password. As with the intervention group, they were supplied with a free helpline number to ring if they had technical problems or needed advice, but they did not receive regular telephone calls. This comparator intervention replicates NHS care in most settings and represents what would happen if a patient were given the website of a cCBT package such as MoodGYM by their GP or primary care mental health worker without being offered proactive support.
Providers
The intervention was delivered by TSWs, a team of people specifically recruited to support the REEACT-2 trial and trained in the delivery of the manualised telephone facilitation intervention. The support workers were not recruited to act as psychotherapists to the participant and were not instructed to replicate or consolidate the therapeutic content of the packages, as they were not trained or instructed to act as cognitive behaviour therapists.
The professional background was mixed and included psychology graduates and people who demonstrated good interpersonal skills who had worked as counsellors, social workers, psychiatric nurses and volunteers with mental health charities (e.g. Samaritans). The telephone facilitation programme was delivered according to a manual developed for the REEACT-2 trial. Full training in delivery of the programme, including the management of information that may be troubling or indicate risk, was given to all potential TSWs and their suitability to take on the role was assessed during the training and with recorded mock facilitation sessions with an experienced TSW.
Follow-up training meetings took place during the trial on a roughly bimonthly basis and TSWs had access at all times by telephone to a supervisor with a professional background in mental health (KL, mental health nursing; DK, primary care physician; and SG, psychiatrist). Between training meetings with KL, regular contact was maintained with the trial manager via e-mail and telephone. TSWs were provided with continuing case supervision every 2 weeks from the trial manager who managed the majority of day-to-day queries. Sessions with participants were recorded (with consent) and early sessions were monitored by a senior trial principal investigator for fidelity to the manual.
Mode of delivery
Participants were given a copy of the MoodGYM booklet at the baseline interview and the MoodGYM program was generally delivered via participants’ own internet-connected computer, enabling participants to log on at their convenience. The default mode of delivery of the support programme was via the telephone. Three participants requested an alternative mode of communication (e-mail) because they felt too anxious to use the telephone.
Locations
Participants could log on to the MoodGYM application anywhere with broadband internet access. They were encouraged to make sure that they had a suitable environment for an appropriate length of time. Researchers could help participants to identify and book time at alternative locations with computer access such as public libraries or GP practices if they had no internet access at home or their home was not a suitable location for any reason.
Participants were encouraged to be at home, or somewhere comfortable and private for the telephone appointments. Whatever the location, to maintain privacy, the participant and TSW agreed a code word in the first session that the participant could use to indicate that it was not appropriate to continue with the conversation and that they would reconvene later.
When/how much
The TSWs contacted the participants at pre-arranged times to suit, as far as possible, the participant. This included some weekend and evening calls. The intervention provided eight telephone calls. The first of these was expected to last between 30 and 45 minutes, the following seven calls between 10 and 15 minutes, and the final call 20 minutes.
Tailoring
Telephone support workers worked to a manual but their conversations were not scripted. The telephone support programme could be tailored to some extent because there was some variation in the severity of participants’ depression, their availability and their willingness or ability to complete a module each week.
Adherence
Adherence by participants to the computer program was measured by requesting information from the website providing MoodGYM (hosted by the developers of MoodGYM at the Australian National University, Canberra, ACT, Australia). We obtained computer usage data on the number of times each participant logged on to the MoodGYM program and whether or not each module was 25%, 50%, 75% or 100% complete.
Telephone support workers kept records of the number and length of telephone sessions as well as detailed records of all attempts to contact the participants.
Fidelity
Fidelity to the telephone support manual was monitored in supervision discussion and from the recordings of the sessions. In keeping with the pragmatic nature of the trial there was a certain amount of variation in the delivery between TSWs and between participants. Telephone sessions were not formally scrutinised or analysed as a research activity, but the recordings have been retained for future reference.
Outcomes
Primary outcome measure
The primary outcome was depression severity and symptomatology as measured by a validated self-report measure (the PHQ-9)14 at 4 months because this is the point at which the largest between-group difference might be expected. The PHQ-9 logs the core symptoms of depression and takes the form of a questionnaire comprising nine sections.
Data were collected in one of four ways according to participant preference:
- by telephone interview with a recruiting researcher
- completion of a client report form (CRF) sent out by post
- online
- at a face-to-face interview with a recruiting researcher.
Secondary outcome measures
The secondary outcome measures comprised the PHQ-9 at 12 months, anxiety [as measured by the Generalised Anxiety Disorder Scale-7 items (GAD-7)];18 somatoform complaints [as measured by the Patient Health Questionnaire-15 (PHQ-15)];19 health state utility [as measured by the European Quality of Life-5 Dimensions (EQ-5D)];20 and service use using the adapted Client Service Receipt Inventory (CSRI)21 and Client Satisfaction Questionnaire-8 items.22 The secondary outcome measures were recorded at 4 and 12 months. A summary of assessments and data collection time points can be found in Table 1.
TABLE 1
Assessments and time points at which they were carried out
Statistical analysis methods
Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) on a two-sided basis and using a 5% significance level.
Primary analysis
All outcomes were summarised descriptively by intervention group and at each time point using mean, median, standard deviation (SD), range and number of patients for continuous outcomes, and number of patients and percentage for discrete outcomes.
Patient Health Questionnaire-9 at 4 months
The primary outcome was the severity of depression as measured by the PHQ-9 at 4 months, a self-reported questionnaire. The primary analysis used the PHQ-9 score as a dichotomous outcome with a score of ≥ 10 meaning depressed and < 10 not depressed; a cut-off point of 10 has been shown to be sensitive for detecting clinical depression in UK primary care.25 The PHQ-9 is a nine-item questionnaire that records the core symptoms of depression and gives a total score ranging from 0 to 27, with a higher score indicating more severe depression. It is categorised as follows: 0–4, none to minimal depression; 5–9, mild depression; 10–14, moderate depression; 15–19, moderately severe depression; and 20–27, severe depression. The cut-off point of 10 was used to categorise participants as depressed or not depressed at 4 and 12 months (PHQ-9 score of ≥ 10 depressed, PHQ-9 score of < 10 not depressed). To be included in the study, participants had to score of ≥ 10 on the PHQ-9 at baseline. The PHQ-9 score at each time point was calculated as the sum of all nine items. If one item was missing it was replaced with the mean of the other eight, but if two or more items were missing, then the whole questionnaire was treated as missing (using the same scoring method used in the REEACT trial).
The number and percentage of participants who were not depressed/depressed were reported at 4 and 12 months and treatment groups were compared using a chi-squared test. The missing responses were summarised and possible reasons explored by summarising and comparing baseline data between those with and without a missing outcome. The primary analysis compared minimally supported cCBT with telephone-facilitated cCBT using a logistic regression model adjusting for the baseline PHQ-9 score, age, sex, baseline GAD-7 score and treatment. Odds ratios (ORs) with 95% CIs are reported.
The primary analysis was on a complete case basis (only including those with a 4-month assessment). As some missing data were expected, sensitivity analyses were performed using imputation. A simple imputation on a worst-case scenario was used assuming that all participants with a missing outcome were still depressed. Anyone with a missing PHQ-9 score was assumed still to be depressed and have a score of ≥ 10.
Secondary analyses
Patient Health Questionnaire-9 at 12 months
The secondary analysis of the primary outcome was the same as for the primary analysis but used the PHQ-9 score of < 10/≥ 10 (not depressed/depressed) at 12 months.
Patient Health Questionnaire-9 as a continuous outcome
The PHQ-9 score was also summarised and analysed as a continuous outcome. This is summarised for each assessment time point (baseline, 4 and 12 months) using mean, SD, median and range, and the number of missing values. Plots are presented showing the mean and 95% CI at each time point.
Means and SDs at the previous assessment were summarised and compared between those with and without the subsequent PHQ-9 score missing, using t-tests, to evaluate whether or not there were differences in scores between those with and without a missing assessment (i.e. whether or not those who dropped out were more depressed).
A repeated measures mixed regression model was used to analyse the change in PHQ-9 score over time. This included all randomised participants and provides reliable estimates assuming the data are missing at random. The outcome was the PHQ-9 score at 4 and 12 months and the model included the baseline score, treatment, age, sex, baseline GAD-7 score and time. The treatment × time interaction was included to evaluate if the difference between treatments changed over time. Different covariance structures were evaluated (e.g. unstructured, compound symmetry) and the one providing the best fit was used. Residual plots were used to check model assumptions. The mean difference, 95% CI and p-values are presented for all terms in the model. Effect sizes (Cohen’s d) were calculated for the between-group differences in mean PHQ-9 score at 4 and 12 months using the difference between the means and corresponding standard errors from the mixed model. The standard errors were converted to SDs using the corresponding sample size in each treatment group.
Other secondary outcomes
The GAD-7 and PHQ-15 scores were analysed as continuous outcomes using the same repeated measures mixed models, as described for PHQ-9 above.
The number of participants taking any medication to help with their depression was summarised descriptively at each time point. cCBT use was summarised descriptively.
Adverse events
Adverse event data were summarised descriptively.
Economic methods
The primary objective of the economic analysis was to assess the relative cost-effectiveness of telephone-facilitated cCBT compared with minimally supported cCBT. The economic analysis was conducted prospectively alongside the REEACT-2 trial.
Health-related quality of life
Decisions concerning resource allocation often need to be taken across specialties and disease areas. If these decisions are to be informed by a cost-effectiveness analysis, then it is crucial that the outcome measure adopted is generic (i.e. that it has meaning outside the clinical area within which it is used). The use of a single generic measure of health benefit enables diverse health-care interventions to be compared, thus enabling broader questions of efficiency to be addressed.
In this study, the main outcome for the cost-effectiveness analysis was the quality-adjusted life-year (QALY), assessed using the EQ-5D. The EQ-5D questionnaire is a standardised generic instrument for measuring health-related quality of life (HRQoL).20 The EQ-5D consists of five health dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has three levels of severity (no problems, moderate problems and severe problems) that generate 245 unique health states into which a patient can be classified. The EQ-5D also provides a single preference weight (also described as a utility or value) for each health state. These can be used as quality-adjustment weights to turn a profile of health states over time into QALYs. The EQ-5D has been validated in UK populations and has been used to measure HRQoL in patients with depression in primary care.26
Resource use and cost data
Information relating to participants’ resource utilisation was obtained via patient self-report using an adapted version of the CSRI.27 The CSRI was administered at baseline and at each follow-up. Participants were asked about their use of services in the previous 6 months (including inpatient and outpatient hospital services, community-based day services, and primary and community care contacts); and whether or not they had incurred any additional costs associated with their depression (e.g. medication or drug costs, child-care costs, travel costs). Participants were also asked to record their use of any medication to help with their depression, including medication name, dose and duration taken.
Unit costs were obtained from routinely published national literature sources, namely the British National Formulary,28 the Personal Social Services Research Unit’s Unit Costs of Health and Social Care29 and the NHS reference costs.30 All unit costs were adjusted to 2012/13 prices using the relevant price indices. Appendix 1 provides sources and details of the unit costs.
The costs of delivering each intervention were limited to those associated with delivering telephone support, as MoodGYM is a free-to-use software package. The cost of telephone support calls was estimated based on mean duration and mean number of support calls recorded as part of the study, and assuming the support was provided by a clinical support worker (band 2). It was assumed, after consultation with our clinical advisers, that a clinical support worker or a professional in the same pay band would provide this service in the NHS, if the intervention was to be rolled out.
The unit costs estimates were then combined with the resource utilisation data to obtain a net cost per patient over the entire follow-up period for the trial. As costs were estimated over a 12-month period, no discounting was applied.
Economic analysis: statistical methods
Overview
A within-trial economic analysis was conducted to evaluate the cost-effectiveness of telephone-facilitated cCBT (MoodGYM) and minimally supported cCBT. Costs and health benefits expressed in QALYs were estimated over the 1-year follow-up. The analysis was conducted on an intention-to-treat basis from the perspective of the UK NHS and Personal Social Services. All analyses were undertaken in Stata® 12.0 (StataCorp, College Station, TX, USA).
Resource use and costs
Descriptive statistics (mean, SD, median and interquartile range) are reported for resource use and costs. The descriptive statistics for resource use presented are based on the available case data set as multiple imputation was only performed for total costs as opposed to individual resource use items. Multiple imputation by chained equations31 was performed for a total of 10 imputations, and costs were imputed at every follow-up time point (baseline, 4 and 12 months) for each resource use category. The independent variables specified in the imputation were baseline EQ-5D score, baseline costs, age, sex, anxiety level at baseline, depression level at baseline and depression duration at baseline. The descriptive statistics for resource use and costs are also reported using unadjusted estimates. Differences in mean costs (and 95% CI) between the groups were subsequently adjusted for baseline costs and additional participant covariates using regression analysis (see Cost-effectiveness analysis).
Health-related quality of life
Health-related quality of life was assessed using responses to the EQ-5D questionnaire applied at baseline and at 4 and 12 months. Missing EQ-5D scores were imputed by multiple imputation by chained equations alongside costs at the same follow-up time points (baseline, 4 months and 12 months) and specifying the same independent variables. The EQ-5D scores were used to estimate patient-specific QALYs using the area under the curve method32 and descriptive statistics were reported. Differences in QALYs (and 95% CI) between the groups were adjusted for additional participant covariates using regression analysis (see Cost-effectiveness analysis).
Cost-effectiveness analysis
Incremental estimates of total costs and QALYs were obtained through regression methods, adjusting for the following baseline characteristics: age, anxiety level, baseline depression severity, depression duration and sex. The costs and QALY estimates were also adjusted for baseline costs and baseline EQ-5D scores, respectively.
Incremental QALYs were estimated using ordinary least squares (OLS) regression, as this method has been recommended for the estimation of QALYs in economic evaluation.33 The regression model applied for the incremental analysis of costs in the base case was a generalised linear model.34 This type of model was preferred to an OLS model, as cost data tend to be heavily skewed and follow a non-normal distribution, which leads to violations of the OLS assumptions. For the analysis of costs, a gamma family distribution was selected. Selection of the family distribution was based on the modified Park’s test35 performed on each imputed data set and complete case data set. An identity link function was selected, thus assuming an additive effect of covariates on costs.
Cost-effectiveness was assessed by comparing the incremental costs and QALYs using standard decision rules.36 An intervention that generates greater mean QALYs and lower mean costs can be considered dominant and, therefore, a cost-effective use of resources when compared with the alternative. When no dominance arises (i.e. one intervention is more costly and more effective than the other), the interventions can be compared by calculating the ratio between incremental costs and QALYs to establish the incremental cost-effectiveness ratio (ICER). When the ICER between two interventions is below the cost-effectiveness threshold that represents the rate at which health-care activities in the NHS (assumed to be cost-effective) generate health at the margin, then the more costly and more effective intervention is considered cost-effective. The cost-effectiveness of the interventions was assessed by comparing ICERs against a cost-effectiveness interval ranging from £20,000 to £30,000 per QALY, in line with NICE cost-effectiveness thresholds for the UK.37
Uncertainty surrounding the decision was assessed using a probabilistic sensitivity analysis and presented through cost-effectiveness acceptability curves that graphically represent the probability of an intervention being cost-effective across a range of cost-effectiveness thresholds. The analysis was performed by simulating random draws of incremental mean costs and QALYs (n = 1000) from a multivariate normal distribution and estimating the proportion of those draws that corresponded to a cost-effective use of resources at cost-effectiveness threshold values ranging from £0 to £60,000 per additional QALY. In order to plot the cost-effectiveness acceptability curve, the variance–covariance matrices from the costs and QALYs regressions were extracted and the corresponding Cholesky decompositions calculated to parameterise multivariate normal distributions.38 This approach is commonly used to ensure that parameters taken from a regression framework are appropriately correlated and not treated as independent when the probabilistic sensitivity analysis is performed (and cost-effectiveness acceptability curves are plotted), but it has the disadvantage of imposing normality on the sampling distribution.
Base-case and sensitivity analysis
The base-case cost-effectiveness analysis was based on a comparison of all participants receiving minimally supported cCBT (n = 187) with telephone-facilitated cCBT (n = 182), and conducted on the multiple imputed data sets. In the base-case analysis, all categories of health-care costs were included, and QALYs estimated from EQ-5D scores were considered.
A separate sensitivity analysis undertaken, which excluded all non-mental health-related hospital costs so as to assess the robustness of base-case results to alternative assumptions in terms of costs, was also considered.
Patient and public participation
Contributors with experience of depression were involved in the design of the trial and the writing of the protocol, and the chief executive of a user-led organisation was a collaborator and coapplicant. Two people with depression read the consent forms and questionnaires and commented on the experience of completing them. All research documentation was designed with patient and public input and the trial oversight committees included members with experience of mental health problems.
- Methods - The second Randomised Evaluation of the Effectiveness, cost-effectiven...Methods - The second Randomised Evaluation of the Effectiveness, cost-effectiveness and Acceptability of Computerised Therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? A randomised controlled trial
- recombining binding protein suppressor of hairless isoform X4 [Rattus norvegicus...recombining binding protein suppressor of hairless isoform X4 [Rattus norvegicus]gi|1958677169|ref|XP_038948336.1|Protein
- Drosophila mojavensis strain OPNM-2 hypothetical protein (GI23324) gene, complet...Drosophila mojavensis strain OPNM-2 hypothetical protein (GI23324) gene, complete cdsgi|744515678|gb|KP236512.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...