Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Heller S, White D, Lee E, et al. A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial. Southampton (UK): NIHR Journals Library; 2017 Apr. (Health Technology Assessment, No. 21.20.)

A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial.
Show detailsMethods for the randomised controlled trial
The trial protocol was published in a separate paper.86
Study design
The REPOSE Trial was a pragmatic, multicentre, parallel-group, open-label, confirmatory cluster RCT. Participants were allocated a place on a week-long DAFNE course, depending on their availability to attend the course. The course (cluster element) groups were then randomly allocated in pairs to either pump or MDI treatment, with allocation concealed. A cluster design was chosen because of the impracticality of randomising individuals and then finding suitable times for that participant to attend a course of the correct allocation.23 Such an approach was more likely to have resulted in significantly higher attrition rates pre course. Following the course, participants received the trial treatment for 2 years and outcome measures were collected at 6, 12 and 24 months post course. Outcome measurement was not blinded (see Data collection).
Approvals obtained
The protocol was approved by the Research Ethics Committee (REC) North West, Liverpool East, on 26 April 2011 (REC reference number 11/H1002/10). Each participating centre gave UK NHS Research and Development (R&D) approval (see Appendix 2). The protocol received Medicines and Healthcare products Regulatory Agency (MHRA) clinical trials authorisation on 26 May 2011 [European Union Drug Regulating Authorities Clinical Trials (EudraCT) reference no: 2010-023198-21].
Setting
The trial was conducted in eight secondary care diabetes centres in Sheffield, Cambridge, Dumfries and Galloway, Edinburgh, Glasgow, Harrogate, London and Nottingham (see Table 11). Participating centres all had experience in delivering high-quality structured education using DAFNE and had variable levels of experience delivering pump therapy; most were established pump centres but some were relatively new to pump therapy. Nottingham was a reserve centre, activated midway through the trial. The seven centres involved from the outset were asked to recruit 40 participants to three pump and three MDI courses (5–8 patients on each course) over 11 months. Owing to a higher than anticipated dropout rate prior to the DAFNE courses we then recruited to an additional pair of courses at Harrogate, and a pair of courses at the reserve centre, Nottingham.
Participants
Participants were eligible for the trial if they met the following inclusion criteria:
- were aged ≥ 18 years
- had T1DM for at least 12 months at the time of the DAFNE course
- were fluent in speaking, reading and understanding English
- were willing to undertake intensive insulin therapy with SMBG, carbohydrate counting and insulin self-adjustment
- had no preference for either pump or MDI, and were happy to be randomised
- were currently using, or willing to switch to, insulin detemir
- had a need for structured education to optimise diabetes control.
Furthermore, participants were excluded if they met any of the following criteria:
- had already completed a diabetes education course
- used a pump in the previous 3 years (defined as > 2 weeks’ use in the last 3 years) or had strong clinical indications for pump therapy in the view of the investigator
- had renal impairment with a chance of needing renal replacement therapy within the next 2 years (enrolment staff to check that creatinine levels not > 200 µmol/l).
- had uncontrolled hypertension (diastolic blood pressure of > 100 mmHg and/or sustained systolic level of > 160 mmHg)
- had a history of heart disease within the past 3 months
- had severe needle phobia (severity of phobia assessed, considering if the phobia might preclude full participation in either treatment arm or influence the participant’s preference for pump therapy)
- had a current history of alcohol or drug abuse
- had serious or unstable medical or psychological conditions that are active enough to preclude the participant safely taking part in the trial (based on investigatory judgement)
- had recurrent episodes of skin infections
- were pregnant or planning to become pregnant within the next 2 years
- had taken part in any other investigational clinical trial during the 4 months prior to screening
- had any other issue that might have precluded them from satisfactory participation in the study based on investigatory judgement
- were unable to give informed consent.
Interventions
Dose Adjustment For Normal Eating with multiple daily injection
Participants on the MDI arm attended a standard DAFNE structured education course, described in detail elsewhere.2 Courses are conducted over 5 consecutive days, providing an average of 38 hours of structured education, delivered to groups of 5–8 adults, aged ≥ 18 years, in an outpatient setting. Courses are delivered by diabetes specialist nurses and dietitians who attend an educator training course, the DAFNE education programme, a seven-part programme consisting of 105 hours of structured training.
The DAFNE curriculum uses a progressive modular-based structure to improve self-management in a variety of medical and social situations. Content is designed to deliver key learning topics at the appropriate time during the week. In this way, knowledge and skills are built up throughout the course with active participant involvement and problem-solving as key methods of learning. The key modules are: ‘What is diabetes?’, ‘Food and diabetes’, ‘Insulin management’, ‘Management of hypoglycaemia’ and ‘Sick day rules’. Lesson plans give guidance on timing and a student activity section serves to give an idea of expected responses. Each meal and snack during the course is used as an opportunity to practise carbohydrate estimation and insulin dose adjustment.
Dose Adjustment For Normal Eating with pump
Participants on the pump arm attended a modified DAFNE course, which had been tested in a pilot study, previously published.27 The 5-day structure of the standard adult DAFNE course was maintained while incorporating the additional skills and learning outcomes that were considered necessary to use pumps successfully. The principles of insulin dose adjustment taught on the standard adult course were maintained.23 The need to introduce ‘pump skills’ required the addition of a pre-course group session, delivered 1–3 weeks before the DAFNE course. This session gave participants the opportunity to learn about the basics of insulin pump therapy, including how to set up the pump, so that they could practise using it with saline before starting on insulin at the beginning of the course. The session included the theory of pump therapy, understanding cannulas and infusion sets, skin care, pump maintenance and the advantages and disadvantages of the insulin pump. Participants switched to insulin on the evening before the DAFNE course or on the first day of the course.
Ongoing treatment
After attending the DAFNE course, participants received the trial treatment for 2 years from the secondary care service. All of the participants in both groups were invited to an additional DAFNE follow-up group session at 6 weeks post course, which is standard for DAFNE course attendees.
Multiple daily injection participants used a combination of quick-acting insulin analogues and twice-daily injections of insulin detemir. Pump participants used a Medtronic Paradigm® VeoTM insulin pump (Model X54) with short-acting analogue insulin, as in a meta-analysis87 this was shown to lower HbA1c to a greater extent than traditional soluble insulin. As insulin is already marketed and licensed for use, and as the participants were already accessing insulin through prescription on a regular basis, there was no need to change how the insulin was accessed for the trial – participants collected insulin from their pharmacist as normal.
The insulin pumps include, as standard, a Medtronic Bolus Wizard (Medtronic, Watford UK) to aid calculation of insulin doses. In order to reduce any potential bias, MDI participants were also given access to a bolus calculator (Accu-Chek Aviva Expert Bolus Advisor System, Roche Diagnostics Ltd, Burgess Hill, UK).
Fidelity testing (FT) of pump courses was undertaken in order to assess whether or not courses were delivered in accordance with DAFNE philosophy and principles, and that the educators had the necessary skills to deliver these principles. The results of the FT are reported in Chapter 5. Standard DAFNE courses were not tested, as there is a rigorous quality assurance programme of MDI courses in standard care.
Treatment was changed (pump to MDI or MDI to pump) at the discretion of the local principal investigator (PI) if self-management of diabetes had become ineffective and was considered a risk to the individual. If the participant failed to attend the pump course then they were withdrawn from pump treatment.
Primary outcomes
The main primary end point was the change in HbA1c at 24 months, in those participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol). The key secondary end point was the proportion of participants reaching the NICE target of a HbA1c level of ≤ 7.5% (58 mmol/mol) at 24 months (of all participants).
Glycated haemoglobin is the accepted gold standard measure of glycaemic control and provides a measure of efficacy. Most health economic models of T1DM estimate the cost-effectiveness by primarily modifying HbA1c levels, which subsequently affect the risk of diabetic complications.88 However, it is important to note that HbA1c may not have fallen in patients who entered the trial with low baseline levels of HbA1c, but who might have been experiencing frequent hypoglycaemia or wished to increase dietary freedom. Success for such individuals would be a HbA1c level that is maintained, or even rises slightly, with a reduction in the frequency of hypoglycaemia.23 We included such patients as they could provide important information about QoL and the potential of pump therapy to reduce rates of hypoglycaemia. However, as their glycaemic control may not alter, including their HbA1c data would have reduced our statistical power to establish improvement in our primary end point. We therefore powered the trial on the number of participants with a baseline HbA1c of ≥ 7.5% (58 mmol/mol) and in whom a fall would reflect a worthwhile improvement in glycaemic control. We ensured standardisation by testing HbA1c in a central laboratory.
Exploratory outcomes on the primary end points
The primary outcome and key secondary outcome were also evaluated at 6 and 12 months in order to explore the short- and medium-term effects of the intervention.
Secondary outcomes
Secondary outcomes were evaluated in all participants and were measured at 6, 12 and 24 months. Blood and urine samples for secondary outcomes were tested in local laboratories.
Hypoglycaemia
We recorded episodes of both moderate and severe hypoglycaemia and specifically recorded episodes at night (those occurring between 23.00 and 07.00). We used a standard definition of severe hypoglycaemia,89,90 being ‘an episode leading to cognitive impairment sufficient to cause either coma or requiring the assistance of another person to recover’. The number of severe episodes are reliably recorded by patients for up to 1 year.91
During the last NICE appraisal of pump therapy, the question of the impact of moderate hypoglycaemia was raised.13 The modelling had included only severe hypoglycaemia, and the point was made that moderate hypoglycaemia, sufficient to interrupt activities of daily living, might, because of greater frequency, have a more cumulative effect on QoL than severe hypoglycaemia. We therefore also recorded rates of moderate hypoglycaemia in an attempt to increase power and identify the ability of pumps to reduce rates of hypoglycaemia. With no standard definition of moderate hypoglycaemia, the Trial Management Group (TMG) agreed to define these as ‘any episodes which could be treated by that individual, but where hypoglycaemia caused significant interruption of current activity, such as having caused impaired performance or embarrassment or having been woken during nocturnal sleep’. As these episodes are more frequent, reliable recall of such events is unlikely to be sustained for more than a few weeks. We therefore asked participants to record the number and timing of moderate episodes over the 4 weeks prior to each follow-up visit. We used this approach successfully to record the frequency of mild episodes in a recent epidemiological study of hypoglycaemic burden in diabetes.89
Insulin dose and body weight
Pump treatment may result in the use of less insulin, leading to a favourable effect on body weight. We recorded total insulin dose at each time point and calculated units per kilogram of body weight.
Lipids and proteinuria
A recent study61 reported little difference in HbA1c on pump therapy compared with MDI but found less progression to microalbuminuria in the pump group, and also lower cholesterol levels. We measured high-density lipoprotein (HDL) cholesterol and total cholesterol (TC). Proteinuria was measured using the albumin-to-creatinine ratio (ACR).
Diabetic ketoacidosis
Diabetic ketoacidosis was measured throughout the trial through the assessment of serious adverse events (SAEs).23 As all significant episodes of ketosis require hospital admission, we were confident in capturing all of the relevant episodes.
Quantitative psychosocial outcomes
The quantitative psychosocial outcomes are described later (see Outcomes).
Sample size
It is generally accepted that a difference of 0.5% (5.5 mmol/mol) in HbA1c is clinically worthwhile. To detect this difference with a standard deviation (SD) of 1% at 80% power and 5% two-sided significance using a t-test requires 64 patients per group, for subjects > 7.5% HbA1c. To allow for a clustering effect of the educators, with an average of seven patients per DAFNE group and a within-course intraclass correlation coefficient (ICC) of 0.05, common in diabetes care, the sample size increases to 84. Allowing for a 10% dropout over 24 months, the sample size per group becomes 93. Audit of the DAFNE database showed us that 75% of subjects had a HbA1c of ≥ 7.5%, therefore requiring 124 subjects per group and 248 in total. We planned to recruit 280 subjects, which increased the power to 85% but allowed for some variation in dropout rates and the proportion of patients with HbA1c ≥ 7.5%. However, monitoring of baseline data showed that the actual proportion of participants with HbA1c ≥ 7.5% was around 90% rather than 75%. A modelling exercise undertaken during recruitment, with conservative estimates of 85% (HbA1c ≥ 7.5%) and dropout rate of 15%, suggested that the trial would require at least 240 participants with primary outcome data at 2 years in order to preserve power of at least 85%.23
Recruitment
A number of methods were used to approach potential participants:
- PIs or educators identified people from DAFNE waiting lists. They then telephoned or wrote to potentially eligible individuals.
- Individuals attending a clinic appointment with a trial PI or educator were offered the option of a future or immediate consultation regarding the trial.
- Clinicians [general practitioner (GP), dietitian, nurse] provided information to patients and referred them to PIs to be screened and enrolled.
- Details of the trial were advertised through the use of posters and leaflets in clinics (diabetes outpatient, dietetic, GP surgery).
- Reception staff in diabetes clinics were informed about the trial and provided with leaflets to give to patients who expressed an interest.
- Participant identification centres were used at some research centres to assist in the identification of suitable participants.
Interested individuals were given the opportunity to discuss the trial with the PI or educator. Those who were still interested in taking part were screened for eligibility. Those who were eligible were either invited to attend a local information meeting, at which the trial was discussed in detail and questions answered, or were provided with a patient information sheet and consent form and given the opportunity to ask further questions. Individuals who were still wanting to take part consented to the trial by one of three methods: (1) by returning a completed consent form (see Appendix 3) in the post, (2) by completing the form with the PI or educator or (3) by completing the form at a local information meeting. The participants’ contact details, GP details and ethnicity were also collected.
Allocation to Dose Adjustment For Normal Eating courses and randomisation
Following consent, participants were allocated to a REPOSE DAFNE course, depending on the participants’ availability.23 Up to eight participants were allocated to each course, with a minimum of five preferable. Courses were randomised, in pairs, to either DAFNE with pump or DAFNE with MDI.23 Participant allocation to courses was finalised for each course pair before randomisation took place, no less than 6 weeks prior to the date of the first DAFNE course in that pair. For the first seven centres, a simple randomisation procedure in block size of ‘2’, stratified by centre, was used for courses 1–4. Courses 5 onwards were allocated in pairs using minimisation of the overall and number of participants, with most recent baseline HbA1c value of ≥ 7.5% or < 7.5% between the treatment groups. Any additional courses were allocated using minimisation. Known dropouts prior to the DAFNE course were excluded from the minimisation algorithm for future course allocation. A validated user-written Stata® 13 (StataCorp LP, College Station, TX, USA) code was produced to generate the allocation by a statistician within Sheffield Clinical Trials Research Unit (CTRU), who implemented the randomisation. The trial co-ordinator revealed the allocation to study centres.23
Blinding of the course allocation was not possible because of the nature of the treatment. Course allocations were revealed to centres 4–6 weeks prior to the date of the first course to allow sufficient preparation time. Participants were informed of the allocation of their DAFNE course no earlier than 4 weeks prior to that course. At this point they were asked to keep a record of any new episodes of moderate hypoglycaemia, which would be collected at the baseline assessment. If the course was a pump course, the participant was booked into a pre-course pump session, up to 3 weeks prior to the course date, in addition to the baseline assessment, which had to take place before the pump session.
If, for any reason, participants were unable to take part in the course at short notice, they could be allocated to a later course date, but only in the same trial arm as in the course to which they were originally allocated. Centres could also keep a list of reserve participants for courses, agreed prior to the time when the course allocation had been revealed to the educators. In the case of participants dropping out, the next person on the reserve list would be invited to participate in that course.
Data collection
Study visits took place at the participants’ diabetes centre. A data collection form (DCF) (see Appendix 4) was completed by the educator with the participant. Blood and urine samples were taken and analysed at local laboratories. Two blood samples were taken for measurement of the primary outcome (HbA1c). One of these was analysed at a central laboratory as the primary measure and the second was tested at the local laboratory as a back-up. DCF data were entered at local centres on to the in-house Prospect web-based electronic data capture system, managed by the CTRU.
Baseline assessments took place up to 3 weeks prior to the DAFNE course. The educator completed the DCF with the participant and handed him/her the self-complete psychosocial questionnaire, asking for return of the completed questionnaire at the forthcoming DAFNE course. Additional demographic data collected at baseline were date of birth, sex, qualifications (highest qualification obtained) and current occupation. Participants were also handed a SAE contact card to aid in contacting their diabetes centre in the event of an AE.
At the DAFNE course, an attendance form was completed, detailing any missed sessions. The completed baseline psychosocial questionnaire was collected and the baseline DCF moderate hypoglycaemic episodes section was updated so that a full 4 weeks of hypoglycaemic episodes were recorded. At all time points, psychosocial questionnaires were posted from centres to Sheffield CTRU and entered on to Prospect by Sheffield CTRU clerical staff.
Participants were followed up at 6, 12 and 24 months after the DAFNE course. Participants were sent the blood glucose diary (see Appendix 5) and instructions for recording moderate hypoglycaemic episodes 4 weeks prior to each visit. Additionally, participants were posted the self-complete psychosocial questionnaire pack prior to the visit and asked to bring their completed questionnaire to the appointment, along with the blood glucose diary and record of moderate hypoglycaemic episodes.
Severe hypoglycaemic episodes or SAEs were collected from participants if reported over the telephone or in clinic. Any additional diabetes-related contacts (DRCs) were also recorded (see Appendix 6 for ongoing data collection booklet).
Blinding of outcome measures was considered impractical because of the intervention-specific nature of outcome measures and the necessity of a local diabetes nurse to collect the data. However, use of an objective outcome (HbA1c) measured in a central laboratory will have minimised bias on the primary end point.
Trial completion
Participants were deemed to have completed the study if they had trial data recorded at baseline and 24 months. Participants were withdrawn from the study if:
- The participant asked to fully withdraw from the trial. On requesting withdrawal from the trial, participants were able to consent to continue to have their routine HbA1c results recorded.
- The participant died.
Participants who were changing treatment continued in the trial unless formally withdrawn. Participants were deemed lost to follow-up if they failed to attend the baseline visit, DAFNE course or 24-month follow-up.
Research governance
The trial sponsor was Sheffield Teaching Hospitals NHS Foundation Trust. The trial was conducted in accordance with Good Clinical Practice (GCP) and the Medicines for Human Use (Clinical Trials) Regulations 2004.92 All staff recruiting participants to the trial had undertaken GCP training. In line with the three-level categorisation of clinical trial risk in the Medical Research Council/Department of Health (DH)/MHRA report on risk-adapted approaches to the management of clinical trials of investigational medicinal products93 (based on the classification by Brosteanu et al.94), the REPOSE Trial was classified as a Type A study: no higher than the risk of standard medical care. The trial treatment in REPOSE was licensed and administered according to its market authorisation. Trial-specific labelling was not used. Given the lack of criticality of the investigational medicinal product (IMP) with the data analysis and trial results, and the design of the trial being equivalent to standard care, there was no IMP tracking and accountability undertaken.
Three committees were established to govern the conduct of the study: an independent Trial Steering Committee (TSC), an independent Data Monitoring and Ethics Committee (DMEC) and a TMG. Full membership of the TSC and DMEC are listed at the end of this report. The committees functioned in accordance with Sheffield CTRU standard operating procedures (SOPs). The TSC was responsible for overall supervision and monitoring of the trial; it considered any recommendations from the DMEC and provided advice on any actions to be taken. The DMEC operated within a charter agreed by all members and was responsible for monitoring efficacy and safety data. Any concerns were reported to the TSC with recommendations. The TMG was responsible for supporting the implementation of the trial.
Reporting of adverse events
Adverse events were defined as any untoward medical occurrence in a participant to whom a medicinal product has been administered, including occurrences that are not necessarily caused by or related to that product. SAEs were defined as any AE that results in death; is life-threatening (subject at immediate risk of death); requires inpatient hospitalisation or prolonging existing hospitalisation; results in persistent or significant disability or incapacity, or consists of congenital anomaly or birth defect; or is another important medical event that may jeopardise the participant. Pregnancy was also recorded as a SAE, so that any AEs could be identified if and when the child was born.
Included as AEs were an increase in frequency of hypoglycaemia, a blood glucose reading > 30 mmol/l, unexplained constantly raised blood glucose readings, suspicion of pump malfunction and pump site infection. Excluded as AEs were non-serious episodes of hypoglycaemia and ketonuria.
Details of AEs were collected during follow-up appointments. Participants were also provided with a contact card and encouraged to get in touch with their diabetes team if they had experienced any adverse health events. SAEs were reported in accordance with the Sheffield CTRU and REPOSE SAE SOPs. SAEs were assessed by the local PI and reported to Sheffield CTRU within 24 hours of becoming aware of the event, with the exception of events that had been stated as exempt from immediate reporting, for which 28 days was allowed. These exemptions were episodes of severe hypoglycaemia requiring hospitalisation, episodes of DKA and pregnancy. SAEs were assessed for seriousness, frequency, intensity, relationship to study product and, when applicable, relationship to pump. The Summary of Product Characteristics for NovoRapid and Levemir (Novo Nordisk, Gatwick, UK) were kept on file as the reference safety information for the assessment of events. AEs were reviewed at regular intervals by the three study oversight committees. The chief investigator and DMEC chairperson were notified of all SAEs on the event being reported.
Reporting of protocol non-compliances
Protocol non-compliances were reported and assessed in accordance with the Sheffield CTRU and REPOSE non-compliances SOPs. A non-compliance was defined as ‘a departure from the protocol or GCP that has been identified retrospectively’. Non-compliances were addressed with staff training or, when appropriate, an amendment to the protocol. In line with MHRA guidance, deliberate prospective protocol non-compliances or ‘waivers’ were deemed to be unacceptable. A prospective list of exemptions from reporting and of pre-specified major and minor non-compliances was drawn up by the CTRU, the chief investigator and the sponsor.
Trial monitoring
Responsibility for monitoring was delegated to the CTRU and conducted in accordance with CTRU SOPs. Both on-site and central monitoring methods were adopted. Onsite monitoring visits took place at all centres at study set-up, prior to delivery of the first DAFNE course, post delivery of DAFNE course 2 and at study closeout. A further monitoring visit took place during follow-up at seven centres. At each visit, the study site file and key essential logs were reviewed for completeness. Source data verification was conducted for 100% of consent and SAE forms. Patient hospital records were reviewed to substantiate participant existence and eligibility (for which criteria were verifiable from hospital records). Monitoring reports were issued after each visit detailing any remedial actions required. Central monitoring tasks included point of entry validation, verification of data and post-entry validation checks. One participant per DAFNE course per centre was randomly selected for verification. Case report forms at all data collection time points were reviewed for completeness and quality, and verified to monitor data entry. Source data verification also took place for 100% of central laboratory HbA1c results. Feedback on verification was provided and additional verification was undertaken when concerns were identified.
Statistical methods
All statistical analyses were performed in Stata 13 onwards. The MDI is the reference group for all treatment comparisons.
Analysis populations
The intention-to-treat (ITT) data set includes all participants who were randomised according to randomised treatment assignments (ignoring any occurrences post randomisation, such as protocol or treatment non-compliance and withdrawals) with at least one HbA1c assessment measure after baseline. Sensitivity analysis of the ITT primary outcome set was performed using six additional analysis sets, as described later in this section.
The per-protocol group is a subset of the ITT group who complied with the protocol. Protocol compliance was defined as adhering to both the DAFNE course and to pump/MDI. Compliance was reviewed and assessed on a case-by-case basis with the following general considerations applied:
- adherence to DAFNE course – in general, a participant was adherent to the course if they attended at least 4 of the 5 days, including the first 2 days (as adjudicated by the course leader)
- adherence to the pump or MDI – a participant was classed as adherent to treatment if he/she adhered to the pump/MDI for the full 2 years (excluding any reasonable temporary interruptions of around 2 weeks).
A review group (SH and JE), ‘blinded’ to patient outcome data, convened to decide any contentious cases for treatment interruptions with the help of the trial statistician (EL).
The complete-case group is a subset of the ITT group who had outcome measurements at a specific follow-up time.
An additional four analysis sets were performed to examine the sensitivity of primary results to multiple imputation and exclusions, as described later in this section.
Data completeness
A CONSORT (Consolidated Standards Of Reporting Trials) flow diagram was used to display data completeness and patient throughput from first contact to final follow-up.
Baseline characteristics
The baseline participant characteristics, diabetes history and laboratory tests were summarised and assessed for comparability between the intervention and control group. No statistical significance testing was carried out to test baseline imbalances between the arms, but any noted differences are reported descriptively.
Primary effectiveness analysis
The primary end point for this study is the change in HbA1c after 2 years in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol). The mean change in HbA1c at 24 months post DAFNE course was compared between participants allocated to pump and participants allocated to MDI using a mixed-effects model. The model was adjusted for clustering by DAFNE course (random effects), centre and baseline HbA1c as a continuous covariate (fixed effects).
The mean (SD) HbA1c change from baseline for the pump and MDI groups, and the number in each group, are displayed. The efficacy of the intervention is reported as mean difference (MD) in HbA1c change at 2 years, with its associated 95% CI and p-value, adjusted for the factors stated above.
Multiple imputation of missing data
Multiple imputation was used to impute missing data on the primary outcome in order to fulfil the ITT principle and for sensitivity analysis. Multiple imputation was used to impute 24-month HbA1c data for patients with at least one assessment after randomisation (i.e. at 6 or 12 months), but without 24-month primary outcome data. Participants’ baseline characteristics were summarised and compared between completers and non-completers. Data were imputed using chained equations (regression) with 50 imputations using baseline, 6- and 12-month HbA1c measurements, DAFNE course, centre, age, sex and HFS behaviour as covariates in the imputation equation. Initially, 10 imputation replicates were planned; however, this was increased to 50 in order to produce a stable and reliable estimate of variability.
The following sensitivity analyses were undertaken on the primary outcome and displayed alongside the ITT results:
- per-protocol cases (subset of ITT who did not deviate from the protocol)
- complete cases (subset of ITT including only participants with complete HbA1c data at 24 months)
- multiple imputation of all missing cases (including those without any follow-up data who are excluded from the ITT analysis)
- horizontal mean value imputation of all missing cases
- excluding participants who withdrew from the study because of pregnancy
- excluding participants with measurements outside a time window of 6 weeks before and after the 24-month follow-up.
A sensitivity analysis on the primary outcome – adjusted for duration of diabetes, number of moderate hypoglycaemic episodes and number of severe hypoglycaemic episodes – was to be performed if notable baseline imbalances were observed; however, none was observed.
An exploratory analysis (on available data) to assess whether or not there were differences in primary outcome between DAFNE lead course educators was conducted using a multilevel model with three levels – patients nested in DAFNE course, which, in turn, are nested within the course lead. Baseline HbA1c, treatment group and centre were treated as fixed effects in the model. The ICCs from this model are presented.
The effect of centre was explored using a mixed-effects regression model. The primary outcome was regressed against treatment, centre (fixed effects) and an interaction term between treatment and centre, and it was also adjusted for course (random effects). The p-value for the interaction between treatment and centre is presented. The MDs between treatment groups with associated 95% CIs, estimated from the mixed-effects model, are presented by centre with the aid of forest plots.
Key secondary effectiveness analysis
The key secondary end point is the proportion of patients reaching the NICE target of a HbA1c level of ≤ 7.5% (58 mmol/mol) at 2 years (including all participants regardless of baseline HbA1c value). The treatment effect was investigated using a mixed-effects logistic regression model adjusted for baseline HbA1c, centre (fixed effect), and a random effect around DAFNE course. The proportion of patients with HbA1c of ≤ 7.5% is presented by treatment group alongside the odds ratio (OR) of HbA1c ≤ 7.5% on pump compared with HbA1c ≤ 7.5% on MDI and its associated 95% CI and p-value.
Secondary effectiveness analysis
Glycated haemoglobin at 6 and 12 months
Secondary analyses on the primary outcome and key secondary outcome were repeated for HbA1c at 6 and 12 months to explore the short- and medium-term effects of the intervention:
- the change in HbA1c at 6 and 12 months in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol)
- the proportion of participants reaching the NICE target of HbA1c level of ≤ 7.5% (58 mmol/mol) at 6 and 12 months.
These outcomes were analysed using statistical models as for the primary and key secondary outcome.
Episodes of severe and moderate hypoglycaemia
The number of episodes of moderate hypoglycaemia reported in the 4-week period prior to the 6-, 12- and 24-month visits were compared between treatment groups using a mixed-effects negative binomial linear regression model, with centre and baseline continuous HbA1c included as fixed effects and course as a random effect. The occurrence of at least one moderate hypoglycaemic episode in the 4 weeks prior to starting the DAFNE course was also included as a covariate.
Each episode of moderate hypoglycaemia was classed as ‘confirmed’ or ‘unconfirmed’ by an educator and the blood glucose level was recorded by the participant. The following outcomes were analysed:
- all recorded episodes
- confirmed episodes, defined as episodes that were confirmed and for which the blood glucose level (if recorded) was < 3.5 mmol/l
- confirmed episodes (US definition), defined as episodes that were both confirmed and for which the blood glucose level (if recorded) was < 4 mmol/l.
Severe hypoglycaemic episodes were collected on an ongoing basis. The number of episodes recorded post baseline was analysed in a similar manner to moderate hypoglycaemic episodes, but with the addition of study follow-up time as the exposure. A sensitivity analysis was conducted in the same manner by excluding the first 6 months of data in order to explore any effect of a ‘settling in’ period on the pump.
The incidence rates of moderate hypoglycaemic episodes in the 4 weeks before each time point are displayed by treatment group, and the treatment effect is reported as an adjusted incidence rate ratio (IRR) with its associated 95% CI and p-value. The incidence rates of severe hypoglycaemic episodes over the study duration are displayed as episodes per patient-year and are reported alongside the IRR, its associated 95% CI and p-value.
The overall change in the rate of episodes of severe hypoglycaemia was estimated for the treatment groups combined using a mixed-effects negative binomial linear regression model. The numbers of episodes were compared pre and post baseline, using participant as the random effect, adjusted for treatment, time by treatment interaction, baseline HbA1c and centre. Length of follow-up was included as the exposure variable. Length of follow-up before baseline was set at 365 days, as participants recorded a 12-month history of severe hypoglycaemic episodes at baseline.
The proportions of participants who experienced at least one moderate hypoglycaemic episode at 6, 12 and 24 months were compared between treatment groups using a mixed-effects logistic regression model adjusted for DAFNE course (random effect), centre, presence of at least one episode before baseline and baseline HbA1c (fixed effects). The proportion of patients who experienced at least one episode of severe hypoglycaemia during the study period was compared between groups using a mixed-effects logistic regression adjusted for DAFNE course, centre and baseline HbA1c. Presence of at least one severe episode before baseline was not used as a covariate in the logistic regression model as all participants with at least one episode before baseline experienced at least one episode post baseline. The proportion of patients reporting hypoglycaemic episodes is presented by treatment group alongside the adjusted OR and its associated 95% CI and p-value.
Insulin dose, body weight and lipids
Insulin dose was calculated as:
In the calculation of insulin dose, weight was taken as the value on the same visit the dose was recorded. If weight was not recorded, it was estimated from other study visits as follows:
- If 24-month weight was missing, 12-month weight was used.
- If 12-month weight was missing, it was imputed as the time-weighted average of 6- and 24-month weight or as 6- or 24-month weight if only one observation was available.
- If 6-month weight was missing, it was imputed as the average of baseline and 12-month data, or imputed as baseline or 12-month data if only one observation was available.
- In all other situations the missing data were left blank.
The analysis of weight was based on available data only.
The mean change from baseline in insulin dose, weight, TC and HDL cholesterol was compared between treatment groups using a mixed-effects linear regression model with independent correlation adjusted for clustering by DAFNE course (random effect), centre and baseline HbA1c (fixed effects). The MD between the groups in change from baseline is displayed with its associated 95% CI and p-value.
Proteinuria
Proteinuria was defined from the ACR at each visit. At each visit a patient was defined as:
- macroalbuminuria – if ACR ≥ 30
- microalbuminuria – if 3 ≤ ACR < 30
- normal – if ACR < 3.
If ACR was missing at a time point, proteinuria status was imputed, based on data from recorded conditions at the same time point.
Proteinuria was analysed using mixed-effects ordered logistic regression adjusted for clustering by DAFNE course (random effect), centre and baseline HbA1c (fixed effects). The OR of being in a higher category (for which macroalbuminuria is the highest category) compared with a lower category is displayed with its associated 95% CI and p-value.
Blood glucose testing
The self-reported number of blood glucose tests performed in the 2 weeks prior to 24-month follow-up was compared between treatment groups, in a post hoc analysis, using a mixed-effects model adjusted for clustering by DAFNE course (random effect), centre and baseline number of blood glucose tests (fixed effects). For both treatment groups combined, the change in the number of tests performed at 24 months compared with baseline was analysed using a paired t-test. Blood glucose testing is presented as number of tests per day, taken as an average over the 2 weeks reported.
Psychosocial questionnaires
Methods for the analysis of questionnaire data are described later (see Methods for the psychosocial evaluation).
Subgroup analysis
Pre-planned subgroup analyses were undertaken and regarded as exploratory; significant results from the analysis were interpreted with caution, as recommended for subgroup analyses.95 The following subgroups were investigated:
- baseline HbA1c (< 7.5% or 58 mmol/mol, ≥ 7.5% to < 8.5% or 69 mmol/mol, ≥ 8.5%)
- duration of diabetes (< 15 years, ≥ 15 years)
- symptoms of hypoglycaemia (do not feel symptoms or < 3 mmol/l, ≥ 3 mmol/l)
- self-reported use of the bolus advisor over the study duration (never or rarely, sometimes, often or always)
- age (< 35, 35–49, ≥ 50 years)
- sex
- body mass index (BMI) (normal, < 25 kg/m2; overweight, 25–29.9 kg/m2; obese, ≥ 30 kg/m2)
- level of education [up to Advanced level (A-level) equivalent, vocational/beyond A-level]
- occupational status (Office for National Statistics levels 1–4)
- socioeconomic status as defined by the Office for National Statistics Index of Multiple Deprivation (above/below median in England, and above/below median in Scotland)
- insulin dose at start of therapy (< 0.7 or ≥ 0.7 IU/weight)
- frequency of moderate hypoglycaemic episodes within the 4 weeks prior to baseline (none, 1, 2 or 3, 4–9, 10+)
- experience of lead DAFNE course educator {‘less experienced’ [six courses or fewer within previous 3 years or completed the DAFNE Educator Programme (DEP) within previous year] vs. ‘higher-level experience [seven or more courses within previous 3 years or had continuous ‘educator’ status for > 6 years]}.
The subgroup analysis used mixed-effects linear regression modelling with the primary outcome, change in HbA1c (%), as the response. The model included main effects of the treatment group and subgroup, an interaction term between treatment and subgroup, and covariates of centre (fixed effect) and DAFNE course (random effect). Treatment effect estimates and 95% CIs are presented within each subgroup category. We used a statistical test for interaction between the randomised intervention group and the subgroup to examine the evidence for treatment effect varying between subgroup; the p-value for this interaction is reported unadjusted for multiple testing. Subgroup analyses were also summarised visually using forest plots.
Safety and harms analysis
Serious adverse events and AEs were summarised and assessed for similarity between the treatment groups. Both SAEs and AEs are reported on an ITT basis (i.e. according to the group to which the participants was randomised), but the number occurring following a treatment switch are highlighted.
Patient and public involvement
As part of our recent work funded by a NIHR programme grant (PGfAR),27 15 DAFNE graduates were recruited to act as a ‘user group’ and contribute to different aspects of the work. We invited two members to join both the steering group and other investigator meetings. In addition, one of the project team is a pump user. They provided input to the trial design, implementation and dissemination, including all participant materials.23 The work supported by the programme grant included qualitative studies in which the barriers to self-management in T1DM were explored. This work led to the development of a pilot study within the PGfAR work, in which a modified DAFNE course incorporating a pump curriculum was developed and piloted in three centres.
Methods of the fidelity assessment
Aim
To ensure that there was consistency in:
- the delivery of the 5-day DAFNE pump curriculum
- the timing and content of pump pre-assessment/setting up on pump session.
Multiple daily injection courses were not included in the FT, as there exists a rigorous quality assurance programme of MDI courses in standard care, and trial centres are routinely audited.
Methods
An experienced DAFNE educator and peer reviewer from Sheffield Teaching Hospitals NHS Foundation Trust was employed as the fidelity assessor (FA); this educator was not directly involved in the delivery of REPOSE courses. The FA assessed whether or not the pump courses delivered the correct DAFNE content and philosophy. The FA visited each centre to observe the ‘Wednesday’ of the pump DAFNE course. Wednesday was chosen as pump curriculum sessions that incorporated key differences to MDI would be delivered by DAFNE educators from both dietetic and nursing specialties. In addition, patients on the course should have settled into the course, be more relaxed and be starting to establish patterns and adjustments to their regimen by the third day. It was planned that the FT take place on the first or second pump course at each centre.
Experienced educators who devised the pump curriculum and the national director of the DAFNE programme discussed which sessions differed most between the pump and MDI DAFNE curricula and, thus, warranted observation. These sessions were decided as follows:
- daily goals, blood glucose results and insulin doses
- insulin dose adjustment theory, basal rate testing
- dose adjustment practice – reducing and increasing insulin
- setting up Bolus Wizard
- sick day rules
- alcohol
- exercise.
All but one session was scheduled for observation on the FT visit, as it was not possible to timetable all sessions that differed between the MDI and pump courses on 1 day. In lieu of observation, the FA reviewed the lesson plan for the sick day rules session.
The following data and documents were requested to be made available for the FT visit:
- pre-course pump session details including patient attendance, session timings and lesson plan
- pump course timetable
- list of course participants and details
- lesson plan for all observed sessions and the sick day rules lesson plan.
A DEP peer support learning outcomes form was completed for each session observed. This form listed the essential learning outcomes for each session and the FA evaluated whether or not these were met (or partially met). For each learning outcome, the FA provided evidence for its achievement. A template report was devised and used to collate the data collected from the FT visit.
Once the FA had completed the assessment, feedback was given immediately so that educators could resolve any problems. The report was completed within 3 days of the visit and sent to the trial management office.
Methods for the economic evaluation
Setting and perspective
The health economic analyses are designed to inform UK decision-makers within the UK NHS on the potential resource implications of choosing to use pump therapy with DAFNE structured education (pump + DAFNE) or MDI with DAFNE structured education (MDI + DAFNE) for the group of adults with T1DM in the REPOSE Trial, comprising adults with T1DM who are naive to pump therapy.
To ensure that all economic analyses were applicable to the UK decision-making setting, all economic analyses took the NHS and Personal Social Services perspective in line with (NICE) guidance.22
Two approaches: economic evaluation alongside the clinical trial and long-term cost-effectiveness modelling
The cost-effectiveness of ‘pump + DAFNE’ compared with ‘MDI + DAFNE’ was assessed using an Economic Evaluation Alongside Clinical Trials (EEACT) and long-term modelling exercise. The EEACT took a 2-year time horizon and the long-term modelling took a lifetime horizon. As the long-term modelling takes a lifetime time horizon, and includes all clinically important complications of diabetes, this should be considered as the primary analysis.
Price year and discounting
All costs are reported in 2013–14 prices; if costs were obtained from a previous financial year they were inflated to 2013–14 prices using the Hospital and Community Health Services Pay and Prices index.96 All costs and QALYs were discounted at a rate of 3.5% in line with NICE guidance.22 All costs and QALYs were assumed to fall at the end of the year, apart from the cost of the structured education courses, which were assumed to occur at the start of the first year.
Population and subgroups for analysis
The individuals in the REPOSE Trial were adults with T1DM who were eligible to receive a structured education course. Furthermore, all individuals were naive to insulin pump therapy and did not have a preference to receive the pump. The average age of participants was 40.4 years and their mean duration of their diabetes was 18.0 years. Data were collected from individuals at baseline and at 6 months, 1 and 2 years post randomisation. In the MDI + DAFNE arm, 6, 3 and 5 individuals out of 135 were lost to follow-up at 6 months, 1 and 2 years, respectively. A further individual in the MDI + DAFNE arm withdrew from the trial at 6 months. In the pump + DAFNE arm, 0, 1 and 2 individuals out of 132 were lost to follow-up at 6 months, 1 and 2 years, respectively. A further individual in the pump + DAFNE arm withdrew from the trial at 1 year.
The data collected in the REPOSE Trial were considered to be the only relevant evidence on the relative effectiveness of pump + DAFNE compared with MDI + DAFNE. This is because REPOSE is the only large study in a UK setting in which adults with T1DM in both trial arms have received equivalent diabetes education in both the pump and MDI trial arms.
There are two populations in the REPOSE Trial: (1) the ITT population and (2) the per-protocol population. The ITT population includes all individuals who graduated their DAFNE course and had follow-up data for at least one data collection period. In the ITT population, individuals were assigned to their randomised treatment irrespective of whether or not they switched to the other insulin delivery mechanism. The per-protocol population includes all of the individuals who were in the ITT population and adhered to their insulin delivery mechanism (either pump or MDI). Unless otherwise stated, all analyses of the REPOSE Trial data to inform the health economic analyses were conducted in the ITT population.
The population analysed in the primary health economic analyses is all individuals in the REPOSE Trial, regardless of whether or not the individual’s baseline HbA1c was ≥ 58 mmol/mol (7.5%). The analysis population differs from the population used in the primary clinical end point statistical analysis, as the base-case health economic analysis focuses on the whole trial population rather than those individuals with a HbA1c of < 58 mmol/mol (7.5%). For the health economic analyses, it is important to assess the cost-effectiveness of pump + DAFNE compared with MDI + DAFNE for all adults with T1DM who would be potentially eligible to receive either treatment if they were adopted as standard practice.
Subgroup analyses 1–6 were conducted in the long-term modelling only, because of concerns about the reduction in sample size potentially producing spurious results in the EEACT. However, subgroup analysis 7 was conducted in the EEACT, as this was an important subgroup analysis for the estimation of treatment effect of HbA1c (see Statistical methods). The subgroup analyses were conducted in following subgroups of the REPOSE Trial participants:
- baseline HbA1c ≥ 58 mmol/mol (7.5%)
- baseline HbA1c ≥ 58 mmol/mol (7.5%) and < 69 mmol/mol (8.5%)
- baseline HbA1c ≥ 69 mmol/mol (8.5%) and < 80 mmol/mol (9.5%)
- baseline HbA1c ≥ 80 mmol/mol (9.5%)
- baseline HbA1c < 69 mmol/mol (8.5%)
- baseline HbA1c ≥ 69 mmol/mol (8.5%)
- all individuals in the per-protocol population.
Cost of the Dose Adjustment For Normal Eating course
A detailed within-trial costing of the DAFNE courses was not undertaken because DAFNE is an already established intervention within the NHS. The cost of DAFNE training for adults with T1DM using MDI has been calculated by DAFNE UK as being £359.10 per course attendee in 2012–13 prices (£363.10 in 2013–14 prices).97
Based on discussion with experts involved in the REPOSE Trial, including a Professor of Clinical Diabetes and Honorary Consultant Physician, and a Professor in Public Health and Health Technology Assessment, it was assumed that the cost of a DAFNE course in the pump + DAFNE arm is identical to the cost of a DAFNE course in the MDI + DAFNE arm, except for the cost of staff time spent conducting an additional pre-course pump-fitting session.
Data were collected on the time spent delivering a pre-course fitting session for pump + DAFNE participants in the FT process. The FT process was conducted for one pump + DAFNE course at each trial centre to ensure that the pump + DAFNE course taught the principles of insulin adjustment in a similar fashion to the MDI + DAFNE course. These data were utilised to estimate the additional cost of the pre-course pump fitting session in the pump + DAFNE arm. Expert advice was sought from two centres in which it was unclear whether reported time use as part of the FT referred to the educator time spent or the total time individuals spent at the venue (which could include non-contact waiting time). To ensure consistency between the estimated costs of a MDI + DAFNE course and a pump + DAFNE course, the cost of staff time was obtained from the estimated cost of staff time for the MDI + DAFNE course. The cost of the pre-course fitting session was estimated to be £28.82 per adult with T1DM.
Economic analysis alongside the clinical trial of pump + Dose Adjustment For Normal Eating versus multiple daily injection + Dose Adjustment For Normal Eating
Resource use by individuals in the REPOSE Trial over the 2-year follow-up period
Resource use was collected either on an ongoing basis or was self-reported by the individuals in the trial at baseline or at a follow-up period (6 months, 1 and 2 years post randomisation). All unit costs used to value the reported resource use in the EEACT, apart from the costs associated with insulin use, are presented later (see Table 3). The unit costs associated with insulin use are reported separately later (see Table 4).
Diabetes-related contacts were collected using two methods in the REPOSE Trial. Patient’s self-reported number and type (either face to face or not face to face) of diabetes-related contacts since the last REPOSE visit (or in the year prior to baseline) were collected in each of the REPOSE DCFs (baseline, 6, 12 and 24 months). Ongoing information was collected from the sites on the number of visits, the type of visit and the time spent at each visit. The self-reported contacts were used in the health economic analysis for two reasons: (1) this method was consistent with the method used to collect information on the baseline number of contacts; and (2) national-level commissioning information provides only a cost per outpatient appointment (rather than for a specified time for a specific health-care professional to conduct an appointment), so from a costing perspective it is the number of contacts that is important rather than the time spent at each contact.
Table 2 shows that numbers of diabetes-related contacts were higher in the CSII + DAFNE arm of the REPOSE Trial than the MDI + DAFNE arm in the first year of the trial. However, most of these differences disappear in the second year of the trial. It should also be noted that the average time spent delivering diabetes-related contacts is higher for pump + DAFNE individuals than MDI + DAFNE individuals, except for telephone contacts delivered between 12 and 24 months post randomisation. This indicates that there are important differences in the number and time spent at diabetes-related contacts for MDI and pump users in the NHS.
TABLE 2
Differences in the number of diabetes-related contacts in the MDI + DAFNE and pump + DAFNE arms of the REPOSE Trial
The unit costs used to estimate the total cost diabetes-related contacts are presented in Table 3.
TABLE 3
Unit costs used in the within-trial analysis of the REPOSE data
The individual’s self-reported resource use was collected on whether they were using lipid-lowering, antiplatelet or depression medication at the time of each REPOSE visit. No information was collected on the type of drug or the quantity used. It was assumed that, if an individual reported use of medication received medication, they had been receiving that specific medication since the last REPOSE visit. The average quarterly cost of each type of medication is reported in Table 3.
Data were collected on an ongoing basis for all inpatient hospitalisations that were not scheduled to treat a pre-existing condition. Therefore, the only missing data were for individuals who were lost to follow-up or withdrew from the trial. At each admission, information was collected on the cause. The possible causes for each admission were DKA, myocardial infarction (MI), severe hypoglycaemia, ischaemic heart disease, unstable angina, heart failure (HF), foot ulcer and renal disease. If the admission was not due to one of these causes, the reason was recorded. This occurred for only one inpatient admission in the REPOSE Trial. The NHS Reference Costs 2013–1498 (and all previous years used to inform the unit costs) present the cost of non-elective inpatient stays as short stays, excess bed-days and long stays. The cost of inpatient stays were estimated as the cost of a short stay if the length of stay was ≤ 1 day. If the length of stay was ≥ 2 days then the cost of the visit was estimated using the following formula:
At baseline, self-reported data were collected on the number of diabetes-related admissions in the past year, days spent in hospital and the reason for admission. The possible causes included DKA, hypoglycaemia, MI, ischaemic heart disease, unstable angina, HF, foot ulcer and renal disease. It was possible that individuals had missing information on the number of days that they were in hospital or the reason for the admission. Mean value imputation was used to impute the number of missing days. All of the admissions for which the reason was missing were treated as an ‘other cause inpatient stay’.
Data were also collected on an ongoing basis for individual’s severe hypoglycaemic events. Severe hypoglycaemia was defined in the REPOSE Trial as been any hypoglycaemic episode that an individual was unable to treat themselves. Information was collected on whether each severe hypoglycaemic event required either a paramedic call-out and/or an inpatient admission. If it was reported that an individual did not have an inpatient admission or a paramedic call-out then it was assumed that a friend or family member provided aid to the individual, which meant that no admission or paramedic call-out was required. This had no implications for NHS resource use, so these episodes of severe hypoglycaemia were assumed to have no monetary cost to the NHS in the EEACT. The unit costs for a paramedic call-out or an inpatient admission for severe hypoglycaemia are presented in Table 3.
Information was collected for all individuals in the REPOSE Trial (at baseline, 6, 12 and 24 months post randomisation) on their current insulin regimen (including type of insulin used), the typical daily insulin dose in the week preceding data collection, the number of injections per day, the type of insulin used by the individual and the method of insulin delivery. As information on insulin type was available, the cost of insulin and insulin pens was estimated separately for each insulin type.
The unit costs associated with insulin use are presented separately from the rest of the unit costs in Table 4. The daily cost of insulin was multiplied by the number of days between each data collection period (6 months, 1 and 2 years) to calculate the cost of insulin in the first and second year. If an individual was receiving insulin pump therapy then the cost of needles, insulin pens and syringes were not applied, as these were already included in the estimates of the cost of insulin pump consumables. From this information a cost of insulin for each individual in the REPOSE Trial was calculated.
TABLE 4
Unit costs of insulin
Cost of the insulin pumps and consumables
The annual cost of an insulin pump and insulin pump consumables was estimated using a survey, which was conducted in all of the trial centres. This survey obtained information on the unit costs and the quantities of insulin pumps and insulin pump consumables purchased by centres in routine clinical practice. Information was also collected on the insulin pump consumables used by participants in the REPOSE Trial. Data were collected over a 6-month period for the insulin pump consumables and a 12-month period for the insulin pumps. The Scottish centres purchased insulin pumps and insulin pump consumables through the Scottish Government. Instead of completing the survey, information was obtained on the average price that the Scottish Government paid for insulin pumps and consumables.
One centre did not report any price information and two centres did not report the quantities of insulin pump consumables used by REPOSE participants. This missingness was addressed by using the mean price and mean resource use at the other trial centres to calculate the cost of insulin pumps and consumables.
For some centres, data collection on individuals’ use of consumables was for a period that was somewhat shorter than 6 months and we estimated their consumables use for 12 months assuming a pro rata uplift. The cost of insulin pumps and insulin pump consumables during the trial duration was estimated by multiplying the annual cost of insulin pumps and consumables by the fraction of each year that each individual spent on insulin pump therapy.
The annual cost of an insulin pump was calculated assuming a pump lifetime of 4.5 years, based on the clinical expert opinion of a diabetes specialist nurse. The annualised cost was multiplied by the number of days that an individual spent on an insulin pump to give the total cost of insulin pump therapy in the trial period.
The effect of a price reduction of insulin pumps and insulin pump consumables of 25% and 50% from the pump costing survey prices was tested in scenario analyses. A further scenario analysis was conducted by using a cost of £2002 per annum for a Medtronic pump and consumables reported in Riemsma et al.8 Riemsma et al.8 conducted an appraisal of integrated sensor-augmented pump therapy systems for managing blood glucose levels compared with stand-alone insulin pumps with a separate CGM system in the UK for NICE’s diagnostics advisory committee. One of the comparators in this appraisal was stand-alone insulin pumps with an additional continuous blood glucose monitoring system. As such, this cost for a Medtronic insulin pump was obtained from the estimated cost of a stand-alone insulin pump estimated in this study. The costs were obtained from the stated prices of an insulin pump from the London New Drugs Group in November 2014.103
Treatment switching
During the REPOSE Trial, it was possible for individuals to switch from insulin delivery mechanism to the other, that is to switch from insulin pump therapy to MDI and vice versa. It is important to include treatment switching in a health economic analysis, as it is unreasonable to assume that (1) people who switch treatment will use the same resources over a lifetime as someone who does not use a pump and (2) someone still receiving an insulin pump has the same benefit from treatment as someone who has switched to using MDI. As a consequence of including treatment switching in the long-term model, the mean cost and QALY gain per patient in the pump + DAFNE arm is more likely to represent the true lifetime costs and QALYs than an analysis that ignored treatment switching.
It was possible to switch treatment twice, and two individuals did so in the REPOSE Trial. The data in the REPOSE Trial were analysed to assess the number of people with diabetes who switched treatment. The estimated cost of insulin and insulin pumps was adjusted to reflect the fact that individuals switch treatments. As the EEACT uses a microcosting approach to estimate costs and obtains QALY data from the self-reported EQ-5D data to calculate QALYs, all other cost and QALY effects due to switching are included in the analysis.
The cost of insulin was adjusted for treatment switching by using the data on an individual’s insulin use. If an individual switched treatment once, insulin use between the last follow-up period and the treatment switching date was estimated using the reported insulin use at his/her last follow-up period (individuals were followed up at 6 months, 1 and 2 years post randomisation). Similarly, insulin use between his/her treatment switching date and the next follow-up period was estimated using the data observed in the next follow-up period. For example, if an individual switched treatment 11 months post randomisation, his/her insulin use reported at 6-month follow-up would be used to estimate the cost of insulin between 6 and 11 months, and insulin use at 1-year follow-up would be used to estimate insulin use between 11 months and 1 year. If an individual switched between the baseline and the 6-month follow-up period then his/her data were treated as missing, as no information was available on insulin use when using his/her randomised treatment allocation, after receiving DAFNE education. Furthermore, if an individual switched treatment twice (n = 2) then the individual was excluded from the EEACT analysis population. This was because individuals both switched and switched back to their original treatment within the time period between two consecutive follow-up periods. Therefore, no information was available on their resource use when they received the other treatment.
The cost of an individual’s use of insulin pumps and consumables in each year was calculated by multiplying the fraction of the year for which they used insulin pumps by the associated yearly cost of insulin pumps and insulin pump consumables.
Estimating the within-trial cost effects
The total cost for each individual consisted of the cost of the following components: inpatient admissions; paramedic call-outs for severe hypoglycaemia; the cost of a pump fitting session for individuals who received pump + DAFNE; the cost of pump-fitting session for individuals who switched from MDI to insulin pump therapy and insulin; annual cost of an insulin pump; annual cost of insulin pump consumables; and the cost of DAFNE course.
In the base-case analysis, complete cost information was used in the EEACT. Complete total cost information was available for 98%, 90% and 92% of individuals in the ITT population at baseline, 1 and 2 years, respectively.
In a scenario analysis, missing cost data were imputed for those individuals who attended at least one REPOSE Trial follow-up visit. Total discounted cost data were imputed using chained equations (predictive mean matching), utilising baseline HbA1c, treatment allocation, age at baseline and baseline cost values as covariates in the imputation equations. Ten different imputed values were calculated for each individual with missing data.
Estimating within-trial quality-adjusted life-year effects using the EuroQol-5 Dimensions and the Short Form questionnaire-12 items
To generate QALY measures over the 2-year trial follow-up, information was collected on an individual’s utility using two different instruments: the EQ-5D and the SF-12. The EQ-5D and SF-12 questionnaires were completed by individuals at baseline and all follow-up visits (6, 12 and 24 months).
In the base-case within-trial analysis, the utility values measured by the EuroQol-5 Dimensions, 3-level version (EQ-5D-3L) were used to calculate QALYs using an area-under-the-curve analysis. EQ-5D utility scores were used in the base case because they are NICE’s preferred utility measure.1 In a scenario analysis, utility values measured using the Short Form questionnaire-6 Dimensions (SF-6D) (a measure derived from the SF-12) were used to calculate QALY values.104
In the base case, only individuals with complete QALY data were included in the analysis. Utilities, as measured by the EQ-5D-3L, were completed by 99%, 93%, 88% and 90% of individuals at baseline, 6, 12 and 24 months, respectively. If an individual had a missing 6-month utility value, then it was assumed that the 6-month utility value would be the average of the baseline and 1-year utility values. If an individual had a missing utility score at 12 or 24 months, then they were excluded from the base-case analysis. The 6-month utility values of individuals with missing utility data at 12 or 24 months were similar in both model arms. The individuals in the pump + DAFNE arm, who did not have 1- or 2-year EQ-5D-3L data, had a mean EQ-5D-3L utility score of 0.8177 [standard error (SE) 0.0602] at 6-month follow-up. The individuals in the pump + DAFNE arm, who did not have 1- or 2-year EQ-5D-3L data, had a mean EQ-5D-3L utility score of 0.904 (SE 0.0256) at 6-month follow-up. The hypothesis that the difference between these two distributions was equal to zero could not be rejected using a two-sided t-test with equal variances at the 10% significance level. Therefore, there is no indication that excluding these individuals from the base case would bias the results.
In a scenario analysis, multiple imputation was used to impute missing QALY values for individuals with assessment data for least one follow-up point. Data were imputed using chained equations (predictive mean matching), utilising baseline HbA1c, treatment allocation, age at baseline and baseline cost or QALY values as covariates in the imputation equations. Ten imputed values were calculated for each individual, with missing data in the analyses using imputed data.
Statistical model used for the within-trial analysis
A seemingly unrelated regression model was used to estimate the costs and QALYs in the EEACT. A seemingly unrelated regression is a type of statistical model that allows for multiple outcome variables to be modelled simultaneously.105 This approach is advantageous, as any covariances between covariates across the different outcome variables are estimated. One seemingly unrelated regression was fitted to four outcome variables: (1) total discounted costs in year 1, (2) total discounted costs in year 2, (3) total discounted QALYs in year 1 and (4) total discounted QALYs in year 2, using the ‘mysureg’ command in the ‘ml_ado’ package in Stata version 13.1. For the QALY outcome variables, baseline HbA1c, treatment allocation and baseline utility were included as covariates, and clustering was controlled for in each DAFNE course. Baseline utility was included as a covariate to estimate QALYs so that any baseline differences in health between the two treatment arms was controlled for.106 For the cost outcome variables, baseline HbA1c, centre, treatment allocation and baseline resource use were included as covariates, and clustering was controlled for in each DAFNE course.
A scenario analysis was conducted in which both missing cost and QALY data were imputed for individuals with at least one assessment during the REPOSE Trial follow-up period. A regression was conducted in each imputed data set and combined using Rubin’s rules.107 Details of the imputation procedures used in this scenario analysis are given in Estimating the within-trial cost effects and Estimating within-trial quality-adjusted life-year effects using the EuroQol-5 Dimensions and the Short Form questionnaire-12 items.
The impact of treatment allocation on total costs was calculated by adding the treatment allocation parameters relating to the cost outcomes in years 1 and 2. Likewise, the impact of treatment allocation on total QALYs was calculated by adding the treatment allocation parameters relating to the QALY outcomes in years 1 and 2. CIs around the effect of treatment allocation on total costs and total QALYs were calculated using the formula for calculating the variance of a variable that is a sum of correlated variables. The variances and covariance used in this calculation were obtained from the variance–covariance matrix of the seemingly unrelated regression.
Analysis
The key measure of cost-effectiveness in the EEACT was the ICER base on the mean incremental effect of pump + DAFNE compared with MDI + DAFNE on total costs and total QALYs. The CIs around these effects were estimated from the variance–covariance matrix of the regression model. The results were presented on a cost-effectiveness plane and the uncertainty around the mean effect was presented using a confidence ellipse.
Long-term cost-effectiveness
The Sheffield Type 1 Diabetes Policy Model Overview
The Sheffield Type 1 Diabetes Policy Model (henceforth, the Model) was used to estimate the lifetime costs and QALYs for individuals receiving MDI + DAFNE and pump + DAFNE. The Model has been developed and used over several years, and a detailed description is provided in a journal article108 and a detailed report to the NIHR on the DAFNE programme grant research.27 In this analysis, we have updated some aspects of the evidence used within the Model. We term the version used here as ‘The Sheffield Type 1 Diabetes Policy Model version 1.3’.
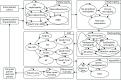
The Model is an individual-level simulation model, which consists of a series of submodels that simulate the progression of diabetic complications (microvascular and macrovascular), SAEs (severe hypoglycaemia and DKA) and mortality in a given population with T1DM. Each of the modelled microvascular (nephropathy, neuropathy, retinopathy and macular oedema) and macrovascular complications (MI, stroke, HF and angina) are included in the model as separate Markov submodels with an annual time cycle. Short-term AEs (severe hypoglycaemia and DKA) are modelled as the annual incidence of these complications, dependent on each patient’s characteristics. The Model structure is also presented in Figure 1. The Model attaches utilities and ongoing costs to health states and one-off costs to events (the move to another health state in a submodel). These costs and utilities are combined with the length of time that a patient spends in a health state to estimate lifetime costs and QALYs. The Model estimates patient’s disease progression over their lifetime.
The disease progression parameters in the Model were not updated in these analyses. However, the costs and utilities associated with health states and events were updated. A full probabilistic sensitivity analysis (PSA) was conducted with 500 probabilistic runs, each with 5000 individuals in each Model arm. All Model runs were conducted using the SIMUL8 2010 professional (Simul8 Corporation, Boston, MA, USA) programme.
Microvascular events and disease progression
For each microvascular complication (retinopathy, neuropathy and nephropathy), individuals progress to the more severe health states within each annual time cycle according to the probabilities reported in table 29 of Heller et al.27 The health states included for retinopathy include no retinopathy, background retinopathy, proliferative retinopathy and blindness. The health states included for neuropathy include no neuropathy, clinical neuropathy, clinically confirmed neuropathy, diabetic foot syndrome and peripheral arterial disease (PAD) with amputation. The health states included for nephropathy include no nephropathy, microalbuminuria, macroalbuminuria, end-stage renal disease (ESRD) and death from ESRD.
Macrovascular events and disease progression
The risks of fatal and non-fatal macrovascular complications (MI, stroke, HF and angina) are modelled in three stages. First, the annual probability of experiencing any cardiovascular event is estimated based on individual characteristics, as per the 5-year cardiovascular risk model of Cederholm et al.109 Second, if the individual is deemed to experience a cardiovascular event, the type of event (MI, stroke, HF or angina) is determined using methods outlined in Palmer,110 based on data from the Diabetes Control and Complications Trial (DCCT) Epidemiology of Diabetes Interventions and Complications study.111 Third, is the issue of fatality. If the event experienced is a MI, stroke or HF, it is determined whether or not the event is fatal using methods outlined in Palmer110 and as shown in table 31 of Heller et al.27
Individuals can also die from other causes; this other-cause mortality is modelled based on UK life tables from 2012–14, adjusted to exclude the causes either attributed to diabetes mellitus (either type 1, type 2 or unspecified) or modelled directly in the microvascular and macrovascular disease components (deaths due to ESRD, MI, stroke and HF).
Utilities: health-related quality of life for health states in the long-term model
Heller et al.27 (pp. 108–9) detail the utility analyses undertaken to inform version 1.2 of the model. Since that report, further analysis has taken place in the course of peer-reviewed journal publication, and the utilities presented in this analysis are based primarily on those revised analyses, which are now published in Peasgood et al.112 The main change in this analysis is that the preferred statistical model to estimate utility values in the publication is a random-effects model rather than a Tobit model. Riemsma et al.8 conducted the independent economic analysis for NICE on the cost-effectiveness of integrate CGM and insulin pump therapy. For the independent analysis, a systematic review of utilities in type 2 diabetes mellitus published in 2014 by Beaudet et al.113 was used for many of the health states of their economic model. The utilities presented in version 1.2 of the model were considered for updating by the new information presented in Peasgood et al.112 and Beaudet et al.113
The following criteria were applied to decide if a utility value should be updated. Utility values estimated in a population with T1DM were preferred to values estimated in a population with type 2 diabetes mellitus. If multiple values were available in a T1DM population, utility values that were estimated using the EQ-5D were preferred to other utility values. If a paper presented more than one parameter value, the parameter from the best-fitting model was the preferred source. If two papers analysed the same data source then the most recent paper was the preferred source. The utility parameters used in the Model version 1.3 base-case analyses, and the distributions used in the PSA, are given in Table 5.
TABLE 5
Base-case utility parameters
Unit costs for health states in the long-term model
The base case unit costs, which are presented in Heller et al.,27 were inflated to 2013–14 prices using the Hospital and Community Health Services Index.2 The base-case health-state costs used, and the distributions used in the PSA, are given in Table 6.
TABLE 6
Base-case health state and transition costs
Pre-specified subgroup analyses
A series of subgroup analyses were conducted in the long-term modelling. The same subgroup analyses were not conducted in the within-trial analysis, as conducting analyses in these subgroups would lead to a reduced sample size and increase the chance that a spurious result would be found. The cost-effectiveness of pump + DAFNE against MDI + DAFNE was compared for subgroups:
- baseline HbA1c ≥ 7.5% (58 mmol/mol)
- baseline HbA1c ≥ 7.5% (58 mmol/mol) and < 8.5% (69 mmol/mol)
- baseline HbA1c ≥ 8.5% (69 mmol/mol) and < 9.5% (80 mmol/mol)
- baseline HbA1c ≥ 9.5% (80 mmol/mol)
- baseline HbA1c < 8.5% (69 mmol/mol)
- baseline HbA1c ≥ 8.5% (69 mmol/mol)
- all individuals in the per-protocol population.
For people with a baseline HbA1c of < 7.5% (58 mmol/mol), there were insufficient numbers (n = 12 people in the MDI + DAFNE arm, n = 13 in the pump + DAFNE arm) to conduct a subgroup analysis.
The subgroup analyses were conducted by changing only the individual characteristics that were inputted into the model. All of the other parameters and assumptions in the model were identical to those in the base case.
Modelled cohort of 5000 simulated individuals
Individual characteristics were drawn from the baseline characteristics of all individuals, irrespective of treatment arm, in the ITT population. The variables included in the baseline individual characteristics are HbA1c, age, diabetes duration, triglycerides, TC, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, systolic blood pressure, baseline cost of insulin, baseline cost of diabetes-related contacts, sex, physical activity (measured as being either low, medium or high, based on the time spent walking, fast walking or running per week), smoking status, ethnicity, history of nephropathy, history of neuropathy, history of retinopathy, history of MI, history of stroke, history of HF and history of angina.
The observed characteristics (including missing values) of the REPOSE Trial individuals were sampled with replacement to generate a cohort of 5000 individuals to be used in the economic model. After the cohort of 5000 individuals was obtained, missing data values were observed for TC, HDL cholesterol, LDL cholesterol, systolic blood pressure and sex. To obtain the missing data, these values were imputed. The data were imputed in the cohort of 5000 individuals, rather than for the individuals in the trial data set, as this allowed the missing data to vary across different replications of an individual with missing data. If the data were imputed before the sampling, then the missing data would take on a fixed value in the cohort of 5000 individuals rather than being uncertain.
The imputation procedure depended on whether the missing value was a continuous or a categorical variable. TC, HDL cholesterol, LDL cholesterol and systolic blood pressure were imputed using chained equations, utilising the truncated regression procedure. Sex was imputed separately from TC, HDL cholesterol and systolic blood pressure, using the Poisson procedure. In both sets of imputation models, all of the complete individual characteristics were included as predictive covariates. LDL cholesterol was calculated using the imputed data for TC and HDL cholesterol, using the following formula:
All of the imputations were performed using single imputation. The reason for using one imputed value was that as more imputations were performed, the average value of these imputations would converge for the different replications of an individual from the trial population. Therefore, the uncertainty in the values of the missing data would not be fully reflected in the model cohort.
Summaries of the baseline characteristics of the 5000 simulated individuals for the base-case cohort and the 260 individuals sampled from the REPOSE Trial are given in Table 7. The summary of baseline characteristics for the 5000 simulated individuals for each of the pre-specified subgroup analyses is provided in Appendix 7.
TABLE 7
The baseline characteristics of REPOSE participants and the simulated cohort
Incorporating estimated clinical effectiveness from the REPOSE Trial: glycated haemoglobin
The probability of switching treatment, changes in HbA1c, the probability of a severe hypoglycaemic event and the probability of the DKA were based on data from the REPOSE Trial. These four clinical effects have been included in the health economic model, as they all would impact on the costs of treatment and QALYs gained by people if either option were to be adopted in routine clinical practice. HbA1c has been included as it is the key driver of all modelled diabetic complications in the Model. Changes in HbA1c were estimated using a beta regression. The probability of severe hypoglycaemia and DKA have been included, so that any benefits of either arm in reducing the incidence of these events is included in the economic model. The probability of severe hypoglycaemia and DKA were estimated using negative binomial models. Treatment switching has been included, as it is expected that when an individual switched treatment from pump to MDI or from MDI to pump that the cost of managing their diabetes and their clinical outcomes would change. The probability of switching treatment was estimated using parametric survival curves, using treatment switching as the event of interest.
Incorporating treatment switching
During REPOSE, individuals in both trial arms could switch their insulin delivery mechanism; because of effects on both costs and clinical outcomes, it was important to incorporate treatment switching into the model. A total of 17 of 132 (12.88%) individuals, initially randomised to the pump, switched once to MDI to deliver their insulin. A further two individuals, initially randomised to the pump, switched from pump to MDI and then switched again from MDI back to pump. A total of 8 of 128 (6.25%) individuals, initially randomised to MDI, switched to pump.
Kaplan–Meier curves were fitted to individual-level data using treatment switching as the event. Parametric survival curves were fitted to the data with HbA1c prior to switching, number of DKAs and number of severe hypoglycaemic events in the year prior to switching (or 2 years’ follow-up if no switching occurred) included as covariates. The SEs of the parametric models were adjusted for clustering within each course. Separate models were fitted to individuals initially randomised to insulin pump therapy and MDI, so no assumption of proportion hazards or accelerated failure time was made. Exponential, Weibull, Gompertz, log-logistic, log-normal and generalised gamma distributions for the parametric curves were considered. The goodness of fit of the different curves was assessed using visual assessment of the Kaplan–Meier plots and the Akaike information criterion (AIC) and the Bayesian information criterion (BIC).
Based on expert clinical opinion of a Professor of Clinical Diabetes and Honorary Consultant Physician, and a Professor in Public Health and Health Technology Assessment, it was assumed, in the base case, that if an individual was on an insulin pump after 2 years then they would remain on the pump; this assumption was made, as, in their experience, once an adult with T1DM was successfully using an insulin pump then they were unlikely to change the method of insulin delivery.
In the model, treatment switching impacted on HbA1c and the cost of pumps, diabetes-related contacts and insulin. Details on how the HbA1c of patients who switched are given later (see Estimation of each individual’s glycated haemoglobin); details on how the costs of treatment were updated for people who switched are also given later (see Cost of insulin, diabetes-related contacts and insulin pumps). No explicit inclusion of treatment switching on the risk of DKA and severe hypoglycaemia was included in the model because of the relatively small numbers of these events in each trial arm (see Table 7). However, the risk of DKA and severe hypoglycaemia does depend on the HbA1c of the individual in the model; therefore, there are differences in the risk of DKA and severe hypoglycaemia between those who switched treatment and those who did not.
Estimation of each individual’s glycated haemoglobin
To develop the method to incorporate HbA1c treatment effect evidence into the model, several factors were considered. Data were collected on each individual’s HbA1c at each follow-up visit. As HbA1c is the key predictor of clinical events in the model, it is important that the distribution of HbA1c is reflective of what was observed in the REPOSE Trial. Because 5000 replicated individuals are included in the model from the n = 260 sample, we are able to incorporate heterogeneity of individual outcomes into the cost-effectiveness analysis using statistical modelling of the REPOSE data set. A clinical expert (Senior Clinical Lecturer/Honorary Consultant) commented that few adults with T1DM were able to sustain a HbA1c level of < 31 mmol/mol (5%) for a full year, and that, in the expert’s experience, no adult with T1DM had a HbA1c of > 200 mmol/mol (20.5%). A final consideration was that the lowest HbA1c observed in the DAFNE research database was 30 mmol/mol (4.9%).27
The effect of pump + DAFNE treatment compared with MDI + DAFNE treatment on HbA1c was estimated using a beta regression.123 Beta regressions estimate outcome parameters, which are bound by, but do include, a range of 0–1. HbA1c from the trial data was transformed so that a HbA1c level of 29 mmol/mol (4.8%) was equal to zero. The upper limit of HbA1c was taken to be 201 mmol/mol (20.5%). A beta regression estimates two parameters of interest for simulating each individual’s HbA1c response to pump + DAFNE in the model, the mean effect (µ) and a dispersion parameter (φ). The expectation and the variance of each individual’s outcome, yi, are estimated using the following formulae:
To estimate the mean effect on 1-year HbA1c, treatment allocation, baseline HbA1c and centre were included as covariates. To estimate the dispersion parameter in the 1-year HbA1c regression, only baseline HbA1c was included as a covariate. All of the parameters that were included in the mean effect regression were tested as covariates, but were not statistically significant at the 5% level.
To estimate the mean effect on 2-year HbA1c, all of the covariates used to estimate the mean effect on 1-year HbA1c were used, and 1-year HbA1c was included as an additional covariate. To estimate the dispersion parameter, HbA1c at 1 year was used. All of the parameters that were included in the mean effect regression were tested as covariates, but were not statistically significant at the 5% level.
The uncertainty in each individual’s outcome was parameterised using a beta distribution, which was individualised, based on their covariates. Independent beta distributions were fitted to 1- and 2-year HbA1c outcomes, as they had different expectations of the mean effect and the variance in the mean effect in the first and second year. For each individual, two independent random draws were taken: one from their individualised beta distribution for 1-year HbA1c and the other from their individualised beta distribution for 2-year HbA1c, to determine their HbA1c at 1 and 2 years, respectively.
In the base case, it was assumed that if an individual switched treatments then they had a change in HbA1c equal to the difference between the predicted mean effect on HbA1c in their randomised treatment arm and the predicted mean effect on HbA1c in their non-randomised treatment arm. The mean effects were obtained from their individualised outcomes from the beta regressions. In the base case, the estimates of changes in HbA1c were obtained from the per-protocol population, as individuals who switched insulin delivery mechanism were not included in this population. Therefore, treatment effect parameters in this population reflect the relative effectiveness of pump + DAFNE versus MDI + DAFNE for those individuals who did not switch insulin delivery mechanism.
The Model is designed to use a mean HbA1c value, using the DCCT% scale, for each individual in each yearly time cycle. In the base-case analysis, an individual’s HbA1c for the first model cycle (0–1 years) is given by their baseline HbA1c, an individual’s HbA1c for the second model cycle (1–2 years) is given by their 1-year HbA1c sampled from their individualised beta distribution for 1-year HbA1c and an individual’s HbA1c for the third model cycle (2–3 years) is given by their 2-year HbA1c sampled from their individualised beta distribution for 2-year HbA1c. These sampled values of HbA1c on the beta scale were then transformed on to the DCCT% scale for use in the long-term modelling.
The trial population – used to estimate HbA1c effect, changes to HbA1c on treatment switching and the timing of changes in HbA1c – was tested in three deterministic scenario analyses. In the first scenario analysis, the treatment effect was estimated in the ITT population, and when an individual switched insulin delivery mechanism his/her HbA1c still changed so that it was reflective of the other trial arm of REPOSE. In the second, the treatment effect was estimated in the ITT population, but there was no variation in HbA1c changes for those individuals who switched. This scenario was conducted as in the ITT analysis population: individuals who switched insulin delivery mechanism remained in the arm to which they were originally randomised. In the third scenario analysis, HbA1c effects were modelled as occurring one model cycle earlier than they did in the base case. For example, an individual’s 2-year HbA1c was used as their modelled HbA1c value in the second model cycle in the scenario analysis rather than the third model cycle in the base case.
Estimating severe hypoglycaemic events and diabetic ketoacidosis events
To develop the method to incorporate severe hypoglycaemic events and DKA treatment effect evidence into the model, several factors were considered. Data on severe hypoglycaemic events and DKA were collected on an ongoing basis throughout the trial. A summary of the numbers of DKAs and severe hypoglycaemic events is given in Table 8. It can be seen that the number of DKAs and severe hypoglycaemic events declines in the second year on every measure except self-reported DKAs in the MDI + DAFNE arm, where the number of events was the same in both years. As such, the statistical models used in the economic data estimated the incidence of severe hypoglycaemia and DKA in the first and second years separately.
TABLE 8
Summary of the observed incidence of DKA and severe hypoglycaemia in the ITT population
Negative binomial regressions were used to predict the number of DKAs and severe hypoglycaemic events in years 1 and 2 for each outcome separately. When the outcome variable was the number of hypoglycaemic events in year 1, year-1 HbA1c and treatment group were included as covariates. When the outcome variable was the number of hypoglycaemic events in year 2, year-2 HbA1c and treatment group were included as covariates. When the outcome variable was the number of DKAs in year 1, year-1 HbA1c and treatment group were included as covariates. When the outcome variable was the number of DKAs in year 2, year-2 HbA1c and treatment group were included as covariates. The possibility of using the number of events in the previous year, baseline events for the 1-year outcomes and year-1 events for the 2-year outcomes, as a covariate was explored. However, because of the low number of events, the negative binomial models often did not converge when this was included as a covariate.
The statistical models did not converge for DKAs reported as SAEs in the first year. This was not the case for self-reported DKAs and there were more self-reported cases of DKA than were picked up through the reporting of SAEs. Therefore, the rates of DKA were estimated using self-reported DKAs as the outcome measure.
The statistical models were fitted using the Zellig package in R version 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria) and using specifications described above; it was used to simulate the predicted number of severe hypoglycaemia and DKA events in each trial arm 10,000 times. The simulations were separately in each trial arm and for HbA1c values every 0.1% between 4% and 20.5%. The number of events observed in the simulations was truncated at 20 events per year to reduce the effect of extreme values in the simulation on the cost-effectiveness results. These simulations were then used to determine the probability that an individual would suffer a given number of severe hypoglycaemic events and DKA events in 1 year, dependent on their HbA1c that year and the trial arm to which they were allocated. The probability that an individual would suffer a given number of events was a fixed parameter in the PSA; therefore, any differences in the rates of DKA or severe hypoglycaemia for an individual between any two model runs will solely be due to differences in their HbA1c.
In the base case, the statistical models fitted to the incidence of severe hypoglycaemia and DKA in years 1 and 2 were used in the first and second model cycles, respectively, to predict the incidence of severe hypoglycaemia and DKA. The statistical models for year 2 were also used in all subsequent model years because we assumed that year 1 models might not be representative of ongoing event rates because of ‘teething problems’ with treatments given, which are in a sense ‘ironed out’ by year 2. This assumption was based on the clinical opinion of the clinical members of the REPOSE TMG, including honorary consultants in diabetes and diabetes nurse specialists.
In a scenario analysis, individuals in both model arms returned to their baseline rate of severe hypoglycaemia and DKA after the second year. Self-reported information was collected at baseline on the number of severe hypoglycaemic events and DKAs experienced by the individuals in the 12 months prior to baseline data collection. The baseline incidence of these events was estimated using the same methods used to estimate the probability of experiencing these events in year 1 or year 2; however, treatment allocation was not included as covariate. This is because all of the events in the baseline rate models occurred prior to an individual’s randomisation in the REPOSE Trial.
Cost of insulin, diabetes-related contacts and insulin pumps
The cost of insulin, diabetes-related contacts and insulin pumps (including consumables) in the long-term model was estimated based on resource use data from the REPOSE Trial data and the unit costs used in the EEACT (see Resource use by individuals in the REPOSE Trial over the 2-year follow-up period). Statistical models were fitted to these subcomponents of total cost in the EEACT, as it is expected that the covariates that predict the cost of insulin in year 1 may be correlated with the covariates that predict the cost of insulin in year 2. It is also expected that this may be true for the cost of diabetes-related contacts and the cost of insulin pumps (including consumables). Therefore, instead of fitting six independent regression models, three seemingly unrelated regressions were fitted [one seemingly unrelated regression for the cost of insulin, another for the cost of diabetes-related contacts and, finally, one for the cost of insulin pumps (including consumables)].
In the ‘cost insulin seemingly unrelated regression model’, the cost of insulin in year 1 and the cost of insulin in year 2 were used as the outcome variables for the seemingly unrelated regression model. Baseline cost of insulin, baseline HbA1c, treatment allocation, whether or not the individual switched from MDI to insulin pump infusion in year 1 and whether or not the individual switched from insulin pump infusion to MDI in year 1 were included as covariates to predict the cost of insulin in year 1. Baseline cost of insulin, baseline HbA1c, the actual method of insulin delivery that an individual was using at the end of the first year, whether or not the individual switched from MDI to insulin pump infusion in year 2 and whether or not the individual switched from insulin pump infusion to MDI in year 2 were included as covariates to predict the cost of insulin in year 2. The SEs were adjusted for clustering in each DAFNE course. For each individual in the model, their baseline cost of using insulin, their HbA1c, their treatment at the start of the year and whether or not they switched treatment were used with the parameter values from the regression to predict their cost of insulin.
In the ‘cost of diabetes-related contacts seemingly unrelated regression model’, the cost of diabetes-related contacts in year 1 and year 2 were used as the outcome variables for the seemingly unrelated regression model. Baseline cost of diabetes-related contacts, baseline HbA1c, and treatment allocation – whether or not the individual switched from MDI to insulin pump infusion in year 1 and whether or not the individual switched from insulin pump infusion to MDI in year 1 – were included as covariates to predict the cost of insulin in year 1. Baseline cost of diabetes-related contacts, baseline HbA1c, the actual method of insulin delivery that an individual was using at the end of the first year, whether or not the individual switched from MDI to insulin pump infusion in year 2 and whether or not the individual switched from insulin pump infusion to MDI in year 2 were included as covariates to predict the cost of insulin in year 2. The SEs were adjusted for clustering in each DAFNE course. For each individual in the model, their baseline cost of diabetes-related contact resource use, their HbA1c, their treatment at the start of the year and whether or not they switched treatment were used with the parameter values from the regression to predict their cost of insulin pump therapy.
In the ‘cost of insulin pump seemingly unrelated regression model’, the cost of insulin pumps and consumables in year 1 and the cost of insulin pumps and consumables in year 2 were the two outcome variables used in the model. No control was made for baseline resource use or baseline HbA1c for either outcome variable, as no individual in the REPOSE Trial had a previous history of using an insulin pump. The individual’s randomised treatment arm, whether or not they switched from pump to MDI in the first year and whether or not they switched from MDI to pump in the first year were included as covariates to predict the cost of insulin pumps and consumables in year 1. An individual’s actual treatment at the end of the first year, whether or not they switched from pump to MDI in year 2 and whether or not they switched from MDI to pump in year 2 were included as covariates to predict the cost of insulin pumps and consumables in year 2. For each individual in the model, their HbA1c, their treatment at the start of the year and whether or not they switched treatment were used with the parameter values from the regression to predict their cost of insulin pump therapy.
Duration of treatment effectiveness beyond the trial period
A key parameter for the long-term cost-effectiveness modelling is the duration of effectiveness of the two interventions and, in particular, the length of time that HbA1c improvements last. The REPOSE Trial provides data only up to 2 years after randomisation. Therefore, the available literature on the long-term duration of treatment effectiveness for MDI individuals taking a DAFNE course and pump + DAFNE individuals needs to be assessed to determine the assumptions to be used for HbA1c progression beyond the 2-year trial period.
A literature search was conducted for studies on the duration of HbA1c improvements for MDI + DAFNE individuals and insulin pump therapy individuals. Seven potentially relevant studies were identified. Two studies124,125 were identified as being potentially relevant for MDI + DAFNE individuals. Five studies56–59,63 were identified as being potentially relevant for insulin pump therapy individuals. Two studies56,59 were excluded: Beato-Vibora et al.56 was not included because fewer than one-quarter of the individuals in the initial sample had follow-up data for any given year; Clements et al.59 was excluded because it was a subgroup analysis of the data presented by Carlsson et al.58 As such, if Carlsson et al.58 was included to estimate the long-term duration of treatment effect of pump therapy, the effect estimated from Clements et al.59 would be given double the weight of the other studies because of a published subgroup analysis being available.
For the five included studies56–59,63 (two studies for adults receiving MDI + DAFNE and three studies for adults receiving pump + DAFNE), the average yearly increase in HbA1c was estimated, pragmatically, using data from the point of largest reduction in HbA1c and the last observation in which the sample size was greater than one-quarter of the initial sample size. A weighted average of these studies’ evidence (using the initial sample size) calculated the mean yearly HbA1c increase for both trial arms (Table 9). The weighted average yearly HbA1c increase for insulin pump therapy individuals was 0.052% per annum. The weighted average yearly HbA1c increase for MDI + DAFNE individuals was 0.054% per annum.
TABLE 9
A summary of the observed changes in HbA1c over time for pump and MDI
The uncertainty in these long-term changes was parameterised using a normal distribution in the PSA. There was no SD for the mean HbA1c increases across the studies in each model arm; data were obtained from the REPOSE Trial on the SD of the mean change in HbA1c between years 1 and 2. The mean observed change in HbA1c between years 1 and 2 for individuals receiving MDI + DAFNE was –0.08%, with a SD of 0.84%. The mean observed change in HbA1c between years 1 and 2 for individuals receiving pump + DAFNE was –0.09%, with a SD of 0.98%. To estimate the SE for each trial arm, the SD associated with each trial arm was divided by the combined sample size of the studies used to estimate the long-term changes in HbA1c. The estimated mean effect and the estimated SE for each model arm were used to parameterise a normal distribution for the PSA.
In the base-case analysis, data from the studies on the HbA1c increases for MDI + DAFNE individuals and pump individuals were used for each individual’s lifetime. To ensure that each individual could not have implausibly high or low HbA1c values, their HbA1c was constrained so that it could not fall below 4.8% or go above 20.5%.
In addition to these five studies, the cost-effectiveness model used by Riemsma et al.8 used an annual progression of 0.045% per annum derived from the DCCT trial. This was assumed to apply equally to all comparators analysed in the study. In a further sensitivity analysis it was assumed that individuals would return to their baseline HbA1c at the end of the third year in the model with no further progression of their HbA1c.
Threshold analysis
A two-way price and effectiveness threshold analysis was conducted to assess the HbA1c reduction and/or annual cost reduction necessary to potentially make CSII cost-effective in the UK for the whole UK population of adults with T1DM who are eligible to receive a structured education course, are naive to pump therapy and do not have a preference to receive the pump. A conservative assumption was made, in that all HbA1c changes did not apply to 1-year HbA1c, but did apply to all future years. The treatment effect associated with pump + DAFNE compared with MDI + DAFNE was varied between HbA1c changes of –0.3% and –1.2%, in –0.1% increments.
There were two methods used for estimating the change in HbA1c due to receiving pump therapy. In the first method, the following steps were taken: (1) all individuals’ HbA1c values were estimated as if they were a MDI + DAFNE recipient and then (2) a treatment effect (HbA1c change) of pump + DAFNE versus MDI + DAFNE was inputted into the model. In the second method (1) the reduction in HbA1c was applied to the individual’s mean effect in the beta regression; (2) this reduction in HbA1c resulted in a different variance to an equivalent MDI patient as their mean effect was lower; and (3) HbA1c was sampled from the individualised beta distribution, which reflected the mean effect and variance parameters. The second method of conducting the threshold analysis will help future investigators to understand the effect of including heterogeneity in an individual’s response to CSII on the HbA1c reductions that are required to make CSII cost-effective. However, it should be noted that this method assumes that the heterogeneity is defined by the equations estimated from the REPOSE Trial and, as such, may not be valid if CSII were to be clinically more effective.
In both scenario analyses, the cost of insulin pumps and insulin pump consumables was changed from 100% of the mean cost obtained from the pump costing survey to 50% of the mean price observed at REPOSE sites in 5% price reduction increments. Deterministic model runs were used to produce all of the results in the threshold analysis.
It should be noted that other than the method used to estimate HbA1c, all of the other parameters were the same, and assumptions were the same as were used in the base case. As all assumptions were the same as those presented for the base-case analysis, all individuals who switched from MDI + DAFNE to pump + DAFNE received the HbA1c associated with pump + DAFNE, and the individuals who switched from pump + DAFNE to MDI + DAFNE received their HbA1c associated with MDI + DAFNE. This means that the modelled HbA1c reductions are equivalent to per-protocol analysis (treatment switchers removed) rather than an ITT analysis (treatment switchers included in their originally randomised groups) of any future study of pump + DAFNE versus MDI + DAFNE.
As no study other than REPOSE has been conducted to assess the cost-effectiveness of pump + DAFNE versus MDI + DAFNE for adults in the UK with T1DM, the results should are indicative of the HbA1c reductions that pump + DAFNE would need to achieve if it were to be deemed cost-effective compared with MDI + DAFNE.
Methods for the psychosocial evaluation
Aims and objectives
As noted in Chapter 2, evidence on QoL effects of the pump has been inconsistent, with some studies reporting no difference between the pump and MDI groups, and others reporting improved QoL on the pump. A previous HTA report identified some gains in QoL that could be described as ‘social related’ rather than ‘health related’.14 These included flexibility of lifestyle and fewer problems dealing with variations in daily life, such as timing of meals. For this reason, we included a range of psychosocial measures alongside embedded qualitative research in the REPOSE Trial.
The psychosocial study employed a mixed-methods quantitative (questionnaires) and qualitative (interviews) approach to:
- establish whether or not, and why, there were any differences in QoL and other psychological or psychosocial outcomes between participants using pump and MDI regimens
- examine whether or not, and why, QoL and other outcomes changed over time
- understand and explore the added benefit (if any) of pump technology over MDI from participants’ and educators’ perspectives
- explore why some patients may do better than others using the pump
- examine acceptability of, and reasons for, discontinuing (pump) treatment
- enhance understanding and assist in the interpretation of trial outcomes.
Quantitative methods
Validated and reliable questionnaires were used to assess generic and health-specific QoL, treatment satisfaction, fear of hypoglycaemia, hypoglycaemia unawareness, self-efficacy, social support, adherence to treatment, emotional well-being and acceptability of technology. A repeated-measures longitudinal questionnaire study explored both differences in outcomes between the two trial arms and the short- and long-term predictors and mediators of outcomes. Outcomes were measured at baseline and at 6, 12 and 24 months after the DAFNE course. These time points were selected to capture both short- and long-term post-treatment changes in psychosocial outcomes. Questionnaires were posted to participants and self-completed within 6 weeks of the specified time point.
Outcomes
Quantitative psychosocial end points were measured via participant self-completed questionnaires, which included items assessing QoL (generic and diabetes specific), fear of hypoglycaemia, treatment satisfaction and emotional well-being. There has been limited examination of the impact of pump therapy on these areas, on how and why these may change over time, and why individuals are able or unable to use pump therapy to improve glycaemic control. Rubin and Peyrot126 reviewed the evidence on ‘patient-reported outcomes’ and concluded that, at present, there is little evidence that pump therapy improves them.
Diabetes-specific QoL was assessed using the DSQOL, a reliable and valid measure.127 Specifically designed for the German study on which UK DAFNE is based, it was included to facilitate important comparisons between the UK and German studies. In addition, generic measures of QoL, the World Health Organization Quality of Life Abbreviated Questionnaire (WHOQOL-BREF)128 and functional health status using the SF-12129 and EQ-5D130 were used. The SF-12 was used to facilitate comparison with ‘healthy controls’ and other long-term conditions.
The HFS131 is a well-validated psychometric tool assessing participants’ fear of hypoglycaemia, both overall and separately, for behaviour and worry. A specific benefit to the survey is that it may be able to identify participants who are likely to maintain high blood glucose levels, thus aiding understanding of potential reasons for poor glycaemic control. A study by Nixon and Pickup,132 in people who had been using a pump for an average of 5 years, found that fear of hypoglycaemic episodes remained a problem.
The DTSQ133 has proven to be highly sensitive in clinical trials.134 Treatment satisfaction refers to an individual’s subjective appraisal of their experience of treatment, including ease of use, side effects and efficacy. Improvements in satisfaction are not necessarily accompanied by improvements in QoL; treatment satisfaction can be high despite diabetes having a negative impact on QoL, which is why it is important to measure both separately.
The Hospital Anxiety and Depression Scale (HADS)135 measures anxiety on one subscale and depression on another through the use of seven questions for each characteristic. It was important to measure emotional well-being in the trial, as participants may find it easier to manage their condition after DAFNE education or with one of the treatments. This could have a substantial effect on their emotional well-being, which the QoL measures are not sensitive enough to pick up.
The DAFNE Principles Questionnaire was completed at 24 months only. This questionnaire (12 items) assesses the impact of the DAFNE course on self-management behaviours, such as bolus and basal rate changes, correction dose practices, timing of injections/bolus doses and review of blood glucose data. It was included partly in order to establish if there were differences in self-management practices between participants in the pump and MDI arms, to aid interpretation of the final trial findings. The DAFNE Principles Questionnaire was administered to all of the participants irrespective of treatment group. This measure was previously used in DAFNE.78
Statistical power was calculated for the primary outcome of HbA1c, thus the psychosocial outcomes are either over- or underpowered, depending on the underlying effect size. Statistically significant results are considered in combination with qualitative data in order to answer the key psychosocial research aims.
Statistical analysis of questionnaire data
Short Form questionnaire-12 items
The Physical Component Summary was calculated using physical functioning, body pain, role physical and general health domain scores. The Mental Component Summary was calculated using vitality, social functioning, role emotional and mental health domain scores. When the questionnaires were only partially completed, missing items were imputed using a single imputation procedure based on the mean calculated from complete items on that domain.136 The scores were standardised and scaled to range from 0 to 100, with higher scores representing better outcomes.129,137
Diabetes-specific quality of life
The DSQOL domain scores (social relations, leisure time restrictions and flexibility, physical complaints, worries about the future, daily hassle or functions, diet restrictions) and DSQOL total score were calculated if at least 80% of the items from the domain were complete, using the following formula:
Preference-weighted treatment satisfaction was calculated by multiplying the various treatment goals with the corresponding degree of satisfaction (scores of –2.5 = totally dissatisfied to 2.5 = extremely satisfied) and summing the results.
Finally, all DSQOL scores were converted to a 0–100 scale, in which a higher value means worse outcome (more burden) on all scores.
World Health Organization Quality of Life Abbreviated Questionnaire
Four subdomains of WHOQOL-BREF were calculated (physical health, psychological, social relationships and environment) if at least 80% of the questions in that domain were present. The domains were scored by calculating the mean of the items within each domain, and scaling to range from 0 to 100,138 with higher scores representing better outcomes.
Hypoglycaemia Fear Survey
The HFS behaviour and worry scores were calculated if at least 80% of items within that domain were complete, using Equation 5 (see Diabetes-specific quality of life).131
The HFS behaviour score ranges from 10 to 50 and the HFS worry score ranges from 17 to 85; in both cases, higher scores represent more fear.
Diabetes Treatment Satisfaction Questionnaire
The DTSQ, which measured satisfaction with diabetes treatment, was administered at baseline and 6- and 24-month follow-up. The DTSQc [Diabetes Treatment Satisfaction Questionnaire (change)], which measures change in satisfaction from pre-trial treatment, was administered at 12 months’ follow-up.
Treatment satisfaction (DTSQ) and treatment satisfaction change (DTSQc) were calculated if at least five of the six items were complete using the following formula:
For the treatment satisfaction domain, higher scores represent higher satisfaction (range 0 to 36 on DTSQ and –18 to 18 on DTSQc). Two further domains, perceived frequency (change) in hyperglycaemia and perceived frequency (change) in hypoglycaemia, were calculated based on single items. Only complete data were used for these scores and low scores represent good perceived blood glucose control (scoring ranges of 0 to 6 in DTSQ, and –3 to 3 in DTSQc).
Hospital Anxiety and Depression Scale
Anxiety and depression domain scores were calculated by summing the items in the respective domains. Mean value imputation based on the other six items of a domain was used to impute missing data if a single item was missing. If more than one item was missing then the domain score was not calculated. The HADS scores range from 0 to 21, with higher scores indicating more anxiety/depression (scoring: normal is 0–7; borderline abnormal 8–10; 11–21 abnormal).135
EuroQol-5 Dimensions
The EQ-5D-3L tariff was derived from five three-level questions using UK norms. The tariff was calculated only if all five questions were answered. It is measured on a scale from –0.56 to 1.00 (good health).
The availability of questionnaire outcome data was summarised for each time point.
The DTSQ domains at 6, 12 and 24 months post course were compared between the treatment groups using a non-parametric Wilcoxon–Mann–Whitney U-test. The median and interquartile range (IQR) of change from baseline for the DTSQ domains at 6 and 24 months, and the median and IQR score for the DTSQc domains at 12 months, are displayed by treatment group. The differences between groups post course are displayed as the median difference (in change from baseline for DTSQ scores) with its associated 95% CI, which was calculated as described in the study by Newson.139
Other QoL outcomes (DSQOL, SF-12, WHOQOL-BREF, HFS, EQ-5D, HADS) at 6 months post course are compared between the treatment groups using a mixed-effects linear regression model of change from baseline adjusted for DAFNE course (random effect), baseline HbA1c, baseline score and centre. The means and SDs for the treatment and control groups with adjusted MDs and associated CIs and p-values (unadjusted for multiple testing) are reported. This analysis is repeated for the 12- and 24-month outcomes. A complementary sensitivity analysis, in which the analysis described above was repeated only including patients with complete data, was performed.
Qualitative methods
Study design
An inductive, thematic approach was used, informed by the principles of Grounded Theory research.140 This entailed concurrent data collection and analysis, allowing findings and themes arising from early phases of data collection to inform the areas explored in later phases, as well as sampling. In-depth interviews, informed by topic guides, were used as the main method of data collection, as these helped to ensure that the discussion remained relevant to areas under investigation, while affording the flexibility needed for participants to raise and discuss issues that they perceived as salient, including those unforeseen at the study’s outset.141,142
Patient participants, recruited from both trial arms, were interviewed at two time points: within 2 weeks of completing their DAFNE courses (round 1) and 6 months later (round 2). This longitudinal design permitted patients’ initial understandings and experiences of using the pump and MDI regimens to be explored, and any continuities and changes in their diabetes self-management practices to be tracked and compared over time. Six months was selected as the time point for follow-up to coincide with collection of 6-month clinical and psychological data, and because previous experience of undertaking longitudinal qualitative research with DAFNE graduates had demonstrated that this allowed sufficient time to establish whether, and for what reasons, patients are able/unable to put their skills training into practice.143–146 In addition, cost considerations (including a request by the funder to reduce the costings for the qualitative component prior to the protocol being finalised) meant that it was not possible to do follow-up interviews with patients at later time points.
Educators were interviewed once, following completion of their centre’s sixth REPOSE DAFNE course. This time point was chosen to avoid any risk of inadvertent contamination of the trial intervention by the qualitative questioning, and also because, at this point, it was anticipated that staff would have had considerable experience of trial recruitment and delivery on which they could reflect.
Recruitment and sampling
As per the original protocol, participants (patients and educators) were recruited from seven of the eight trial centres (with roughly equal numbers recruited from each centre); recruitment to the qualitative research was not undertaken in the eighth centre (Nottingham), as this centre came on board only in the later phases of the trial and recruited patients to only one set of courses.
When they were consented to take part in the trial, patient participants were asked whether or not they would be willing to be approached to take part in the qualitative research (see Appendix 8). Of the 317 patients who were randomised, 315 (99.37%) agreed to be approached. Participants who gave this agreement were purposively sampled so that both those randomised to pump and MDI arms of the trial were recruited and there was broad, and roughly equal, representation of ages, sex, diabetes duration and occupational/socioeconomic groups in the final sample.
It was initially planned that one nurse and one dietitian would be recruited and interviewed from each of the seven main trial centres. However, after initial interviews had been conducted and analysed, a decision was made to increase the number of nurse educators interviewed. This is because the initial interviews had made apparent that these staff members tended to have the greatest involvement in recruitment and notifying patients of the outcome of randomisation, and, as reported elsewhere,147 these aspects of trial work proved to be particularly challenging for staff. Educators were sent recruitment packs and invited to ‘opt in’ to the study; all of those approached agreed to take part.
Recruitment of patients and educators continued until data saturation occurred, that is until no new findings or themes were identified in new data collected. All participants provided written consent prior to their interviews.
Data collection
Baseline interviews with patients were undertaken face to face to establish rapport and were conducted at a time and location convenient to them (mostly their own homes). Follow-up interviews were done by telephone (again at a time most convenient to the interviewee). There was no apparent difference in the quality and disclosure of information between interviews undertaken face to face and those done on the telephone. All staff opted to be interviewed by telephone.
Topic guides for the patient interviews were developed in light of literature reviews, course observations, inputs from the trial team and patient representatives, and revised in light of emerging findings. Full details of the topics explored in patients’ round 1 and round 2 interviews are provided in Appendix 9. In brief, round 1 interviews explored patients’ understandings of the trial and the pump, and their reasons for agreeing to take part; their views about the outcome of randomisation; and their early experiences of using a MDI or pump regimen to undertake diabetes self-management practices following course attendance and training in DAFNE principles. Round 2 interviews were used to explore whether or not, how and why patients’ experiences of managing their diabetes had changed since their last interview (including reasons for adhering or not adhering to treatment recommendations, discontinuing treatment, etc.); how the use of their regimen (pump or MDI) had impacted on their perceptions of their diabetes, their confidence and perceived ability to undertake diabetes self-management practices; and their everyday (work and family) lives. Although broadly the same areas were explored in the follow-up interviews, each participant’s round 1 interview account was reviewed before their round 2 interview was undertaken to enable follow-up of specific issues raised by particular individuals.
Staff interviews explored their experiences of recruiting into the REPOSE Trial, delivering the 5-day courses and undertaking patient follow-up as part of the trial; perceptions of patients’ engagement with pump therapy compared with MDI during the trial; previous experiences (if any) of using insulin pumps in routine clinical practice; and views about the potential benefits of the pump compared with MDI regimens. In light of emerging findings, staff were also invited to reflect on whether or not their views about the potential benefits, and beneficiaries, of insulin pumps had changed in light of their experiences of delivering, and observing, patients during the REPOSE Trial. Full details of the areas explored in the staff interviews are also contained within Appendix 9.
Interviews with patients were conducted between June 2012 and June 2013, and those with staff between December 2012 and April 2013. All interviews averaged 1 hour, were digitally recorded and transcribed in full for in-depth analysis.
Data analysis
Data were analysed thematically using the method of constant comparison.148 This entailed members of the qualitative research team reading patient and educator transcripts (which were treated as ‘stand-alone’ data sets) repeatedly before cross-comparing them to identify issues and experiences that cut across different patient and educator accounts. To address the study aims and objectives, a longitudinal analysis of the patient data was also undertaken. Each individual’s round 1 and round 2 accounts were cross-compared and attention paid to continuities and changes in their experiences, views and diabetes self-management practices (using pump or MDI) over time, and the reasons for these. A key aspect of this analysis also focused on comparison of the (longitudinal) accounts of patients using pump and MDI regimens. This was done to better understand the impact of using pump (compared with MDI) regimens on patients’ diabetes self-management practices, disease perceptions and everyday life.
Members of the qualitative team undertook their own independent analyses and wrote separate reports before meeting (both during and after data collection) to compare their interpretations, discuss discrepant cases, and reach agreement on recurrent themes and findings. For both patient and educator interviews, a final coding frame, which reflected the original study aims/questions and emergent themes, was developed once all of the data had been reviewed and consensus reached on key themes and findings. NVivo9 (Doncaster, VIC, Australia), a qualitative software package, was used to facilitate data coding and retrieval. Coded data sets were subjected to further analyses to allow for the identification of subthemes and illustrative quotations.
Confidentiality
To protect participants’ identities, each individual was allocated a unique identifier and these identifiers are used in the reporting of interview data. In the case of staff, ‘N’ is used to refer to a nurse and ‘D’ to a dietitian. In the case of patients, data are tagged with the participant’s treatment arm (‘M’ for MDI, ‘P’ for pump), identifying number and interview round (e.g. ‘M7.2’ refers to the second interview with MDI participant 7).
- Methods - A cluster randomised trial, cost-effectiveness analysis and psychosoci...Methods - A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial
- Overall conclusions - Risk assessments and structured care interventions for pre...Overall conclusions - Risk assessments and structured care interventions for prevention of foot ulceration in diabetes: development and validation of a prognostic model
- Conclusions - Adalimumab, etanercept and ustekinumab for treating plaque psorias...Conclusions - Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation
- Definition of the decision problem and review question - A systematic review and...Definition of the decision problem and review question - A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome
- List of abbreviations - Treatment of extravasation injuries in infants and young...List of abbreviations - Treatment of extravasation injuries in infants and young children: a scoping review and survey
Your browsing activity is empty.
Activity recording is turned off.
See more...