Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Heller S, White D, Lee E, et al. A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial. Southampton (UK): NIHR Journals Library; 2017 Apr. (Health Technology Assessment, No. 21.20.)

A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial.
Show detailsTrial recruitment
Participant recruitment initially took place at seven centres between November 2011 and December 2012. A review of recruitment and retention in August 2012 revealed higher than expected dropout rates prior to the DAFNE courses. The recruitment target was therefore increased to a maximum of 340, but with no more than 280 in the ITT population. In order to achieve this we facilitated an additional pair of DAFNE courses at an existing centre (Harrogate) and introduced a reserve centre (Nottingham) to facilitate a further two courses. Recruitment continued until April 2013. Figure 2 illustrates recruitment and course attendance rates against targets. Table 10 summarises course attendance by treatment group, with similar mean participant numbers per course. Table 11 shows recruitment details by centre.

FIGURE 2
Participant recruitment and attendance targets and rates.
TABLE 10
Summary of course attendance
TABLE 11
Recruitment by centre
Participant flow
Figure 3 shows the CONSORT flow of participants through the trial. In total 1278 people were invited to take part, of which 885 responded. Of these responders, 362 were interested in taking part. Reasons given for non-participation are listed in Table 12. Of those interested, 334 were assessed as eligible and 321 of these consented to take part. Four of these dropped out prior to randomisation. Forty-six courses (23 course pairs) were randomised, comprising 317 participants (156 pump and 161 MDI). Fifty patients were excluded from the analysis: 40 patients withdrew before baseline data were collected and 10 withdrew before they attended a DAFNE course. All randomised courses were delivered.23 One participant was deemed protocol non-compliant, as he/she had not adhered to the DAFNE course (as adjudicated by the course leader). Of the 267 participants (132 pump and 135 MDI) who were randomised, attended baseline visit and attended a DAFNE course, 260 (132 pump and 128 MDI) had HbA1c data for at least one post-baseline follow-up visit and these make the ITT set. A total of 248 participants (128 pump and 120 MDI) had complete primary outcome data at 24 months’ follow-up.23
TABLE 12
Reasons for non-participation (footnote to CONSORT flow diagram)
Baseline data
Table 13 shows the baseline demographics and characteristics of the trial population. Overall, eight centres recruited to the study contributing between 10 and 51 participants per centre. Patients were more likely to be male (60%) and were generally white British (91%). The average age of participants was 41 years.
TABLE 13
Demographics and characteristics of participants at baseline
Table 14 shows the history of diabetes among study participants at baseline. The median (IQR) duration of diabetes was 16 (8–26) years, 12% of the participants had an episode of severe hypoglycaemia in the 12 months prior to baseline and around half of the participants had a prior history of complications (55%).
TABLE 14
History of diabetes among study participants at baseline
Table 15 shows the history of severe hypoglycaemic episodes by baseline HbA1c category: 5 (20%) of the 25 participants with HbA1c of < 7.5% had an episode in the 12 months prior to baseline; 26 (11%) of the 242 participants with baseline HbA1c of ≥ 7.5% had an episode in the 12 months prior to baseline.
TABLE 15
History of severe hypoglycaemic episodes at baseline by baseline HbA1c category
Table 16 shows laboratory results of participants at baseline. The mean HbA1c was 9.3% or 77.9 mmol/mol in the pump group and 9.0% or 74.8 mmol/mol in the MDI group. Other than this difference in baseline HbA1c, the data appear to be well balanced across treatment groups.
TABLE 16
Laboratory test results of participants at baseline
Table 17 summarises the proportion of participants with above and below 7.5% HbA1c at baseline in each centre, stratified by treatment group.
TABLE 17
Baseline HbA1c level by centre
Protocol deviations
One participant was excluded from the per-protocol analysis set, as they did not adhere to the DAFNE course (this is not including the dropouts prior to the DAFNE course).
Twenty-five patients had a single treatment change form that recorded change across study treatments; 17 patients switched from pump to MDI and eight patients switched from MDI to pump. Two patients on the pump arm changed to MDI and back again (recorded on treatment change forms), and other participants recorded temporary treatment breaks at the follow-up appointments. After review, excluding any reasonable temporary treatment interruptions, 236 out of the 260 ITT participants were considered as compliant with the protocol. Of the 235 ITT participants with baseline HbA1c of ≥ 7.5%, 18 were considered protocol deviations, leaving 217 in the per-protocol analysis set. Participants who deviated from the protocol started with higher baseline HbA1c across both the treatment groups (Table 18); however, greater improvement was seen for the protocol deviants in the MDI group. The reasons for protocol deviation/treatment change are shown in Table 19.
TABLE 18
Glycated haemoglobin (% and mmol/mol) by treatment group and protocol adherence
TABLE 19
Details of treatment change
Primary outcome
Table 20 shows the primary outcome, change in HbA1c at 24 months in participants whose baseline HbA1c was ≥ 7.5%. The mean change in the pump group was a decrease of 0.85% or 9.3 mmol/mol, whereas the mean decrease in the MDI group was 0.42% or 4.5 mmol/mol. After adjusting for centre, course and baseline HbA1c, the MD in HbA1c change from baseline was –0.24% (95% CI –0.53% to 0.05%) or –2.7 mmol/mol (95% CI –5.8 to 0.5 mmol/mol; p = 0.098).23 Figure 4 shows the distribution of HbA1c change at 2 years, by treatment group.
TABLE 20
Effectiveness of the intervention: MD in change in HbA1c (% or mmol/mol) at 24 months in participants whose baseline HbA1c was ≥ 7.5%

FIGURE 4
Changes in HbA1c (%) at 24 months in participants whose baseline HbA1c was ≥ 7.5%. (a) Pump; and (b) MDI.
The treatment difference was larger for the per-protocol analysis: MD –0.36% (95% CI –0.64% to –0.07%) or –3.9 mmol/mol (95% CI –7.0 to –0.8 mmol/mol) in favour of the pump; p = 0.015. However, the observed point estimate was still smaller than the minimum clinically important difference of 0.5% or 5.5 mmol/mol,23 although the 95% CI includes this clinically important effect.
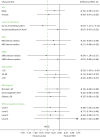
Table 21 shows sensitivity analysis on the primary outcome; the analysis was repeated for complete case, imputing data for all participants, excluding mistimed measurements and excluding pregnant women. The results from Tables 20 and 21 are presented graphically in Figure 5. All sensitivity analyses show similar results to the primary analysis shown in Table 20.
TABLE 21
Sensitivity analysis on the primary outcome (change in HbA1c at 24 months in participants whose baseline HbA1c was ≥ 7.5%)

FIGURE 5
Forest plot of MD in change from baseline in HbA1c (%) at 24 months between groups for the sensitivity analysis samples. MCID, minimum clinically important difference, adjusted for baseline HbA1c, centre and DAFNE course.
Notes: (1) Thirteen local laboratory HbA1c values were used in the final analysis (two at 6 months, one at 12 months, 10 at 24 months), seven HbA1c values were taken from patient notes (one at 6 months, four at 12 months, two at 24 months). (2) ICC from complete case model at 24 months is 0.005. If centre is excluded from the model (as a fixed effect), the ICC is 0.08.
The change in HbA1c for participants with data at all four time points is displayed, by treatment group, in Figure 6. The majority of improvement in HbA1c occurred in the first 6 months; HbA1c stayed roughly constant between 6 and 24 months. The change in HbA1c over time is also displayed in Figure 7, but here all of the participants with post-baseline data are included. Each coloured line represents a participant, and the thick black line is the mean for each treatment group.

FIGURE 7
Glycated haemoglobin (mmol/mol) over time in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol) (including participants with any post-baseline HbA1c data, n = 235). (a) Pump; and (b) MDI.
Table 22 shows the mean change at 24 months for the treatment groups combined; the change in all participants with complete 24-month HbA1c data was a decrease of 0.54% (95% CI 0.38% to 0.69%) or 5.9 mmol/mol (95% CI 4.2 to 7.6 mmol/mol). For participants with baseline HbA1c ≥ 7.5%, the reduction was slightly bigger, of 0.64% (95% CI 0.48% to 0.80%) or 7 mmol/mol (95% CI 5.2 to 8.8 mmol/mol).23
TABLE 22
Change in HbA1c (% or mmol/mol) from baseline to 24-month follow-up, treatment groups combined
Sensitivity analysis: effect of centre and lead Dose Adjustment For Normal Eating course educator
We undertook a further analysis that used a nested model of patients within courses, which, in turn, are nested within course lead educators, to investigate differences in outcomes between lead educators. For this nested model, the ICC of the lower-level clustering variable, DAFNE course, is 0.5%; for the upper-level clusters, lead educator, ICC < 0.1%. We found no evidence of notable differences in outcomes between lead course educators. This analysis was performed for available data only.
We explored the centre effect through an interaction test between centre and treatment group. Results of estimated MD in HbA1c change (% or mmol/mol) at 24 months are presented by centre with the aid of forest plots (Figure 8). The overall p-value for the interaction between treatment and centre was 0.565, suggesting that there is no centre effect. The centre with the largest difference between treatments was Nottingham, although the CI for this centre is large because of the small number of participants with outcome data at that centre.
Secondary outcomes
Glycated haemoglobin
The proportion of participants reaching the NICE target of HbA1c of ≤ 7.5% (58 mmol/mol) after 2 years is displayed in Table 23 (including all participants regardless of baseline HbA1c value). The proportion of patients with HbA1c ≤ 7.5% was similar across the groups. The results are very similar at 6 and 12 months (Table 24).
TABLE 23
Effectiveness of the intervention: proportion of participants with HbA1c of ≤ 7.5% (58 mmol/mol) at 24 months (including all participants regardless of HbA1c at baseline)
TABLE 24
Effectiveness of the intervention: proportion of participants with HbA1c ≤ 7.5% (58 mmol/mol) at 6 and 12 months
Table 25 shows the distribution of HbA1c categories at baseline and 24 months. Of the participants who ended with HbA1c of ≤ 7.5% at 24 months, 12 began the study with baseline HbA1c of ≥ 8.5%.
TABLE 25
Distribution of HbA1c categories at 24 months for all participants
The primary analysis at 24 months displayed above (see Primary outcome) is repeated for 6- and 12-month follow-up visits among participants with complete data. The results for these interim follow-ups are consistent with the primary outcome analysis and are displayed in Table 26. The largest MD in HbA1c change from baseline was observed at 6 months, –0.25% (95% CI –0.52% to 0.02%) or –2.7 mmol/mol (95% CI –5.6 to 0.2 mmol/mol), but is not clinically relevant or statistically significant at the 5% nominal level.
TABLE 26
Effectiveness of the intervention: MD in change in HbA1c at 6 and 12 months in participants whose baseline HbA1c was ≥ 7.5%
Episodes of moderate and severe hypoglycaemia
Few severe hypoglycaemic episodes were observed post baseline; 49 episodes recorded from 25 participants23 (Table 27). All severe hypoglycaemic episodes occurred while participants were on their allocated treatment. Across both treatment groups the number of severe hypoglycaemic episodes reduced: the average number of episodes per patient-year in the study reduced from 0.17 before baseline to 0.10 during follow-up. The IRR for the number of severe hypoglycaemic episodes in the 24-month follow-up, compared with the year before baseline, is 0.46 (95% CI 0.24 to 0.89; p = 0.021).23 Therefore, compared with the year before baseline, the number of severe hypoglycaemic episodes per year were roughly halved in the 2 years of follow-up post baseline. There was no statistically significant difference in the rate of severe hypoglycaemia during follow-up between the treatment groups having adjusted for centre, DAFNE course, baseline HbA1c and presence of at least one severe hypoglycaemic episode in the 12 months before baseline (IRR 1.13; 95% CI 0.51 to 2.51; p = 0.766). The comparison of severe hypoglycaemic episodes between groups was repeated excluding the first 6 months of follow-up, which is the ‘settling in’ period on the pump. This time the estimated IRR was almost equivocal, but the large CI around this reflects the amount of uncertainty as a result of these analyses being based on so few episodes from few participants (IRR 1.05; 95% CI 0.44 to 2.53; p = 0.912).
TABLE 27
Severe hypoglycaemic episodes per patient-year in study (n = 267, all participants with baseline data and who attended a DAFNE course)
Across both treatment arms, on average, three moderate hypoglycaemic episodes were recorded per patient over a 4-week history at 6 months (Table 28). By 24 months, the average number of recorded moderate hypoglycaemic episodes during a 4-week history was slightly lower (2.6 for pump, 2.3 for MDI). There was no statistically significant difference between the groups in the rate of moderate hypoglycaemic episodes at any time point.23
TABLE 28
Moderate hypoglycaemic episodes: IRR between pump and MDI
Few participants reported one or more severe hypoglycaemic episode during study follow-up: 14 (10.6%) in the pump group and 11 (8.6%) in the MDI group (Table 29). There was no evidence that the number of patients reporting at least on severe hypoglycaemic episode was different in the two groups: OR 1.22 (95% CI 0.49 to 3.03). More than half of the participants reported at least one moderate hypoglycaemic episode in the 4 weeks prior to follow-up at each time point and across both treatment groups. Slightly more participants reported at least one episode at 6 months in the pump group than in the MDI group (p = 0.088). However, a smaller proportion of participants reported episodes in the pump group at 12 and 14 months, although not statistically significant.
TABLE 29
Effectiveness of the intervention: proportion of participants who experienced at least one moderate or severe episode of hypoglycaemia
Figure 9 shows the distribution of the number of severe hypoglycaemic episodes for those who had one or more episodes post baseline. The majority of participants had only one severe hypoglycaemic episode, 10 participants recorded more than one episode during the follow-up period and the maximum recorded by a participant was seven. The number of patients who had an episode makes up a small proportion of the study population (10%).

FIGURE 9
Distribution of number of severe hypoglycaemic episodes in participants with at least one episode post baseline, by treatment group. (a) Pump, n = 14; and (b) MDI, n = 11.
Figure 10 shows the timing of severe hypoglycaemic episodes. Each dot represents a severe hypoglycaemic episode. Dots connected by a line represent severe hypoglycaemic episodes experienced by the same person.
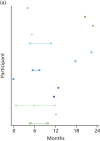
FIGURE 10
Severe hypoglycaemic episodes over time per participant with at least one episode post baseline, by treatment group. (a) Pump, n = 14; and (b) MDI, n = 11.
There is no statistically significant difference in the odds of proteinuria between the treatment groups (Table 30). At 6 months, the odds of being in a higher proteinuria category (where macroalbuminuria is the highest category) are estimated to be 21% lower in the pump group than the MDI group (OR 0.79), but 14% higher at 12 months, and almost identical at 24 months.
TABLE 30
Secondary outcomes: proteinuria – proportion of participants in each proteinuria category at 6, 12 and 24 months
Table 31 shows exploratory descriptive analyses of self-reported physical activity for the two groups at each study visit; no formal statistical tests have been performed on these data. The amount of physical activity appears similar across the groups.
TABLE 31
Physical activity level by treatment group at baseline, 6, 12 and 24 months
Table 32 shows the results of comparing secondary continuous outcomes across treatment groups. Weight remained roughly constant throughout the study duration, and was not statistically significantly different between the treatment groups at any follow-up.23 A slight increase in HDL cholesterol and a slight decrease in TC was observed across both treatment groups.23 There was no evidence of a difference between treatment groups in cholesterol change from baseline, with p-values ranging from 0.219 to 0.856. Insulin dose decreased across both pump and MDI arms. There was evidence of a difference in the mean change in insulin dose at 12 months between treatment groups; on average, participants in the pump group had a 0.07-IU/weight larger decrease (95% CI 0.01 to 0.013 IU/weight; p = 0.017) in insulin dose than those in the MDI group. However, the difference between treatments in insulin dose was slightly smaller at 6 and 24 months, but not statistically significant.
TABLE 32
Secondary continuous outcomes: MD in change from baseline at 6, 12 and 24 months
Table 33 summarises blood glucose testing per day averaged over a 2-week recorded period, stratified by the baseline HbA1c category. A post hoc analysis indicated that there was no difference in the mean blood glucose testing frequency between treatment groups at 24 months, having adjusted for baseline number of blood glucose tests, centre and DAFNE course.23 The adjusted MD in blood glucose tests (95% CI) was 0.22 (–0.24 to 0.68) per day or 3.1 (–3.4 to 9.6) over 2 weeks; p = 0.352. Overall, the number of blood glucose tests increased from 3.6 at baseline to 4.1 per day at 24 months (95% CI 0.33 to 0.82; p < 0.001).23
TABLE 33
Blood glucose testing frequency over a 2-week period at baseline and 24 months, comparison by treatment group and baseline HbA1c (%) category
Subgroup analysis
The potential moderating effects of subgroups were explored using mixed-effects linear regression, with an interaction between treatment and subgroup. Results of the subgroup analyses are presented in Tables 34–37, and the results are summarised graphically using forest plots in Figures 11 and 12.
TABLE 34
Subgroup evaluation (demographics): change in HbA1c (%) at 24 months within subgroup and subgroup treatment interaction tests
TABLE 35
Subgroup evaluation (demographics): change in HbA1c (mmol/mol) at 24 months within subgroup and subgroup treatment interaction tests
TABLE 36
Subgroup evaluation (diabetes characteristics): change in HbA1c (%) at 24 months within subgroup and subgroup treatment interaction tests
TABLE 37
Subgroup evaluation (diabetes characteristics): change in HbA1c (mmol/mol) at 24 months within subgroup and subgroup treatment interaction tests
We found no reliable statistical evidence of any subgroup effects or interactions between the pump and MDI groups. However, there was some indication that participants with qualifications up to A-level/equivalent did better in the pump arm than in the MDI arm – MD in HbA1c change (95% CI) at 24 months of –0.67% (–1.21% to –0.14%) vs. –0.07% (–0.47% to 0.33%) or –7.4 mmol (–13.2 to –1.5 mmol) vs. –0.8 (–5.1 to 3.6 mmol) – although the interaction test was not statistically significant (p = 0.07).23
Ancillary analyses
Adverse events
Table 38 shows the AEs recorded throughout study follow-up. More participants in the pump arm (66%) reported AEs than in the MDI arm (37%). However, part of this difference can be attributed to the 23 cases of suspicion of pump malfunction, which, by definition, could occur only for participants using pump therapy. Table 39 shows the AEs that were recorded over different time periods during study follow-up. During each time period, more participants in the pump arm experienced AEs than in the MDI arm. A total of 142 AEs were recorded for the pump group during the first 6 months of follow-up in comparison with 84 in the following 6 months, and 94 in the final 12 months of follow-up, suggesting that more AEs occurred during the early ‘settling in’ period on pump therapy.
TABLE 38
Safety analysis: AEs
TABLE 39
Safety analysis: AEs by post-course time window
Table 40 shows the AEs that were classified as being SAEs. The distribution of SAEs was similar across the treatment groups, with the exception that more participants experienced DKA in the pump group. Table 41 shows SAEs by study time period. Again, for the pump group, more SAEs were recorded in the first 6 months (n = 17) than in the following 6 months (n = 11) or when compared with the last 12 months (n = 17).
TABLE 40
Safety analysis: SAE
TABLE 41
Safety analysis: SAEs by post-course time window
Note: All of the DKAs that occurred were reported as SAEs and resulted in hospitalisation. All of the SAEs have a corresponding AE; however, in some cases, a DKA SAE had a corresponding AE that was not labelled as DKA, which is why there are more DKA SAEs recorded than DKA AEs.
Characteristics of participants by missing data status
Tables 42 and 43 show baseline characteristics of patients with missing data.
TABLE 42
Continuous baseline characteristics by treatment group and 24-month missing data status
TABLE 43
Categorical baseline characteristics by treatment group and 24-month missing data status
Findings of the fidelity assessment
Course characteristics
All eight REPOSE centres were fidelity tested. Four centres were fidelity tested on their second pump course and two centres were fidelity tested on their first pump course. One centre was fidelity tested on their third and final course as a result of personal circumstances of the FA precluding the assessment being undertaken on the second pump course. Nottingham ran one pair of courses and, thus, FT took place on its only pump course.
The number of REPOSE participants on the fidelity-tested pump courses ranged from 3 to 7 (for course sizes, see Table 44). The range of participants on the remaining pump courses was 3–8, with a mean of 5.7.
TABLE 44
Size of fidelity-tested pump courses
One pump course (Cambridge) included a non-REPOSE participant who had been on a pump for 10 years and was very keen to do DAFNE.
Pump pre-course session
All participants attended the pump pre-course session to learn the mechanics of pump therapy and to programme and load the pump with saline to enable practice and familiarisation prior to undertaking the course. This session was scheduled to run for 2 hours and 30 minutes (± 15 minutes). Seven out of the eight centres ran sessions within this duration window. The centre that did not (Nottingham) ran a pump pre-course session of 2 hours and so was 15 minutes short of the specified duration window.
The majority of centres delivered the pump pre-course session solely by REPOSE educators (diabetes specialist nurses and dietitians). Two centres (Glasgow and Edinburgh) had a Medtronic representative present to provide technical support and help with elements of pump set-up. One centre (Glasgow) also had the PI present.
All pump pre-course session lesson plans were evaluated by the FA as relating to the objectives set for this session.
Insulin switchover
Participants were asked to switch over their pump from saline to insulin the evening before their pump DAFNE course if they felt happy to do so.
All course participants switched the evening before their course at three centres (Nottingham, Edinburgh and Harrogate). At Glasgow, all participants switched to insulin on the morning of course. This was a decision taken by the personnel at that centre who, after already having run one pump course, felt that this approach worked best, and course participants had not expressed any preference for the Sunday evening. At the remaining centres, the majority of participants switched to insulin the night before their course. Those who did not cited the following reasons:
- unsure of how to fit reservoir
- started in previous week but stopped, as wanted support from health professionals
- anxiety regarding change
- timing issues and technical problems with pump
- pump failure/motor alarming problem
- ran out of consumables and had cannula problems.
Pump courses
The pump course timetable was reviewed by the FA. All centres provided timetables that were evaluated as incorporating all elements of the pump DAFNE curriculum in a logical order. Based on the times allocated for sessions on the pump course timetable, all centres planned to deliver the curriculum in the specified duration window of ≥ 1870 minutes but ≤ 2280 minutes. The mean course duration was 2006 minutes, that is 33 hours and 26 minutes.
All sessions planned to be observed were reviewed during the fidelity visit and their lesson plans were reviewed.
The sick day rules lesson plan was reviewed for each centre. Seven of the eight centres were evaluated as having no issues with this session lesson plan, with only minor problems noted, for example no aims or objectives listed, timings not written on. One centre (Glasgow) was evaluated as having an issue with the sick day rule lesson plan. The lesson plan was lifted directly from the pump DAFNE curriculum without personalisation. The Glasgow educator explained that there was no time to personalise the lesson plan but agreed to remedy for future courses.
Essential learning outcomes
The FA recorded (with evidence) if all essential learning outcomes were met in the sessions observed. Sessions were recorded as having met all learning outcomes: ‘yes’ or ‘no’ or partially achieving essential learning outcomes. Table 45 provides a summary.
TABLE 45
Summary of essential outcomes achieved
For three sessions (‘Insulin dose adjustment theory and basal rate testing’, ‘Setting up the bolus wizard’ and ‘Alcohol’) all of the centres met all of the essential learning outcomes. For the ‘Exercise’ session, seven centres met the essential learning outcomes and the remaining centre (Sheffield) met 95% of learning outcomes.
For the dose escalation and reduction sessions, all of the centres either met or partly met all of the essential learning outcomes. For the centres that partly met the learning outcomes for these sessions, 80–98% of learning outcomes were met.
The essential learning outcomes for the session ‘Daily goals, blood glucose results and insulin doses’ were partly met at seven of the eight centres and fully met at one centre (100%). It is important to note for this session, which is delivered at the beginning and end of each day, it is expected that some essential learning outcomes will not be covered in one session, as it is guided by situations that the patients have recorded in their diaries. During the DAFNE course, as new situations are observed, further essential learning outcomes are generally covered.
Although not essential for the FT, the FA observed the ‘Lunchtime CP (carbohydrate portion)’ and the ‘Corrections’ sessions at some centres.
Overall fidelity assessment concerns and action plans
The FA was asked to make an overall assessment of whether or not there were any major concerns about the delivery of the pump course and, if there were, any recommended actions to be taken. These are summarised by centre in Table 46.
TABLE 46
Concerns and actions required per centre
Conclusion
Overall, the pump courses appear to have been delivered according to the pump course curriculum. The pump courses observed seem representative of pump courses on REPOSE in terms of course characteristics. The pre-course session was delivered consistently and met the objectives set. All pump courses were planned to run in a logical order within the time frame specified. There were problems with the term ‘rebound hyperglycaemia’ being used (three centres) and non-personalisation of lesson plan (one centre).
Generally, essential learning outcomes were consistently delivered during the sessions. The session ‘Daily goals, blood glucose results and insulin doses’ had the lowest percentage of essential outcomes met. This is not unusual for this session, when learning outcomes are met during the week of the course. In standard care, learning outcomes may also be omitted in other sessions during the week of the DAFNE course, but are subsequently covered in other sessions. This can be for various reasons, for example more pressing issues and questions raised by the participants. With appropriate timetabling and timings allocated to sessions, there should be sufficient time for experienced educators to deliver all of the essential learning outcomes for all sessions. The key thing is that educators have awareness of any learning outcomes that have been missed and can produce a strategy for how they will incorporate the missed content at another relevant stage of the week, or indeed at the 6-week follow-up session if necessary.
The quality assurance programme for MDI DAFNE courses in standard care audits the entire DAFNE course week, whereas the REPOSE FT was restricted to 1 day of the course. Therefore, although the quality assurance programme of MDI courses can examine if missed learning outcomes are covered in later sessions, this was not possible for the REPOSE FT of the pump courses, and is a limitation. Nevertheless, the number of missed learning outcomes was still low.
- Results of the randomised controlled trial - A cluster randomised trial, cost-ef...Results of the randomised controlled trial - A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial
- Assessment of factors relevant to the NHS and other parties - Adalimumab, etaner...Assessment of factors relevant to the NHS and other parties - Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation
- Economic model: evidence-based pathway - Risk assessments and structured care in...Economic model: evidence-based pathway - Risk assessments and structured care interventions for prevention of foot ulceration in diabetes: development and validation of a prognostic model
- Introduction - Clinical effectiveness and patient perspectives of different trea...Introduction - Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis
- Trial management, governance and conduct - The Age of BLood Evaluation (ABLE) ra...Trial management, governance and conduct - The Age of BLood Evaluation (ABLE) randomised controlled trial: description of the UK-funded arm of the international trial, the UK cost–utility analysis and secondary analyses exploring factors associated with health-related quality of life and health-care costs during the 12-month follow-up
Your browsing activity is empty.
Activity recording is turned off.
See more...