Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Heller S, White D, Lee E, et al. A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial. Southampton (UK): NIHR Journals Library; 2017 Apr. (Health Technology Assessment, No. 21.20.)

A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial.
Show detailsCost of insulin pumps and consumables
The weighted average cost of an insulin pump from the pump costing survey was £2571. The cost of insulin pumps was converted into a yearly cost using annuitisation. The lifetime of the insulin pumps was taken to be 4.5 years and the discount rate was that used by NICE (3.5%).22 This gave a weighted average yearly cost of insulin pumps to be £627. The weighted average yearly cost of insulin pump consumables was £1433.
Within-trial cost-effectiveness analysis
Base-case analysis
The results of the within-trial cost-effectiveness analysis are presented using a confidence ellipse in Figure 13. In the base case, pump + DAFNE was dominated by MDI + DAFNE, as pump + DAFNE produced fewer mean QALYs at a higher mean cost. The confidence ellipse shows that pump + DAFNE was associated with statistically significantly higher costs than MDI + DAFNE at the 5% significance level, as the confidence ellipse does not cross the x-axis at £0. The confidence ellipse also shows that pump + DAFNE was not associated with statistically significantly lower QALYs than MDI + DAFNE at the 5% significance level. This is because the confidence ellipse crosses the y-axis of the graph at 0. Another point to note is that the confidence ellipses do not cross a threshold ICER of £20,000 per QALY gained; therefore, the ICER of pump + DAFNE compared with MDI + DAFNE is greater than £20,000 per QALY gained at the 95% confidence level.

FIGURE 13
Confidence ellipse for the base-case within-trial analysis: controlling for baseline utility.
The cost-effectiveness acceptability curve is presented in Figure 14. It shows that pump + DAFNE has a 0.0% chance of being considered cost-effective at threshold ICERs of £20,000 per QALY gained and £30,000 per QALY gained. This is important as, based on the data in the REPOSE Trial, pump + DAFNE has a 0% probability of being cost-effective at the thresholds used by NICE in the UK for decision-making.22
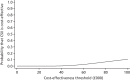
FIGURE 14
Cost-effectiveness acceptability curve for the base-case within-trial analysis: controlling for baseline utility.
Table 47 presents the incremental cost and QALY outcomes of pump + DAFNE compared with MDI + DAFNE in each year of the trial and for both years combined. In the base case, the incremental cost in year 2 is lower than the cost in year 1. This is probably due to (1) treatment switching and (2) the rate of DKAs and severe hypoglycaemic events being noticeably lower in the pump + DAFNE arm in the second year than in the first year. The incremental QALYs are negative in the first year and positive in the second year. However, in neither year is this result statistically significant and, in both years, the central estimates are less than one-hundredth of a QALY. This is not unusual in diabetes trials, in which the crucial QALY gains due to an intervention come much later in the patient experience because of a reduced risk of long-term complications.
TABLE 47
Within-trial cost-effectiveness analysis results of pump vs. MDI, both with DAFNE structured education
Summary of the scenario analyses
The following scenario analyses were undertaken to explore structural uncertainty in the base-case analysis:
- per-protocol population
- missing cost and QALY data were imputed
- QALYs measured by the SF-6D were used instead of QALYs measured using the EQ-5D
- imputed data and QALYs measured by SF-6D QALYs
- pump costs measured by Riemsma et al.8 were used
- Riemsma et al.8 pump costs were used and missing data were imputed
- the cost of pumps and consumables are 25% lower
- the cost of pumps and consumables are 25% lower in a per-protocol population
- the cost of pumps and consumables are 50% lower
- the cost of pumps and consumables are 50% lower in a per-protocol population.
In the first scenario, EEACT was conducted in the per-protocol population as this was a pre-specified subgroup analysis (see Chapter 2, Population and subgroups for analysis). In the second scenario, missing cost and QALY data were imputed to explore the uncertainty that may result from having incomplete data, as in the base-case analysis only 78.85% of people had complete cost and QALY data. Details of the imputation procedure used are provided in Chapter 3 (see Estimating the within-trial cost effects and Estimating within-trial quality-adjusted life-year effects using EuroQol-5 Dimensions and Short Form questionnaire-12 items). A further sensitivity analysis was conducted, for which the SF-6D measure, instead of the EQ-5D, was used to calculate QALYs. This scenario analysis was conducted to explore if changing the preference-based measure of health changed the estimated QALY values significantly enough to potentially change the conclusions on cost-effectiveness (see Chapter 3, Estimating within-trial quality-adjusted life-year effects using EuroQol-5 Dimensions and Short Form questionnaire-12 items). Uncertainty in insulin pump costs was also explored, to see if significant discounts from the prices observed at the REPOSE Trial sites would lead to pump + DAFNE being considered to be cost-effective compared with MDI + DAFNE. Several of these uncertainties were also combined in other scenarios to determine if the joint effect of the uncertainties had any meaningful effect on the conclusions.
Results of the scenario analyses
The results of the scenario analyses are also presented in Table 47. It is clear that pump + DAFNE compared with MDI + DAFNE generated fewer QALYS at a higher cost in all analyses, apart from those scenarios conducted in the per-protocol population. The lowest ICER is observed in the scenario for which the per-protocol population is used, and there is a cost reduction in insulin pumps and consumables of 50%. The ICER in this scenario is £552,866, which is above the £20,000–30,000 per QALY gained threshold considered by NICE.22 Therefore, based on the data observed directly in the REPOSE Trial, pump + DAFNE would be unlikely to be considered cost-effective if it were to be assessed by NICE.
Clinical evidence used to inform the cost-effectiveness of pump + Dose Adjustment For Normal Eating compared with multiple daily injection + Dose Adjustment For Normal Eating
This section details the results of the statistical models fitted to estimate the incidence of treatment switching, HbA1c, the risk of severe hypoglycaemia, the risk of DKA, the cost of insulin, the cost of diabetes-related contacts and the cost of insulin pumps. The parameters presented in these statistical models were directly included in the long-term health economic model, except for the risk of severe hypoglycaemia and the risk of DKA, for which simulations of the expected number of events were inputted into the long-term health economic model. In the PSA, the uncertainty in the parameters of these statistical models was assumed to follow a multivariate normal distribution. Variance–covariance matrices are available from the authors on request.
Treatment switching
The results of the exponential and Weibull parametric survival models for individuals randomised to MDI + DAFNE and pump + DAFNE are given in Table 48. The results for the Gompertz, log-logistic and log-normal parametric models are given in Appendix 14. It was not possible to estimate a survival curve using a generalised gamma distribution, as the model did not converge in either trial arm.
TABLE 48
Results of the exponential and Weibull parametric survival models fitted to individuals in both arms of the REPOSE Trial
In the pump + DAFNE arm, it was predicted that an individual was more likely to switch treatment if they had a severe hypoglycaemic event or if they had a higher HbA1c. It was also observed that an individual was less likely to switch from CSII to MDI if they had experienced a DKA event in the previous year. All of these results are statistically significant at the 5% level in the Weibull and exponential models, except for the effect of HbA1c on the probability of switching in the exponential model.
In the MDI + DAFNE arm, the relationships between HbA1c, number of severe hypoglycaemic episodes in the year prior to switching and the number of DKAs in the year prior to switching worked in a similar way to the pump + DAFNE arm. It should be noted that the effect sizes are different in the two arms for different covariates. In the MDI + DAFNE arm, all of the coefficients were statistically significant at the 5% level.
Table 49 shows the AIC and BIC for the different survival models fitted to pump + DAFNE individuals and MDI + DAFNE individuals. In the pump + DAFNE arm, the curve with lowest AIC and BIC was the exponential model. For the MDI + DAFNE individuals, the curve with the lowest AIC was the Weibull model and the curve with the lowest BIC was the exponential model.
TABLE 49
Summary of the AIC and the BIC for the fitted survival curves used in the long-term modelling
The visual plot of the survival curves for remaining on the initially allocated treatment for the pump + DAFNE and MDI + DAFNE individuals are presented in Figures 15 and 16, respectively. The curves fitted to the treatment switching data show a reasonable fit to the Kaplan–Meier curves for individuals who were randomised to pump + DAFNE and MDI + DAFNE. A visual inspection of curves showed that the exponential curve for pump + DAFNE showed the best fit to the Kaplan–Meier curve at the 1- and 2-year time points, although a visual check does not indicate that it has the best fit for all of the time points. A visual inspection of the curves in the MDI + DAFNE arm shows that all curves had a reasonable fit to the Kaplan–Meier curve, except the exponential curve, which had a poor fit to the Kaplan–Meier curve, especially in the first year.
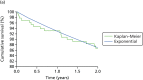
FIGURE 15
A visual plot of the Kaplan–Meier and parametric survival curves for those individuals who were randomised to pump with DAFNE. (a) Exponential curve; (b) Weibull curve; (c) Gompertz curve; (d) log-logistic curve; and (e) log-normal curve.
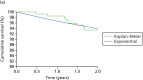
FIGURE 16
A visual plot of the Kaplan–Meier and parametric survival curves for those individuals who were randomised to MDI with DAFNE. (a) Exponential curve; (b) Weibull curve; (c) Gompertz curve; (d) log-logistic curve; and (e) log-normal curve.
In the base case, the exponential model will be used to model the treatment switching of individuals in the pump + DAFNE arm, and the Weibull model will be used to model the treatment switching of individuals in the MDI + DAFNE arm. Uncertainties in the coefficients of these models were included in the PSA using a multivariate normal distribution. Scenario analyses were conducted when the risk of switching treatment was estimated directly from the Kaplan–Meier curves at years 1 and 2. The risk of switching treatment for a pump individual, given that he/she was receiving pump therapy at the start of the year, was 6.94% at year 1 and 6.89% at year 2. The risk of switching for a MDI individual, given that they were receiving MDI at the start of the year, was 1.58% in year 1 and 5.13% in year 2.
Glycated haemoglobin
The results of the beta regressions used to model the effectiveness of pump + DAFNE versus MDI + DAFNE in the ITT population is given in Table 50. Pump + DAFNE has a coefficient on HbA1c reduction of –0.056 at year 1 and –0.018 at year 2; neither result was statistically significant at the 5% significance level. These coefficients are not easily interpretable, as changes in HbA1c, as the mean effects are estimated using a logit link function.
TABLE 50
Effect of pump compared with MDI for all individuals in the ITT population
In the per-protocol population as in this group, the statistical analysis showed a significant improvement in HbA1c for pump + DAFNE. The results of the beta regression fitted to the per-protocol population is given in Table 51. Pump + DAFNE was associated with a coefficient of –0.056 in year 1 and –0.047 in year 2. Neither of these coefficients was statistically significant at the 5% level. The uncertainty in the coefficients in these statistical models was included in the PSA of the Sheffield Type 1 Diabetes Model by sampling the coefficients from a multivariate normal distribution using the known variance covariance matrices.
TABLE 51
Effect of pump compared with MDI for all individuals in the per-protocol population
Severe hypoglycaemia and diabetic ketoacidosis
The results of the negative binomial regressions for the incidence of severe hypoglycaemia are given in Table 52. The regression predicts that the number of severe hypoglycaemic events increases as a patient’s HbA1c decreases; however, this result is not statistically significant at the 5% level in the second year. Pump + DAFNE compared with MDI + DAFNE was associated with a higher incidence of severe hypoglycaemia in year 1 and a lower incidence of severe hypoglycaemia in year 2. Neither result was statistically significant at the 5% level.
TABLE 52
Negative binomial model fitted to the incidence of severe hypoglycaemia at baseline, 1 and 2 years
The results of the negative binomial regressions for the incidence of DKA are given in Table 53. The predicted number of DKAs increase with a patient’s HbA1c. This result is statistically significant in the first year, but not in the second year. Pump + DAFNE when compared with MDI + DAFNE was associated with a higher incidence of DKA in year 1 and a lower incidence of DKA in year 2. Neither result was statistically significant at the 5% level.
TABLE 53
Negative binomial model fitted to the incidence of DKA at baseline, 1 and 2 years
Cost of insulin, diabetes-related contacts and insulin pumps
The results of the analyses on the cost of insulin used in the long-term modelling are given in Table 54. Pump treatment was associated with a reduction in insulin costs of around £500 per annum in years 1 and 2 compared with MDI treatment. This result was statistically significant in both years. Switching from pump to MDI treatment was associated with an increase in insulin costs of around £550 in year 1 and £150 in year 2. No coefficient could be estimated on whether or not a MDI individual switched to pump, as this parameter was collinear with model parameters. Switching from MDI to pump was associated with a decrease in insulin costs of around £350 in year 2. All of these results were statistically significant at the 5% level.
TABLE 54
Result of seemingly unrelated regression on insulin costs (£) in years 1 and 2
The model uses the parameters in the regression model presented in Table 53 to estimate their cost of insulin. For example, the formula used to estimate the cost of insulin beyond the second year in a deterministic analysis is as follows:
The results of the analyses on the cost of diabetes-related contacts are given in Table 55. Pump + DAFNE was associated with an increase in the cost of diabetes-related contacts of £130 per annum in year 1 and £90 per annum in year 2 compared with MDI. These results were not statistically significant in year 1 or 2. Switching from insulin pump therapy to MDI was associated with an increase in diabetes-related contact costs of £280 per annum in year 1 and a decrease of £50 per annum in year 2. Switching from MDI to insulin pump therapy was associated with an increase in diabetes-related contact costs of £730 per annum in year 1 and £300 per annum in year 2. None of the treatment switching coefficients was statistically significant at the 5% significance level.
TABLE 55
Result of seemingly unrelated regression on diabetes-related contact costs (£) in years 1 and 2
The cost of DRCs for each individual was predicted using the values in Table 55. For example, in a deterministic model run, a patient’s cost of DRCs in the first year was given by the following formula:
The results of the analyses on the cost in insulin pump therapy (includes the yearly cost of the pump and the associated consumables) is given in Table 56. Insulin pump therapy was associated with a cost per annum of £2056 in year 1 and £2051 in year 2. Switching from insulin pump therapy to MDI was associated with a decrease in insulin pump therapy costs of £1140 in year 1 and a reduction of £910 in year 2. Switching from MDI to insulin pump therapy was associated with an increase in costs of £840 in year 1 and £130 in year 2. All of these results were statistically significant at 5% level.
TABLE 56
Result of seemingly unrelated regression on insulin pump therapy costs (£) in years 1 and 2
The coefficients in these statistical models were included in the model to predict the cost of insulin, diabetes-related contact and insulin pump therapy. The uncertainty in these parameters was included in the PSA by using a multivariate normal distribution for each regression equation.
Long-term cost-effectiveness
Base-case analysis
The results of the long-term cost-effectiveness analysis base case results using the PSA is shown in Table 57. For the pump arm, the mean costs of the intervention are £42,143 discounted over the lifetime horizon, which compares with £20,398 for the MDI arm. The difference between the intervention costs for the two arms is £21,745. AE costs are slightly lower in the pump arm, £1040 versus £1509, a mean lifetime saving of £470 per person. Complication costs are also lower £57,435 versus £59,877, a mean lifetime saving of £2443 per person, which is mostly due to reductions in the occurrence of end-stage renal failure in the nephropathy complications. The net incremental lifetime cost of pump versus MDI is therefore estimated as £18,832 (95% CI £535 to £34,978) per person.
TABLE 57
Base-case PSA results from the Sheffield Type 1 Diabetes Policy Model
The ‘QALYs lived without diabetic complications’ captures all of the QALYs gains from the increased life expectancy of patients who receive pump + DAFNE prior to adjusting their utility downwards for the incidence of diabetic complications. The ‘QALYs lived without complications’ in the pump + DAFNE arm is 14.3898 QALYs compared with 14.2894 QALYs in the MDI + DAFNE arm, a mean increase of 0.1005 QALYs. The QALYs lost because of AEs are slightly lower in the pump + DAFNE arm than in the MDI + DAFNE arm, –0.0068 versus –0.0102 QALYs, leading to a mean increase of 0.0034 QALYs in favour of pump + DAFNE. The overall QALYs lost because of the incidence of diabetic complications was again slightly lower in the pump + DAFNE arm than in the MDI + DAFNE arm, –1.2725 versus –1.2947 QALYs, a mean increase in lifetime QALYs of 0.0222. However, pump + DAFNE is not associated with a mean increase in QALYs for each of the individual long-term diabetic complications. This is because although the incidence of the complications is expected to be lower in the pump + DAFNE arm, as they have a lower HbA1c, people are also expected to live longer in the pump + DAFNE arm, so they may be at a greater overall risk of suffering a diabetic complication within their lifetime. The net incremental QALY gain per person is 0.1260 QALYs (95% CI –0.7381 to 0.9705 QALYs) per person.
Pump + DAFNE generated more QALYs – 0.1260 QALYs (95% CI –0.7381 to 0.9705 QALYs) – at a higher incremental cost of £18,832 (95% CI £535 to £34,978) than MDI + DAFNE. The ICER associated with pump + DAFNE was £149,483 per QALY gained. This is outside the range of £20,000–30,000 per QALY gained at which NICE would usually consider to be cost-effective. Figure 17 shows the base-case cost-effectiveness plane for the PSA. It is clear that, when using the £20,000 per QALY gained threshold, most PSA runs lie in the region where pump + DAFNE would not be considered to be cost-effective, as they are above the £20,000 per QALY gained line. The cost-effectiveness acceptability curve presented in Figure 18 shows the probability that pump + DAFNE and MDI + DAFNE are cost-effective across a range of cost-effectiveness thresholds.152 It is clear that MDI + DAFNE has a higher probability of being cost-effective than pump + DAFNE at all cost-effectiveness thresholds in the range of £0–50,000 per QALY gained.
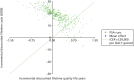
FIGURE 17
Cost-effectiveness plane of the base-case analysis using the Sheffield Type 1 Diabetes Policy Model.
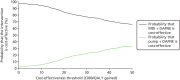
FIGURE 18
Cost-effectiveness acceptability curve of the base-case analysis using the Sheffield Type 1 Diabetes Policy Model.
The modelled lifetime incidence of diabetic complications in the PSA is given in Table 58. Pump + DAFNE was associated with fewer diabetic complications than MDI + DAFNE. However, this is to be expected, as the treatment effect coefficient was negative in the beta regression that was used to estimate HbA1c in the model (see Tables 50 and 51). It should also be noted that the incidence of proliferative retinopathy, macular oedema and blindness were higher in the pump + DAFNE arm than the MDI + DAFNE arm. This seems to be counterintuitive; however, there are two effects. The first is that, in a given year, patients in the pump + DAFNE arm are at a lower risk of these complications. The second effect is that as the HbA1c of patients in the pump + DAFNE arm is, on average, lower than the MDI + DAFNE arm then they are expected to live longer, increasing their absolute risk of experiencing a complication. For the proliferative retinopathy, macular oedema and blindness complications, the increased risk as a result of living longer outweighs the decreased annual risk of a complication as a result of these patients having a lower HbA1c value.
TABLE 58
Lifetime incidence of diabetic complications per 100 years for an adult with T1DM in the base-case economic model
Summary of the scenario analyses in the long-term model
The following scenario analyses were conducted in the long-term modelling:
- pump costs estimated using data in Riemsma et al.8 on the yearly cost of insulin pump therapy
- a 25% price reduction in insulin pumps and consumables
- a 50% price reduction in insulin pumps and consumables
- the ITT estimate of treatment effect was used
- the ITT estimate of treatment effect was used and there was no change in HbA1c if an individual switches treatment
- post-trial HbA1c progression in both arms is estimated from the DCCT at +0.045% per annum
- individuals return to their baseline HbA1c after 3 years and experience no progression in their HbA1c thereafter
- HbA1c effects occur one model cycle earlier
- individuals return to baseline risk of hypoglycaemic episodes and DKA at 3 years
- treatment switching probabilities were estimated directly from the Kaplan–Meier curves
- subgroup – individuals with a baseline HbA1c of < 8.5% (69 mmol/mol)
- subgroup – individuals with a baseline HbA1c of ≥ 8.5% (69 mmol/mol)
- subgroup – individuals with a baseline HbA1c of ≥ 7.5% (58 mmol/mol)
- subgroup – individuals with a baseline HbA1c of ≥ 7.5% (58 mmol/mol) and < 8.5% (69 mmol/mol)
- subgroup – individuals with a baseline HbA1c of ≥ 8.5% (69 mmol/mol) and < 9.5% (80 mmol/mol)
- subgroup – individuals with a baseline HbA1c of ≥ 9.5% (80 mmol/mol)
- subgroup – individuals in the per-protocol population
- subgroup – individuals in the per-protocol population and no treatment switching is included in the model.
Structural uncertainty and potential subgroup effects were explored in the scenario analyses with the long-term model.
Much like the EEACT, uncertainty due to potential decreases in price of insulin pumps was explored in these scenario analyses.
Four further scenario analyses were conducted around the different methods that could be used to estimate each patient’s HbA1c in the model. A scenario analysis was conducted in which HbA1c was estimated using beta regression in the ITT population rather than the per-protocol population. As the ITT population includes switchers in their originally assigned treatment groups, a further scenario analysis was conducted using the regression estimated in the ITT population where the individuals in the model did not experience a change in HbA1c when they switched treatment, as these effects were already included in the estimate of the relative treatment effect of pump + DAFNE versus MDI + DAFNE. Uncertainty in the long-term changes in HbA1c was explored by using data observed in the DDCT trial for both of the model arms. As there was no information in the DCCT trial on different HbA1c trajectories for pump or MDI users, the same trajectory was used in both model arms, which effectively assumes that the treatment effect for pump users in the REPOSE Trial is maintained for a lifetime. Uncertainty in the HbA1c of individuals after the REPOSE Trial was also explored by assuming that all individuals returned to their baseline HbA1c after the third model year. This is a very conservative assumption, but gives some idea of the least favourable scenario to pump + DAFNE. The effect of assuming that changes in HbA1c occurred one model cycle (1 year) earlier than the base case on the model outcomes was explored. Full details on the reason for and rationale behind scenario analyses 4–8 are given earlier (see Chapter 3, Estimation of each individual’s glycated haemoglobin and Duration of treatment effectiveness beyond the trial period).
The effect of assuming that the second-year risk functions for severe hypoglycaemia and DKA were applied for the rest of an individual’s lifetime was tested by instead assuming that individuals in both arms returned to their risk of severe hypoglycaemia and DKA at baseline. Full details on this scenario analysis is given earlier (see Chapter 3, Estimating severe hypoglycaemic events and diabetic ketoacidosis events).
Finally, the validity of the treatment switching models was testing by assuming directly using the risks of switching observed in the Kaplan–Meier curves. In this scenario, treatment switching was a random event that did not depend on HbA1c, number of severe hypoglycaemic episodes in the last year and number of DKAs last year, as was used in the base case. Full details on this scenario analysis are given earlier (see Chapter 3, Incorporating treatment switching).
Further to the one-way scenario analyses, a threshold analysis was conducted to determine the HbA1c fall that future pumps would need to have to be considered cost-effective. Full details on this threshold analysis are given earlier (see Chapter 3, Threshold analysis).
Results of the one-way scenario analyses
The one-way scenario analyses are presented in Table 59. The ICER did not fall below £30,000 per QALY gained in any of the scenario analyses. Furthermore, the subgroup analyses did not indicate that the ICER for pump + DAFNE compared with MDI + DAFNE will fall below £30,000 per QALY gained for any identified pre-specified subgroup in the REPOSE Trial patient population. The most favourable ICER to pump + DAFNE was observed when a 50% reduction in the price of insulin and insulin pump consumables was modelled; however, the ICER in this scenario was £46,578, which is above the maximum acceptable ICER range of £20,000–30,000 that is usually used by UK decision-makers when deciding whether or not a health technology is cost-effective. Although the ICERs are more favourable to pump + DAFNE in the long-term modelling than in the EEACT, the long-term modelling does not indicate that pump + DAFNE is likely to be considered a cost-effective treatment pathway if it were to be appraised by NICE.
TABLE 59
One-way sensitivity analyses and subgroup analyses conducted using the Sheffield Type 1 Diabetes Policy Model
An important scenario to note is the one in which the HbA1c effects occur one model cycle earlier.
Results of the threshold analysis
The results of the two-way price and effectiveness threshold analysis for a certain reduction in HbA1c are given in Table 60. When the annual pump cost is assumed to be £2060 then the analysis shows that the reduction in HbA1c (for CSII compared with MDI) would need to be ≥ 11 mmol/mol (1.0%) for pumps to be considered cost-effective (ICER £22,757). When the annual cost is 25% lower (£1545) then a HbA1c reduction of > 7.7 mmol/mol (0.7%) would be needed to have an ICER of < £20,000 per QALY gained. When the annual cost is halved (£1030) then a HbA1c reduction of 4.4 mmol/mol (0.4%) would be sufficient to have an ICER of < £20,000 per QALY gained.
TABLE 60
Incremental cost-effectiveness ratio associated with CSII for different HbA1c reductions (for all adults with T1DM) and annualised prices (£) of insulin pumps and insulin pump consumables when no uncertainty in the HbA1c reduction is assumed
The results of the two-way price and effectiveness threshold analysis for when the uncertainty in the treatment effect is estimated using the dispersion parameter formula used in the REPOSE Trial is given in Table 61. When the annual cost is assumed to be £2060 then the analysis shows that the reduction in HbA1c (for pumps vs. MDI) would need to be > 9.8 mmol/mol (0.9%) for pumps to have an ICER of < £30,000 per QALY gained. When the annual cost of insulin pumps and consumables is 25% lower (£1545), then a HbA1c reduction of 7 mmol/mol (0.6%) would be needed to have an ICER of < £30,000 per QALY gained. When the annual cost is halved (£1030) then a HbA1c reduction of 4.4 mmol/mol (0.4%) would be sufficient to have an ICER of < £20,000 per QALY gained.
The threshold analysis indicates if a future study of pumps + DAFNE versus MDI + DAFNE were to be conducted then the cost of insulin pumps and their associated consumables should be taken into account when determining the appropriate effect size to power the study on. At current prices, per-protocol effect sizes of > 5.5 mmol/mol would be required in the whole population who would be eligible for pump therapy for insulin pumps to have an ICER in the £20,000–30,000 per QALY gained range at which NICE is likely to consider them to be a cost-effective use of NHS resources.
Summary of the economic analysis results
None of the analyses conducted in the EEACT or the long-term modelling had an ICER of < £30,000 per QALY gained. Furthermore, no subgroup was identified for which the ICER was < £30,000 per QALY gained. This indicates that pump + DAFNE is unlikely to be considered to be a cost-effective use of NHS resources by NICE in the UK compared with the current practice of MDI + DAFNE, as the ICERs are all above the ICER range of £20,000–30,000 per QALY gained, which is usually used by NICE to determine the cost-effectiveness of health technologies. The findings of this analysis are consistent with the current recommended care pathway for adults with T1DM in the UK, who should be offered structured education with MDI, ideally around 12 months after diagnosis (but failing that at any later stage).
- Cost of insulin pumps and consumables
- Within-trial cost-effectiveness analysis
- Clinical evidence used to inform the cost-effectiveness of pump + Dose Adjustment For Normal Eating compared with multiple daily injection + Dose Adjustment For Normal Eating
- Long-term cost-effectiveness
- Summary of the economic analysis results
- Results of the economic evaluation - A cluster randomised trial, cost-effectiven...Results of the economic evaluation - A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial
- Discussion - The Effectiveness, cost-effectiveness and acceptability of Communit...Discussion - The Effectiveness, cost-effectiveness and acceptability of Community versus Hospital Eye Service follow-up for patients with neovascular age-related macular degeneration with quiescent disease (ECHoES): a virtual randomised balanced incomplete block trial
- Health economics analysis: results - Standard threshold laser versus subthreshol...Health economics analysis: results - Standard threshold laser versus subthreshold micropulse laser for adults with diabetic macular oedema: the DIAMONDS non-inferiority RCT
- Clinical effectiveness and patient perspectives of different treatment strategie...Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis
- Adenoidectomy with or without grommets for children with otitis media: an indivi...Adenoidectomy with or without grommets for children with otitis media: an individual patient data meta-analysis
Your browsing activity is empty.
Activity recording is turned off.
See more...