NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walsh TS, Stanworth S, Boyd J, et al. The Age of BLood Evaluation (ABLE) randomised controlled trial: description of the UK-funded arm of the international trial, the UK cost–utility analysis and secondary analyses exploring factors associated with health-related quality of life and health-care costs during the 12-month follow-up. Southampton (UK): NIHR Journals Library; 2017 Oct. (Health Technology Assessment, No. 21.62.)

The Age of BLood Evaluation (ABLE) randomised controlled trial: description of the UK-funded arm of the international trial, the UK cost–utility analysis and secondary analyses exploring factors associated with health-related quality of life and health-care costs during the 12-month follow-up.
Show detailsApprovals
The UK ABLE trial required a complex set of approvals to be in place to commence recruitment. These reflected the international nature of the trial, the large number of centres involved, and the need to set up both ICUs sand blood banks to enable randomisation and intervention management (Table 2). In total, over 25 separate contracts/agreements were required to be set up and agreed between the sponsor’s legal department and various organisations. This took considerable time and effort, and resulted in delays while agreements were finalised.
TABLE 2
Summary of the range of contracts and approvals
One practical issue related to the definition of the UK protocol. The Canadian co-ordinating centre ran the international trial and acted as sponsor for the Canadian sites, and a Canadian version of the protocol was used. In the UK, with separate funding from the NIHR HTA programme to the University of Edinburgh, the UK co-sponsors (University of Edinburgh and NHS Lothian) required a separate document representing the UK protocol that was legally distinct from the Canadian protocol, despite the unification of protocols into a single international trial. A separate UK protocol therefore needed to be written and approved by the ethics committee.
Ethics considerations
The different legal frameworks for incapacitated patients in England and Scotland required separate ethics applications and approval processes, with trial materials using appropriate terminology for surrogate decision-makers. In many international centres, a true waiver of consent was granted because the two intervention groups were both part of standard care, and the decision to transfuse RBCs was determined by clinicians and not in accordance with the trial protocol. In addition, delays to transfusion were considered unacceptable, as these might have delayed treatment and reduced patient safety. UK law did not allow this approach, but resulted in different approaches in England/Northern Ireland from those used in Scotland (see Chapter 2). These differences made recruitment more difficult for some patients in Scotland, which adversely affected recruitment rates. Approval of consent by telephone was important in Scotland to decrease this impact. In all cases, the patient was approached wherever possible to obtain permission to continue in the trial if they survived their ICU admission and regained capacity. This took considerable research nurse resource and, in some cases, was difficult to achieve before patients were discharged home. The different approaches required to obtain consent or lack of objection to participate and remain in the trial illustrate the complex and time-consuming processes involved in trials recruiting critically ill, incapacitated patients with time-sensitive recruitment windows and interventions.
Site set-up
Site set-up required both the clinical (in the ICUs) and blood bank teams to be trained and SOPs established to execute the randomisation and group allocation. Blood bank set-up was challenging, but was facilitated by specialist blood bank co-ordinators employed for the purpose of set-up, monitoring and support throughout the trial. A detailed set of protocols and procedures was developed to enable potential participants to be rapidly screened to ascertain if allocation to ‘fresh’ RBCs was feasible based on blood group, cross-match and blood availability. In addition, procedures to ensure checking, blinding and modified blood issue procedures were established. There were frequently delays in this set-up as a result of the intense pressure many NHS blood banks worked under, and competing activities such as inspections and audits. A limited number of technician staff were generally available to undertake randomisation and group allocation procedures, which limited recruitment periods to weekdays in most centres. Despite dedicated funding for this activity, many blood banks did not have access to additional staff to support the trial beyond routine NHS work. This resulted in many potential participants being ‘missed’, and limited participation to mainly patients in whom first RBC transfusions were prescribed during weekday working hours. In addition, procedures to ‘tag’ participants on local blood bank systems to ensure that subsequent requests for RBCs maintained both group allocation and blinding were necessary, but did not delay blood issue. A modified blood-checking procedure at the bedside by clinical staff that ensured that national standards were adhered to but that maintained the blinding of RBC storage age was also needed in all centres.
Although the ABLE study was a trial based in the ICU, the major logistic challenges were in the blood banks. This was the first large, multicentre UK trial that required multiple NHS blood banks to allocate trial participants to receive different blood products under emergency conditions while maintaining blinding from clinical teams. The success of the trial reflected very considerable effort and support by blood bank staff and the blood bank co-ordinators working on the trial.
Timelines
A summary of the major trial set-up timelines is shown in Figure 4. We found a wide variation between sites for times to R&D approvals, and times to recruitment of the first patient from final approval (Figure 5). The reasons were multifactorial and varied between centres. Delays with final contracts between the sponsor and study site were prevalent, as a result of the slow responses from legal teams. These resulted in a ‘knock-on’ effect on final R&D approval; this was also delayed in some centres until clinical sites were ready to start screening, to minimise the impact of NIHR metrics that recorded time to first recruit. For clinical set-up, delays in blood bank training were a major source of delay in some centres. Frequent reasons were competing priorities, such as national inspections of the laboratory service (especially the Medicines and Healthcare products Regulatory Agency), unrelated to the ABLE trial, and staff shortages such that the organisational changes required to run the trial were delayed. The funding of dedicated blood bank co-ordinators in the trial was vital to minimise these delays.

FIGURE 4
Summary of the major logistic milestones during the set-up of the UK ABLE trial. CSP, Coordinated System for gaining NHS Permission.
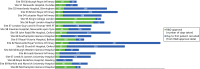
FIGURE 5
Summary of the time (days) for R&D approval and the time (days) from approval to first recruitment for the 20 ICUs that participated in the UK ABLE trial. FT, foundation trust.
Trial management
A TMG met every 4–6 weeks throughout recruitment by teleconference. These meetings were supplemented by regular investigator teleconferences with research staff (from both ICUs and blood banks), at which representatives from all sites were encouraged to share both positive and negative experiences of recruitment and trial conduct. These meetings were supplemented by regular newsletters and recruitment tables circulated by e-mail. Screening logs were returned and examined in real time. This was useful for a number of reasons: (1) it provided a rapid indication of when staffing or other problems were affecting recruitment; and (2) it provided valuable data on the reasons that potential patients were ‘missed’, which were collated and shared with all sites. This enabled a focus on solving common recruitment problems that occur across all trial sites. For example, it rapidly became apparent that the most common reason for missed recruitment was that the first transfusion took place out of hours when research staff and/or blood bank staff were unavailable. Several ICUs used local audit to change their practice and minimise night-time transfusions, consistent with current guidance.
These data enabled the reasons for non-enrolment of eligible patients to be clearly tracked during trial conduct (Table 3) and were used to implement improvements in real time.
TABLE 3
Reasons why eligible patients were not able to be considered for the UK ABLE trial based on screening log information collected during the trial
A continuously updated ‘top tips for recruitment’ checklist and sharing of solutions to problems was a focus of these meetings, which maintained momentum. The ‘top tips’ checklist was used as an audit tool to help each site explore whether or not they could optimise recruitment (Table 4).
TABLE 4
The ‘top tips’ checklist that was developed during the trial based on regular engagement with trial centres
Follow-up
A predefined strategy for follow-up at 6 and 12 months post randomisation was used. First, the patient’s GP was contacted to ascertain survival status. Survivors were sent questionnaires by post, accompanied by a £5 gift token. At 12 months, non-response to postal questionnaire was followed by a second postal questionnaire. Failure to respond to postal follow-up was followed by up to three attempted contacts by telephone to complete the questionnaires. In addition, any queries were resolved by telephone contact, with up to three attempted contacts. As follow-up is known to be challenging in critical care survivors, we collected data to summarise the total time needed to achieve follow-up data. We also analysed the effectiveness of different approaches to follow-up in this population.
Audit data indicated that the average time required for each follow-up was 26 minutes at 6 months and 25 minutes at 12 months. Table 5 shows the success rate of the different follow-up strategies. Telephone contact was attempted at least three times, and this significantly improved follow-up response rates, especially at the 6-month time point.
TABLE 5
Summary of process of follow-up with response rates
Audit of blood transfusion in participating intensive care units
In order to understand how the patients recruited to the ABLE trial compared with all patients receiving blood transfusion in UK ICUs, we undertook an audit of 489 sequential ICU admissions to 15 out of 20 ABLE trial sites. Each site audited at least 30 sequential admissions, and used blood bank data to ascertain and record all RBC transfusions during the hospital stay, including the numbers of transfusions and whether or not the transfusion occurred pre, during or post ICU care.
Transfusion data were unavailable for six patients. The audit showed that 222 out of 483 (46%) of all patients admitted to the typical UK ICUs received a RBC transfusion during their hospitalisation. Transfused patients received a mean of 6.8 RBC units during hospitalisation. Data showed that 110 out of 483 (23%) patients received RBCs prior to ICU admission [49/483 (10%), exclusively pre ICU admission]. These patients were ineligible for the ABLE trial. A total of 135 out of 483 (28%) patients received RBCs during ICU stay [66/483 (14%), exclusively during ICU stay]. The mean RBC use for transfused patients during ICU care was 4.4 RBC units per patient. A total of 73 out of 483 (15%) patients received RBCs during the post-ICU discharge period [26/483 (5%), exclusively during the post-ICU period]. Many patients received RBCs at multiple time points during their hospitalisation.
These data, collected during the ABLE trial recruitment, indicated that many patients received RBCs but were either not eligible for the ABLE trial or were not included.
Concurrently, we examined the screening logs for the ABLE trial and found that of 3754 patients who received RBCs during their ICU care, only 1035 (28%) were eligible for the ABLE trial.
Together, these data clearly showed that, despite the pragmatic design of the trial, the ABLE trial population was only around 25% of ICU admissions, and this accounted for a minority of patients receiving RBCs during ICU care.
- Trial management, governance and conduct - The Age of BLood Evaluation (ABLE) ra...Trial management, governance and conduct - The Age of BLood Evaluation (ABLE) randomised controlled trial: description of the UK-funded arm of the international trial, the UK cost–utility analysis and secondary analyses exploring factors associated with health-related quality of life and health-care costs during the 12-month follow-up
- Assessment of cost-effectiveness - Biomarkers for assessing acute kidney injury ...Assessment of cost-effectiveness - Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis
- Development and validation of pre-eclampsia prediction models - Validation and d...Development and validation of pre-eclampsia prediction models - Validation and development of models using clinical, biochemical and ultrasound markers for predicting pre-eclampsia: an individual participant data meta-analysis
- Danio rerio hexosaminidase B (beta polypeptide) (hexb), transcript variant 2, mR...Danio rerio hexosaminidase B (beta polypeptide) (hexb), transcript variant 2, mRNAgi|836467586|ref|NM_001309863.1|Nucleotide
- Biomarkers for assessing acute kidney injury for people who are being considered...Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis
Your browsing activity is empty.
Activity recording is turned off.
See more...