NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Picot J, Rose M, Cooper K, et al. Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2017 Dec. (Health Technology Assessment, No. 21.79.)

Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation.
Show detailsResults
Quantity and quality of research available
A total of 2068 references were identified by searches (after de-duplication) and two additional references were identified through other sources (Figure 4). We screened the titles and, where available, abstracts of the 2070 references and retrieved full copies of 125 references. We excluded 63 full-text references, the majority because either the intervention (n = 28) or comparator (n = 29) did not meet the inclusion criteria (a list of the excluded studies with reasons for exclusion is presented in Appendix 4). Twenty-four references were designated as ‘unclear’, all of which were conference abstracts (seven47–53 of these could be linked to full papers already either included or excluded and 17 appear to be ongoing or recently completed studies; see Ongoing studies). The remaining 32 references met the inclusion criteria of the systematic review and were included. These 32 references describe 30 separate studies.

FIGURE 4
Flow chart for the identification of studies.
The majority of the 30 studies which met the inclusion criteria for this systematic review evaluated NBI (n = 24), with two of these also evaluating one of the other interventions of interest (NBI and i-scan, n = 1; and NBI and FICE, n = 1). A further four studies evaluated i-scan and a further two studies evaluated FICE. The final tally of included evidence is as shown in Table 4.
TABLE 4
Evidence meeting the criteria for the systematic review
Narrow-band imaging
Twenty-four studies20,54–78 included in the systematic review provided data on the use of NBI for VCE of colorectal polyps. From here on in the report, Kaltenbach and colleagues57,72 and Gupta and colleagues68,73 will be identified by a single study reference to the main source of data (Kaltenbach and colleagues57 and Gupta and colleagues68). Two of these studies, a prospective cohort study by Lee and colleagues77 and a RCT by Kang and colleagues,78 also reported on i-scan and FICE, respectively, and so are also included in our report in the i-scan and FICE sections.
An overview of the characteristics of the included NBI studies is presented in Table 5 (more detailed information is available in the data extraction forms presented in Appendix 3). More than half of the studies were conducted in the USA (14 studies20,54,55,57,58,61,63,64,66,68,69,74–76). Five studies were conducted in Europe (one in the UK,70 two in Italy,59,60 one in Italy and the Netherlands62 and one in Spain65). The remaining five studies were conducted in Asia: two in Japan,56,71 two in South Korea77,78 and one in Australia.67 Seven of the studies focused on diminutive polyps,55,57,59,67,68,76,77 nine focused on small polyps (< 10 mm in size)20,56,60,62,65,70,71,75,78 and eight included polyps of any size.54,58,61,63,64,66,69,74 The studies that included polyps larger than diminutive polyps provided at least one outcome of interest for the subgroup of diminutive polyps. One study, by Hewett and colleagues,54 was restricted to polyps in the rectosigmoid colon.
TABLE 5
Overview of NBI studies
Half of the studies enrolled participants undergoing colonoscopy either for screening, surveillance or because of symptoms,20,57,59–63,65,67,69,70,74 with all but two (Hewett and colleagues20 and Patel and colleagues55) reporting the proportions of participants in each category. Five studies enrolled participants undergoing colonoscopy for either screening or surveillance reasons,54,68,75–77 but not because of symptoms, with one more study66 including participants presenting for elective screening or follow-up colonoscopy (reasons for the follow-up colonoscopy not provided). In two studies the entire sample of participants was drawn from a screening population.71,78 In the remaining three studies the types of participants enrolled is not known because it was not reported in the publications.56,58,64
The male-to-female ratio of participants in the included studies lay between 1 : 1 and 2 : 1 in 13 studies,54,56,59–63,65,66,69,74–76 and between 2 : 1 and 3 : 1 in three studies.70,77,78 In the remaining four studies that reported the male-to-female ratio it was approximately 4 : 1,71 10 : 1,68 23 : 157 and, the highest reported male-to-female ratio, 35 : 1.67 The male-to-female ratio of participants was not reported by four studies.20,55,58,64
The mean age of participants, if it was reported, lay between 54 and 67 years (16 studies54,56,57,59–62,65,66,68,70,71,74,76–78) or the median age lay between 60 and 69 years (four studies63,67,69,75). The age of participants was not reported by the remaining four studies.20,55,58,64
The majority of the studies were conducted in a single centre,54,56,59,60,63–65,69,70,74–78 four were conducted in two centres61,67,68,71 and one each at three centres,57 four centres55 and five centres.62 The number of centres was not reported by three studies.20,58,66
Study colonoscopies were undertaken by more than one endoscopist in most studies: one endoscopist in five studies,54,64,69,75,77 two in one study,20 three in one study,67 four in four studies,59,70,74,78 five in four studies,56,57,62,65 six in three studies,60,68,76 seven in three studies,63,66,71 10 in one study,61 12 in one study58 and, the largest number of endoscopists, 26 in one study.55 In eight studies, all the endoscopists had prior experience of using NBI,54,59,60,62,67,68,71,77 and in four studies some of the endoscopists had prior experience of using NBI.56,57,65,70 Only four studies stated that the endoscopists involved had no prior experience of using NBI to characterise colorectal polyps,55,58,61,78 but in a further eight studies it was not clear what experience of using NBI, if any, the endoscopist(s) may have had.20,63,64,66,69,74–76 The majority of the studies included an element of training for the endoscopist(s) in the characterisation of colorectal polyps using NBI, either training all endoscopists20,55,57–67,69,74,76,78 or the non-experts.70 In the study by Gupta and colleagues, which is a reanalysis of three earlier studies, training occurred in one of the three studies.68 In five studies54,56,71,75,77 it was not stated if any training had taken place. In three of these, the endoscopists had prior experience of NBI.54,71,77 In the Iwatate and colleagues study56 the five endoscopists had mixed levels of NBI experience, and it was unclear what NBI experience the single endoscopist in the Shahid and colleagues study had.75
A variety of different systems were used to classify polyps as adenomas or hyperplastic polyps (see Table 5). The most commonly used systems were the NBI International Colorectal Endoscopic classification scheme or a version of this, which was cited by eight studies,20,56–59,63,65,66 and the criteria proposed by Rex,64 which were cited by four studies.54,60,64,78 Two studies67,69 cited the Sano–Emura classification system, two74,75 based characterisations on modifications of the Kudo criteria and two55,68 on work by Rastogi and colleagues,73,86,87,93 with one further study76 also citing a Rastogi and colleagues publication,96 although it is not known in this case whether or not the criteria were the same. One study70 used vascular pattern intensity97 to classify polyps, one61 polyp colour, vessels and mucosal pattern,94 and one77 the author’s own system. In the final two studies either criteria were reported but not attributed to any named system62 or no criteria were reported or cited.71
The QUADAS assessments of the NBI studies indicates that the studies were at a low risk of spectrum, verification, disease progression, incorporation, test review and clinical review biases (Table 6). Supporting information for the judgements shown in Table 6 is provided in the data extraction form for each study (see Appendix 3). Note that ‘yes’ answers to QUADAS questions 1–9 (see Table 3) imply a low risk of bias, whereas ‘yes’ answers to QUADAS questions 10 and 11 reflect adequacy of reporting and further supporting information is required to assess the risks of bias associated with these questions. For five studies55,56,58,64,66 the risk of spectrum bias (QUADAS question 1) was unclear because the reason(s) for patients having a colonoscopy were not reported. In two studies57,63 not all the polyps received verification by histopathology. In the Kaltenbach and colleagues study57 this was because, when two or more non-neoplastic polyps were identified in the rectosigmoid colon in any one patient, a ‘representative sample’ was resected for histopathological analysis. How often this circumstance arose was not reported. In the Wallace and colleagues study,63 10 polyps (from 321 polyps, therefore representing 3% of the total) were not assessed by histopathology (and whether or not one further polyp had been assessed by histopathology was unclear). Overall, it is our opinion that the risk of differential verification bias in these two studies was probably very low.
TABLE 6
Overview of NBI QUADAS assessments
In all but four studies59,61,63,69 the risk of diagnostic review bias was rated as low (QUADAS question 7). The risk of bias was rated as unclear in the studies by Henry and colleagues,69 Paggi and colleagues,59 Pohl and colleagues61 and Wallace and colleagues63 because they did not report whether or not the histopathologist(s) were blinded to the NBI prediction for each polyp. The majority of studies did not report on uninterpretable/intermediate test results, probably because there were no uninterpretable/intermediate test results because of the nature of the NBI assessments (studies typically required a decision to be made, although this could be assigned as low confidence in some studies). In the studies by Gupta and colleagues and Iwatate and colleagues, there was evidence of uninterpretable or intermediate test results.56,68 An optical diagnosis could not be determined for four polyps (0.3%) in the study by Gupta and colleagues,68 and Iwatate and colleagues56 excluded two patients with ‘unevaluable material’. Patel and colleagues55 reported that polyps were excluded from the analysis if a confidence level was not assigned or if histopathology was missing or ‘other’, or if the polyp could not be retrieved, so it seems likely that there were also some uninterpretable or intermediate test results in this study. The outcome for QUADAS item 10 was judged unclear for the Wallace and colleagues study because not all patients who were randomised completed the study, so it is possible that uninterpretable test results were the reason for the missing data.63
For the final QUADAS item (question 11, attrition bias), the judgement was ‘yes’ for the majority of studies either because no withdrawals were apparent in the study20,54,56,59,60,64–67,69,71,74–77 or because withdrawals or other missing data were explained.57,61–63,70,78 For two studies the judgement was ‘unclear’.55,58 In the Ladabaum and colleagues study,58 the subjects of the study were endoscopists, and it was unclear whether or not any of them had dropped out of the study; there was little reporting on those undergoing colonoscopy. Patel and colleagues55 did not report the number of participants selected to take part or the number of patients included in the data analyses, so it was unclear whether or not there had been any withdrawals. For one study, by Gupta and colleagues,68 this question was not applicable because the included data were drawn from records of participants in three earlier trials that met the inclusion criteria for a retrospective analysis and, therefore, no participants were able to withdraw.
In addition to the assessment of the QUADAS items, the generalisability of each study was also briefly summarised during data extraction (the summary of reviewers’ comments can be seen in full in the data extraction forms in Appendix 3). The overall impression from the included NBI studies is that they enrolled participants likely to be representative of the types of participants who would receive colonoscopy in the UK for screening, surveillance or on account of symptoms experienced (in line with the inclusion criteria for this systematic review). However, only one study was conducted in the UK,70 and just four elsewhere in Europe,59,60,62,65 where it might reasonably be assumed that populations might be most similar to those in the UK. Most studies were conducted in a single centre,54,56,59,60,63–65,69,70,74–78 so inherently these results may not be transferable to other centres. In contrast, in most studies more than one endoscopist was involved in conducting colonoscopies and characterising polyps.20,55–63,65–68,70,71,74,76,78 Across all the studies the experience of endoscopists covered the whole range from those who were less experienced in conducting colonoscopy generally and had little or no experience using NBI to very experienced endoscopists who also had extensive experience of using NBI. Training for endoscopists (which may have been to train those with no prior experience of NBI or to ensure that all endoscopists at a centre were characterising polyps to the same standard) formed a part of the majority of studies, but how relevant this training may have been to current UK practice is unknown. Finally, a variety of classifications systems were used to determine whether polyps were adenomas or hyperplastic. The assessment group understands that, in countries where polyp characterisation is conducted without magnification, such as the UK, the NBI International Colorectal Endoscopic classification is becoming widely accepted. It is unclear how generalisable the results obtained using other polyp classifications are to UK practice.
i-scan
Five studies77,79–82 included in the systematic review provided data on the use of i-scan for VCE of colorectal polyps. An overview of the characteristics of the included i-scan studies is presented in Table 7 (more detailed information is available in the data extraction forms presented in Appendix 3). Four of the studies were conducted in Europe (those by Basford and colleagues in the UK,79 Hoffman and colleagues80 and Rath and colleagues82 in Germany and Pigo and colleagues81 in Italy) and one, by Lee and colleagues,77 was conducted in South Korea. Basford and colleagues79 and Hoffman and colleagues80 enrolled all their participants from a screening population, whereas the other three studies77,81,82 enrolled participants receiving colonoscopy for screening or surveillance purposes, with one81 also including participants with gastrointestinal symptoms. In the three studies77,81,82 that enrolled different types of participants, the proportions of participants receiving colonoscopy for screening, surveillance or symptoms was not reported. The Pigo and colleagues study81 enrolled almost equal proportions of men and women, whereas more men than women were enrolled in the other four studies. Four studies77,80–82 reported the mean age of the participants, which ranged from 55 years to 66 years. The two studies conducted in Germany did not report data on polyp characterisation for the whole colon: Hoffman and colleagues80 reported on polyps only in the last 30 cm of colon, and Rath and colleagues82 characterised polyps in the distal colon (the descending colon, the sigmoid colon or the rectum). Three of the studies (i.e. those by Hoffman and colleagues,80 Lee and colleagues77 and Rath and colleagues82) focused on the characterisation of diminutive polyps, whereas Basford and colleagues79 focused on small polyps (< 10 mm) and Pigo and colleagues81 included polyps of all sizes (and their data on diminutive polyps were limited to the rectosigmoid colon). Consequently, for the three studies that focused on the characterisation of diminutive polyps, data are drawn from the whole patient population, whereas it is not clear what proportion of the patients contributed data on diminutive polyp characterisation in the Basford and colleagues79 and Pigo and colleagues81 studies. All the studies were conducted in single centres, and in all but one study a single endoscopist performed the study colonoscopies and characterised polyps. In the Hoffman and colleagues study,80 three endoscopists were involved. It was clearly reported in three of the five studies (i.e. by Basford and colleagues,79 Hoffman and colleagues80 and Lee and colleagues77) that the endoscopist(s) had prior experience using i-scan but, because of an absence of reported details, it is not clear whether or not study endoscopists underwent any specific training with i-scan prior to the start of the studies. Only two studies77,82 used the same system, which was developed for the Lee and colleagues study,77 to classify polyps as adenomas or hyperplastic polyps (see Table 7); the remainder all used different systems. One study81 cited the NBI International Colorectal Endoscopic classification system, one80 used surface pit pattern, citing studies by Kudo and colleagues among others, and Basford and colleagues79 developed their own system for their research.
TABLE 7
Overview of the i-scan studies
The QUADAS assessments were conducted for each study and supporting information for the judgements shown in Table 8 is provided in the data extraction form for each study (see Appendix 3). Note that ‘yes’ answers to QUADAS questions 1–9 imply a low risk of bias whereas ‘yes’ answers to QUADAS questions 10 and 11 reflect adequacy of reporting and further supporting information is required to assess the risks of bias associated with these questions. The QUADAS assessments of the i-scan studies indicate that the studies were at a low risk of spectrum, verification, disease progression, differential verification, incorporation, diagnostic review, test review, clinical review and test classification biases (see Table 8). An exception is that, in the Hoffman and colleagues study,80 it was unclear how representative the patients were of those who would receive the test in practice because few details about the participants were reported, although it is known that they fulfilled the criteria for screening colonoscopy.
TABLE 8
Overview of i-scan QUADAS assessments
None of the studies indicated that any uninterpretable or intermediate test results had been reported. Hoffman and colleagues80 reported results for normal mucosa in addition to adenomatous and hyperplastic polyps, but there is no indication in the paper that this was as a result of any difficulty in interpreting the index test.
No withdrawals (of patients or of polyps from the analysis) were apparent in the Hoffman and colleagues80 and Lee and colleagues77 studies. The exclusion of patients screened for inclusion was explained by Basford and colleagues.79 Pigo and colleagues81 recruited 78 patients and 150 polyps were included in the analysis, but it was not clear whether or not the 150 polyps were from the full sample of 78 recruited participants. Rath and colleagues82 recruited 224 patients to their study, but the analysis included only 77 of these (all were described as having distal diminutive polyps). It is possible that the remaining patients in these studies had larger polyps located other than in the distal colon, but this is not explicitly stated. Therefore, the Pigo and colleagues81 and the Rath and colleagues82 studies are rated as being at possible risk of attrition bias.
In addition to the assessment of the QUADAS items, the generalisability of each study was also briefly summarised during data extraction (the summary of reviewers’ comments can be seen in full in the data extraction forms in Appendix 3). The overall impression from the included i-scan studies is they enrolled participants likely to be representative of the types of participants who would receive colonoscopy in the UK for screening or surveillance or on account of symptoms experienced. However, only one study was conducted in the UK,79 with three out of the remaining four conducted in Europe (two in Germany80,82 and one in Italy81), whereas the final study was conducted in South Korea.77 Three of the five studies were conducted by endoscopists with prior experience of i-scan,77,79,80 and all took place in single centres often described as academic or specialist centres. The results of these studies may therefore not be applicable to less experienced endoscopists working in more generalist or community settings. Only one study used the NBI International Colorectal Endoscopic classification system (which is becoming widely accepted for polyp characterisation without magnification) to determine whether polyps were adenomas or hyperplastic.81 It is unclear how generalisable the results obtained using other polyp classifications are to UK practice.
Flexible spectral imaging colour enhancement
Three studies included in the systematic review (Kang and colleagues,78 Longcroft-Wheaton and colleagues83,84) provided data on the use of FICE for VCE of colorectal polyps (Table 9). Two of the studies were conducted in the UK83,84 and the other was conducted in South Korea.78 In all three of these studies, all the included participants were undergoing colonoscopy for screening purposes. The Longcroft-Wheaton and colleagues83 study enrolled a slightly higher proportion of women than men, whereas the other two studies enrolled a higher proportion of men than women. All three studies reported the mean age of participants, which ranged from 5478 to 65 years.84 All three studies focused on the real-time diagnosis of colorectal polyps sized < 10 mm and provided subgroup analyses of diminutive polyps. All the studies were conducted in single centres. In the Kang and colleagues78 study, four endoscopists carried out the colonoscopies, whereas the other two studies each involved one endoscopist. Kang and colleagues78 reported that the study endoscopists had no prior experience with FICE, whereas Longcroft-Wheaton and colleagues83,84 reported that the endoscopist in each of these studies had previous experience of in vivo diagnosis of polyps, although the authors did not specify endoscopists’ experience with FICE. Longcroft-Wheaton and colleagues83 stated that the study endoscopist had had prior training in real-time diagnosis. In the other studies,78,84 the endoscopists’ prior training in both real-time diagnosis and, more specifically, the use of FICE was unclear. Kang and colleagues78 noted, however, that the endoscopists received feedback every 2 weeks during the study about the accuracy of their endoscopic predictions compared with the histopathological diagnosis. The study by Kang and colleagues78 (which also included a NBI arm), used a classification system for polyp characterisation based on colour, vascular density and vascular pattern.64,90,91,98 The two studies by Longcroft-Wheaton and colleagues83,84 both used a characterisation system based on vascular patterns that was developed by Teixeira and colleagues.99
TABLE 9
Overview of the FICE studies
Table 10 shows the quality assessments of the three FICE studies.78,83,84 Reviewers considered all three studies to be at a low risk of bias across most of the QUADAS items assessed. None of the studies, however, reported the number of uninterpretable test results, but reviewers believed this to be zero in two studies.78,84 Two studies explained participant withdrawals.78,83 Longcroft-Wheaton and colleagues84 did not state whether or not there were any withdrawals.
TABLE 10
Overview of QUADAS assessments for the FICE studies
In addition to the assessment of the QUADAS items, the generalisability of each study was also briefly summarised during data extraction (the summary of reviewers’ comments can be seen in full in the data extraction forms in Appendix 3). Reviewers noted that two of the studies were conducted in the UK83,84 and so are likely to be representative of a UK population (although it is noted that these studies included a small number of participants – 50 and 89 participants each). It was also noted that it is unclear how representative participants in the South Korea study78 would be of the UK population and how similar the endoscopists’ training in this study would be to endoscopists’ training in the NHS in the UK. As all the studies were conducted in single centres it is unclear how the results would generalise to other centres and settings.
Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy)
Narrow-band imaging
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps
All but one of the included NBI studies reported sensitivity74 or both sensitivity and specificity20,54–71,75,77,78 of NBI for the characterisation of diminutive colorectal polyps as adenomas or hyperplastic polyps compared with the characterisation verified by histopathological assessment of the resected polyps. Only Vu and colleagues76 did not report on either sensitivity or specificity (this study was included in the systematic review because it reported accuracy in terms of the proportion of correctly classified polyps and data on surveillance intervals). The way in which data were reported by the studies varied and is shown in Table 11. Some studies reported on all the polyp characterisations made by study endoscopists. In other studies, the endoscopist indicated how confident they were in their NBI characterisation of the polyp as adenomatous or hyperplastic, and results were reported separately for high- and low-confidence characterisations. Some studies reported data on all the characterisations and also the subsets of data for high- and low-confidence characterisations (data on low-confidence characterisations are available in the data extraction forms in Appendix 3). One study, by Hewett and colleagues,54 was restricted to the rectosigmoid colon. As can be seen in Table 11, several other studies also reported data for subsections of the colon as well as for the whole colon. One study, by Iwatate and colleagues,56 included a subgroup analysis by type of endoscopist (specialist or generalist).
TABLE 11
Overview of the available data on sensitivity and specificity
The subsections that follow report on the:
- sensitivity and specificity of NBI for the characterisation of diminutive polyps in the whole colon (first, data on all characterisations, then the separate subset of data on the polyp characterisations made with high confidence by the endoscopists), with accompanying meta-analyses (including a post hoc analysis of high-confidence characterisations made by endoscopists with prior experience of NBI)
- sensitivity and specificity of NBI for the characterisation of diminutive polyps in the rectosigmoid colon (again, for all characterisations and separately for the subset of high-confidence characterisations), with accompanying meta-analyses (including a post hoc analysis of high-confidence characterisations made by endoscopists with prior experience of NBI)
- sensitivity and specificity of NBI for the characterisation of polyps in parts of the colon other than the rectosigmoid colon (too few studies to meta-analyse)
- NPV of NBI for the characterisation of diminutive colorectal polyps; accuracy of NBI (proportion of correctly classified polyps).
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps in the whole colon
Twenty-three studies20,54–71,74,75,77,78 reported on the characterisation of diminutive polyps within the whole colon, although five of these reported data only from high-confidence characterisations.20,57,59–61
The results for all characterisations of diminutive polyps in the whole colon (i.e. not separated by confidence level), where 2 × 2 table data were reported or calculable, are shown in Figure 5.
The ability of NBI to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) ranged from 0.55 to 0.97 (i.e. 55–97%) across the 17 studies that reported this outcome. Sensitivity was above 90% in seven studies55,64,66–68,70,71 (and in two of these it was ≥ 95%55,67) between 80% and 90% in six other studies56,58,62,69,77,78 and was < 80% in four studies.63,65,74,75
The ability of NBI to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) was typically lower than the sensitivity of the test, ranging from 0.62 to 0.95 (i.e. 62% to 95%) across the 16 studies that reported this outcome. Specificity was above 90% in just two studies,69,75 between 80% and 90% in seven studies62,64–66,70,71,77 and was below 80% in seven studies.55,56,58,63,67,68,78
It was possible to run a bivariate meta-analysis (using Stata/IC14 and metandi45) for the 16 studies that reported both sensitivity and specificity. This produced a summary value for sensitivity of 0.88 (95% CI 0.83 to 0.92) and for specificity of 0.81 (95% CI 0.75 to 0.85). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 6. The 95% confidence region around the summary point indicates where we have 95% confidence that the summary point lies. The 95% prediction region illustrates the extent of statistical heterogeneity among the studies. If the bivariate model for sensitivity and specificity is correct, we have 95% confidence that the true sensitivity and specificity of a new study in the future will lie within the 95% prediction region. As can be observed from Figure 6, the 95% prediction region is large.

FIGURE 6
Summary receiver operating characteristic curve plot from the meta-analysis of NBI for all characterisations of polyps in the whole colon.
In order to investigate the heterogeneity between studies, a covariate for endoscopist experience with NBI was added to RevMan and separate SROC curves were drawn as shown in Figure 7. Although caution must be taken when interpreting this figure, because of the small number of studies for each subgroup, it nevertheless appears to support the hypothesis that endoscopists with prior experience of using NBI to characterise diminutive colorectal polyps achieve higher sensitivity and specificity than endoscopists who have had no prior experience of using NBI to characterise diminutive colorectal polyps (other than any training that they undertook at the start of the study).
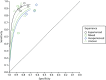
FIGURE 7
Summary receiver operating characteristic curve plots for all characterisations of polyps in the whole colon by endoscopists’ level of experience using NBI.
The results for studies that reported results from polyp characterisations using NBI that were designated as high-confidence decisions, and where 2 × 2 table data were reported or calculable, are shown in Figure 8.
The ability of high-confidence characterisations made with NBI to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) was ≥ 0.90 (i.e. ≥ 90%) in 9 of the 13 studies20,55–57,59,60,62,64,77 (in four of these it was ≥ 95%20,55,57,64) and between 80% and 90% in three other studies.58,61,63 The lowest sensitivity value reported was 59%, by Sola-Vera and colleagues.65 Some studies reported the sensitivity obtained from all characterisations and the sensitivity from only the high-confidence characterisations. In all studies in which both these values were reported, the sensitivity was higher when obtained from high-confidence decisions (difference ranging from an increase of 1.5% to 5.8%).
The ability of NBI to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) from high-confidence polyp characterisations was just > 90% (i.e. > 0.90) in three studies,64,65,77 but did not exceed 92% in any study. In just three studies, specificity lay between 80% and 90%,61–63 but in the majority of the studies it lay < 80%,55–60 with the lowest specificity just 44.1%, reported by Ladabaum and colleagues.58 Specificity was higher when obtained from high-confidence decisions in seven of the eight studies that reported both the specificity obtained from all characterisations and the specificity from only the high-confidence characterisations, with the increase ranging from 3.5% to 7.3%. The one exception was the study by Ladabaum and colleagues58 in which the specificity calculated from high-confidence characterisations was lower than that obtained from all characterisations (44.1% vs. 64.4%, respectively).
A bivariate meta-analysis (using Stata/IC14 and metandi45) was run for the 11 studies that reported both sensitivity and specificity from polyp characterisations made with high confidence. This produced a summary value for sensitivity of 0.91 (95% CI 0.85 to 0.95) and for specificity of 0.82 (95% CI 0.76 to 0.87). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 9. The effect of reporting only on high-confidence characterisations rather than all polyp characterisations is to move the summary estimate up (increasing sensitivity) and slightly to the left (increasing specificity).
The impact of restricting the analysis to high-confidence characterisations rather than including all characterisations can be observed in Figure 10, which shows both summary curves on the same plot. As already stated, the effect of reporting only on high-confidence characterisations rather than on all polyp characterisations is that the summary estimate moves up (increasing sensitivity) and slightly to the left (increasing specificity).
Seven studies55,56,58,62–65,77 reported both sensitivity and specificity from all diminutive polyp characterisations and separately for only high-confidence diminutive polyp characterisations, although for one of the these studies58 2 × 2 table data were not available for the high-confidence characterisations [which had a reported sensitivity of 88.4% (95% CI 82.2% to 94.7%) and specificity of 44.1% (95% CI 26.5% to 61.6%)]. The pairs of results from these studies are shown in Figure 11 and forest plots in Figure 12.
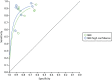
FIGURE 11
Plot showing paired data from the studies that reported on all diminutive polyp characterisations and separately on high-confidence diminutive polyp characterisations.
To obtain data for a scenario analysis within the economic model (see Chapter 5, Scenario analyses), a post hoc bivariate meta-analysis (using Stata/IC14 and metandi45) was run for a subgroup in which endoscopists experienced in the use of NBI characterised the polyps in the whole colon (Figure 13). Four such studies were included in this analysis.59,60,62,77
The meta-analysis produced a summary value for sensitivity of 0.92 (95% CI 0.89 to 0.94) and for specificity a value of 0.82 (95% CI 0.72 to 0.89). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 14. Restricting the meta-analysis from 11 studies reporting different levels of NBI experience (experienced, n = 4;59,60,62,77 mixed experience, n = 3;56,57,65 inexperienced, n = 2;55,61 and unclear, n = 263,64) to the four studies that reported endoscopists experienced in the use of NBI narrowed the 95% CI for sensitivity [11 studies with a variety of experience, 0.91 (95% CI 0.85 to 0.95); four studies with prior NBI experience, 0.91 (95% CI 0.89 to 0.94)] and widened the 95% CI for specificity [11 studies with a variety of experience, 0.82 (95% CI 0.76 to 0.87); four studies with prior NBI experience, 0.82 (95% CI 0.72 to 0.89)]. The changes in the 95% CIs are reflected in the change in the size and shape of the 95% confidence region and 95% prediction region in Figure 14 in comparison with Figure 9.

FIGURE 14
Summary receiver operating characteristic plot showing the summary point on the summary curve from the meta-analysis of NBI for high-confidence characterisations of polyps in the whole colon when made by endoscopists experienced in the use of NBI.
Colonoscopies in one study, by Iwatate and colleagues,56 were conducted by five endoscopists. Two of the five endoscopists were described as specialists in colonoscopy and they had extensive experience in magnifying colonoscopy with NBI (> 1000 cases). The other three endoscopists were described as general endoscopists with limited experience in magnifying colonoscopy with NBI (≤ 1000 cases). As shown in Table 12, the two specialist endoscopists achieved higher sensitivity and specificity than the three general endoscopists, but the difference between the two was statistically significant only for specificity (p = 0.007).
TABLE 12
Sensitivity and specificity according to experience with NBI of the endoscopists
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps in the rectosigmoid colon
As shown in Table 11, four studies54,55,58,63 reported sensitivity and specificity following characterisation (any level of confidence) of diminutive polyps in the rectosigmoid colon, with three of these reporting sufficient data for a 2 × 2 table to be constructed for entry into the meta-analysis.54,58,63
Three of the four studies54,55,63 that reported results for all characterisations also reported sensitivity and specificity following high-confidence characterisations of polyps in the rectosigmoid colon, with two further studies61,62 reporting only high-confidence characterisation data. Four of the five studies reporting on high-confidence characterisations provided sufficient data for 2 × 2 tables to be constructed for entry into the meta-analysis.54,61–63
The results from the studies that used NBI to characterise polyps in the rectosigmoid colon, where 2 × 2 table data were reported or calculable, are shown in Figure 15. The results from Patel and colleagues55 are not represented in Figure 15 because it was not possible to impute values into a 2 × 2 table that provided a solution for the reported outcomes in the paper (accuracy, sensitivity, specificity, PPV and NPV).
Bivariate meta-analyses were conducted (using Stata/IC14 and xtmelogit or using Stata/IC14 and metandi45) of the studies where 2 × 2 table data were available. For all characterisations of diminutive polyps in the rectosigmoid colon, the summary value for sensitivity is 0.85 (95% CI 0.75 to 0.91) and for specificity is 0.87 (95% CI 0.74 to 0.94). For high-confidence characterisations of diminutive polyps in the rectosigmoid colon, the summary value for sensitivity is 0.87 (95% CI 0.80 to 0.92) and for specificity is 0.95 (95% CI 0.87 to 0.98). The parameter estimates for the bivariate model from these two meta-analyses were entered into RevMan to produce the SROC plot shown in Figure 16. As seen with the results for the whole colon, the effect of reporting only high-confidence polyp characterisations rather than all polyp characterisations is to increase sensitivity and specificity (summary point moves up and to the left on the SROC plot).
Note that one study was not included in either meta-analysis, that is, Patel and colleagues,55 with all characterisations of polyps with a sensitivity of 88.4% (95% CI 84.8% to 92.0%) and a specificity of 78.3% (95% CI 71.8% to 84.9%) and high-confidence characterisations of polyps with a sensitivity of 90.9% (95% CI 87.4% to 94.4%) and a specificity of 88.6% (95% CI 81.0% to 96.1%). The large 95% confidence and a 95% prediction regions, which were generated for the high-confidence characterisation plot, are not shown on this figure and the software used to draw the SROC plot (RevMan) did not generate a 95% confidence region or a 95% prediction region for the other data set.
To obtain data for a scenario analysis within the economic model (see Chapter 5, Scenario analyses), a post hoc bivariate meta-analysis (using Stata/IC14 and xtmelogit) was run for a subgroup of studies in which the endoscopists were experienced in the use of NBI. Two such studies54,62 were included in the analysis (Figure 17).
The meta-analysis produced a summary value for sensitivity of 0.90 (95% CI 0.71 to 0.97) and for specificity of 0.98 (95% CI 0.91 to 1.00). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 18. Restricting the meta-analysis from the four studies reporting different levels of NBI experience (experienced, n = 2; inexperienced, n = 1; and unclear, n = 1) to only two studies in which endoscopists had experience in the use of NBI increased the summary value for sensitivity while widening the 95% CI [four studies with a variety of experience, 0.87 (95% CI 0.80 to 0.92); and two studies with prior NBI experience, 0.90 (95% CI 0.71 to 0.97)] and increased the summary value for specificity while narrowing the 95% CI [four studies with a variety of experience, 0.95 (95% CI 0.87 to 0.98); and two studies with prior NBI experience, 0.98 (95% CI 0.91 to 1.00)].
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps in parts of the colon other than the rectosigmoid colon
Five studies55,57,58,61,68 provided data on the characterisation of diminutive polyps in regions of the colon other than the rectosigmoid colon (see Table 11). The results of these studies are summarised in Table 13.
TABLE 13
Summary of the sensitivity and specificity of NBI for the characterisation of diminutive colorectal polyps in parts of the colon other than the rectosigmoid colon
Negative predictive value of narrow-band imaging for the characterisation of diminutive colorectal polyps
The NPV is the probability that subjects with a negative screening test (i.e. colorectal polyp is characterised as hyperplastic) truly do not have an adenoma. However, it must be borne in mind when viewing these results that the NPV is influenced by the prevalence of disease (i.e. in this case the prevalence of adenomas in the tested populations). When prevalence is increased, the result is a decrease in the NPV. Owing to the importance of NPV within the PIVI statement (see Chapter 1, Diagnostic thresholds and requirements for use of virtual chromoendoscopy), consideration was given to meta-analysing NPVs from the included studies even though this is not advised by either the National Institute for Health and Care Excellence’s Diagnostics Assessment Programme Manual37 or the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.36 However, because it is clear that the prevalence of adenomas and hyperplastic polyps is likely to vary between studies [e.g. because of differences in case mix (screening, surveillance and symptomatic populations) and patient characteristics (age, sex)], we chose not to pool NPVs across studies. Instead, we have provided forest plots for these outcomes and marked the 90% threshold value on each plot.
For the characterisations of diminutive polyps in the whole colon (made with any level of confidence), the NPV ranged from 43% to 96.1% (Figure 19 and Table 14). The study by Sola-Vera and colleagues65 is noteworthy because this study reported the lowest NPV – far lower than in any other study. All the other studies reported NPVs of > 70%, with five studies reporting NPVs of ≥ 90%.55,64,66,67,69 However, it should be noted that the lower limit of the 95% CI fell below 90% in every study except Patel and colleagues.55

FIGURE 19
Negative predictive values of NBI for all characterisations of diminutive polyps in the whole colon (made with any level of confidence).
TABLE 14
Negative predictive values of NBI for the characterisation of diminutive polyps in the whole colon
Limiting the assessment of NPV to high-confidence polyp characterisations increased the NPVs, which ranged from 48% to 98.3% in the studies that reported this outcome (Figure 20 and Table 14). Again, the study by Sola-Vera and colleagues65 had the lowest NPV of any study by a considerable margin. All other studies reported NPVs for high-confidence assessments of > 78%, with five studies reporting NPVs of ≥ 90%.20,55,57,64,77 Once again, however, inspection of the 95% CIs reveals that the lower limit of this fell below 90% in all but two studies.55,64

FIGURE 20
Negative predictive value of NBI for high-confidence characterisations of diminutive polyps in the whole colon. a, Note that no 95% CI was reported for the Hewett and colleagues study.
One study, by Iwatate and colleagues,56 compared differences in NPVs achieved by specialists in colonoscopy and general endoscopists. Specialists in colonoscopy achieved NPVs of > 90% (mean value 90.9%, 95% CI 70.8% to 98.9%), whereas the NPVs achieved by general endoscopists were lower, with a mean value of 71.4% (95% CI 47.8% to 88.8%); however, the difference between the groups was not statistically significant.
Seven studies54,55,58,61–63,68 reported on the NPVs for the characterisation of diminutive polyps in the rectosigmoid colon (top section, Table 15). Five of these studies54,55,58,63,68 reported data for all diminutive polyp characterisations in the rectosigmoid colon and NPVs ranged from 87.4% to 98.4%. In four54,55,63,68 of these five studies the NPVs were > 90%. Only in the study by Ladabaum and colleagues58 was the 90% threshold not reached.
TABLE 15
Negative predictive values of NBI for the characterisation of diminutive polyps in the rectosigmoid colon and other regions of the colon
Data for high-confidence characterisations of polyps in the rectosigmoid colon were reported by five of the seven studies (Figure 21).54,55,61–63 In three of these five studies,54,55,63 the data on high-confidence characterisations were provided in addition to data on all polyp characterisations in the rectosigmoid colon. In these studies the high-confidence results led to NPVs that remained at > 90% and were slightly increased. Two studies61,62 provided high-confidence results only for the rectosigmoid colon and in both the NPV was over the 90% threshold. It is worth noting, however, that in two62,63 of the five studies that report NPVs for high-confidence characterisations of diminutive polyps in the rectosigmoid colon, the lower limit of the 95% CI falls below 90%.

FIGURE 21
Negative predictive values of NBI for high-confidence characterisations of diminutive polyps in the rectosigmoid colon.
The NPVs of NBI for characterisation of diminutive polyps in other regions of the colon (where reported by studies) is also presented in Table 15. Although the mean NPV was above the 90% threshold in some instances, none of the lower limits of the 95% CI was > 90%.
One study61 reported the NPV for characterisations of diminutive polyps in the rectosigmoid colon achieved by endoscopists with prior optical diagnosis experience in colonoscopy and by endoscopists without prior optical diagnosis experience. Endoscopists with prior optical diagnosis experience achieved a NPV of 96.6% (95% CI 92.7% to 98.7%), whereas the NPV achieved by endoscopists without prior optical diagnosis experience was lower at 93.5% (95% CI 88.7% to 96.7%).
Accuracy of narrow-band imaging
As well as measures such as sensitivity, specificity and NPV reported above, another global measure, diagnostic accuracy, can be calculated from the 2 × 2 table data. This is expressed as the proportion of correctly classified polyps (the sum of the TP and TN results) among all the polyps (TP + TN + FP + FN). Like NPV, diagnostic accuracy is affected by disease prevalence such that at the same sensitivity and specificity diagnostic accuracy increases as disease prevalence decreases.
Accuracy of polyp characterisations in the whole colon was reported by, or could be calculated for, 16 studies (Table 16).55,56,58,62–71,75,77,78 Accuracy was ≥ 90% in five studies,66,67,69–71 between 76% and 89% in 10 studies55,56,58,62–64,68,75,77,78 and only 63.9% in the final study.65
TABLE 16
Accuracy (proportion of correctly classified polyps) with NBI
Thirteen studies20,55–65,77 reported on the accuracy of high-confidence polyp characterisations in the whole colon (see Table 16). Accuracy was ≥ 90% in two studies,64,77 between 81% and 90% in 10 studies20,55–63 and only 68.5% in the final study.65
Accuracy of polyp characterisation was typically 3–5% higher among high-confidence characterisations than among all polyp characterisations in the eight studies55,56,58,62–65,77 that reported both values.
i-scan
Sensitivity and specificity of i-scan for the characterisation of diminutive colorectal polyps
Five studies77,79–82 provided data on the characterisation of diminutive polyps as adenomas or hyperplastic polyps using i-scan, with the characterisation verified by histopathological assessment of the resected polyps. The way in which data were reported by the studies varied. Two studies, by Basford and colleagues79 and Lee and colleagues,77 reported on the characterisation of diminutive polyps within the whole colon. Basford and colleagues79 presented data only from the polyp characterisations that the endoscopist had high confidence were correct, whereas Lee and colleagues77 provided data for all characterisations and then separately for characterisations made with either high or low confidence (data for low-confidence characterisations are available in Appendix 3). The other three studies presented data on the characterisation of diminutive polyps from within a part of the colon: the distal colon (Rath and colleagues82), the last 30 cm of colon (Hoffman and colleagues,80 who did not present a per-polyp analysis, only an analysis per patient) and the rectosigmoid colon (Pigo and colleagues81 and Rath and colleagues,82 although it was not possible to impute the 2 × 2 table data for the latter study). Rath and colleagues82 also provided data separately for the polyp characterisations they had made with high confidence.
The results for all characterisations (i.e. not separated by confidence level) are shown in Figure 22. The ability of i-scan to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) was > 90% in three of the four studies that reported results for all characterisations (i.e. Lee and colleagues,77 Pigo and colleagues81 and Rath and colleagues82), whereas sensitivity was only 82% in the per-patient analysis reported by Hoffman and colleagues.80 The ability of i-scan to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) was more variable across the studies, ranging from 83% (Rath and colleagues,82 results for polyps in the distal colon) to 96% (Hoffman and colleagues80).
The results for studies that reported results from polyp characterisations with i-scan that were designated as high-confidence decisions are shown in Figure 23. The ability of high-confidence characterisations made with i-scan to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) in the three studies that provided data was 0.94 (i.e. 94%; Lee and colleagues77), 0.97 (97%; Basford and colleagues79) and, in the Rath and colleagues’ study,82 0.98 for distal polyps and 0.96 in the analysis limited to polyps in the rectosigmoid colon. In the Lee and colleagues study,77 the sensitivity achieved from high-confidence polyp characterisations was slightly lower than that obtained from all the polyp characterisations, 0.94 (95% CI 0.84 to 0.99) versus 0.95 (95% CI 0.87 to 0.99), whereas the reverse was true for the Rath and colleagues study82 for both the data set for distal polyps and that for rectosigmoid colon polyps (distal polyps: high confidence 0.98, 95% CI 0.90 to 1.00, vs. overall 0.93, 95% CI 0.83 to 0.98; rectosigmoid colon: high confidence 0.96, 95% CI 0.80 to 1.0, vs. overall 0.90, 95% CI 0.73 to 0.98). The ability of i-scan to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) when the characterisation was made with high confidence was ≥ 0.90 (i.e. 90%) in all three studies. Furthermore, the specificity of i-scan arising from high-confidence decisions was greater than the specificity observed when all the polyp characterisations were taken into account in the two studies that reported both sets of data (Lee and colleagues,77 92% vs. 86%; Rath and colleagues,82 distal polyps 95% vs. 83%, rectosigmoid colon polyps 95.5% vs. 87.5%). The 2005 Rath and colleagues82 study, which was conducted in Germany among patients attending for screening or surveillance colonoscopy and which reported on characterisation of distal polyps (polyps in the descending colon, the sigmoid colon or the rectum), achieved the best sensitivity (98%), which was coupled with the second highest value for specificity (95%). However, in common with the other studies providing data on i-scan, a single endoscopist working in what appears to be a specialist endoscopy centre achieved these results, so it is not clear how transferable these results would be to less experienced endoscopists working in less specialist settings.
A bivariate meta-analysis was run (using Stata/IC14 and xtmelogit) to provide a summary estimate for the two studies that reported high-confidence characterisations of polyps in the whole colon, which could be used in the economic model. This produced a summary value for sensitivity of 0.96 (95% CI 0.92 to 0.98) and for specificity of 0.91 (95% CI 0.84 to 0.95). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 24.
Negative predictive value of i-scan for the characterisation of diminutive colorectal polyps
As previously stated, the NPV is the probability that subjects with a negative screening test (i.e. colorectal polyp is characterised as hyperplastic) truly do not have an adenoma. However, it must be borne in mind when viewing these results that the NPV is influenced by the prevalence of disease (i.e. in this case the prevalence of adenomas in the tested populations). When prevalence is increased, the result is a decrease in the NPV.
Two studies77,80 reported NPVs for the characterisations of diminutive polyps in the whole colon (made with any level of confidence), although one of these studies, by Hoffman and colleagues,80 reported only a per-patient analysis. Although the mean NPV was > 90%, the lower limit of the 95% CI fell below 90% in both studies (Table 17). High-confidence characterisation of polyps in the whole colon was reported by two studies.77,79 Basford and colleagues79 reported a NPV of 95.4% (95% CI 87.1% to 99.0%) and Lee and colleagues77 a NPV of 94.7% (95% CI 85.4% to 98.9%).
TABLE 17
Negative predictive values of i-scan for the characterisation of diminutive polyps
Three studies reported on the NPV for the characterisation of diminutive polyps in the distal portion of the colon82 or the rectosigmoid colon,79,81,82 with Rath and colleagues82 also reporting on high-confidence characterisations and Basford and colleagues79 reporting only on high-confidence characterisations. In all cases, although the point estimate for the NPV lay above the 90% threshold, the lower limit of the 95% CI fell below this.
Accuracy of i-scan
Diagnostic accuracy (the proportion of correctly classified polyps among all the polyps) was reported for all diminutive polyp characterisations,80,81 for only high-confidence polyp characterisations79 or for both77,82 (Table 18), with three studies providing data for the characterisations of polyps in the whole colon77,79,80 and a single study for polyps in the rectosigmoid colon81 or distal polyps.82 Like NPV, diagnostic accuracy is affected by disease prevalence. At the same sensitivity and specificity, diagnostic accuracy increases as disease prevalence decreases.
TABLE 18
Accuracy (proportion of correctly classified polyps) with i-scan
Accuracy was ≥ 90% in all the studies77,79–82 and the accuracy of high-confidence polyp characterisations was higher than among all polyp characterisations in the two studies that reported both values.77,82
Flexible spectral imaging colour enhancement
Sensitivity and specificity of flexible spectral imaging colour enhancement for the characterisation of diminutive colorectal polyps
Three studies 78,83,84 provided data on the characterisation of diminutive polyps as adenomas or hyperplastic polyps using FICE compared with characterisation verified by histopathological assessment of the resected polyps. In all three studies the characterisations were made on polyps in any part of the colon, and in all three the level of confidence with which the characterisation was made was not stated. The results of the polyp characterisations are shown in Figure 25.

FIGURE 25
Accuracy of FICE for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps. a, 2 × 2 table data imputed.
The ability of FICE to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) ranged from 74% to 88% across the studies. The ability of FICE to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) had a narrower range across the studies, from 82% to 88%.
It was possible to run a bivariate meta-analysis (using Stata/IC14 and xtmelogit) with data from the three studies. This produced a summary value for sensitivity of 0.81 (95% CI 0.73 to 0.88) and for specificity of 0.85 (95% CI 0.79 to 0.90). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 26.
Negative predictive value of Flexible Spectral Imaging Colour Enhancement for the characterisation of diminutive colorectal polyps
Table 19 reports the NPVs for the three FICE studies. These ranged from 70% to 84%.
TABLE 19
Negative predictive value of FICE for the characterisation of diminutive colorectal polyps
Accuracy of Flexible Spectral Imaging Colour Enhancement
The three studies that reported on the use of FICE provided diagnostic accuracy (the proportion of correctly classified polyps among all the polyps) for all diminutive polyp characterisations in the whole colon (Table 20).78,83,84 The reported diagnostic accuracy values ranged from 80% to 85%.
TABLE 20
Accuracy (proportion of correctly classified polyps) with FICE
Post hoc pooled analysis of all virtual chromoendoscopy technologies
The appropriateness of pooling evidence from different VCE technologies together is uncertain. The technologies certainly all aim to enhance surface vessel patterns, but the technologies use different methods to achieve this. We have therefore assumed that there is a ‘class effect’ and that evidence from different VCE technologies can be meaningfully pooled.
A pooled analysis of the studies included in this assessment for which 2 × 2 data were available was undertaken in order to inform a scenario analysis using the economic model (see Chapter 5, Sensitivity analyses). Data for high-confidence assessments of polyps in the whole colon were available from 11 NBI studies and two i-scan studies (note that Lee and colleagues77 contribute data on NBI and i-scan) (Figure 27). No FICE data were available to include in this analysis because the FICE studies did not report high-confidence polyp characterisations separately.

FIGURE 27
Accuracy of VCE high-confidence decisions for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps in the whole colon.
A bivariate meta-analysis (using Stata/IC14 and metandi45) was carried out, which produced a pooled summary estimate for sensitivity of 0.92 (95% CI 0.87 to 0.95) and for specificity of 0.83 (95% CI 0.78 to 0.87). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 28. The VCE pooled estimates for sensitivity and specificity do not differ greatly from the NBI pooled estimates (see Figure 9), which is unsurprising given that the bulk of the evidence comes from studies of NBI.
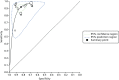
FIGURE 28
Summary receiver operating characteristic curve plot showing the summary point on the summary curve from the meta-analysis of VCE high-confidence decisions for characterising diminutive colorectal polyps in the whole colon.
A pooled analysis of the virtual chromoendoscopy studies for high-confidence assessments of polyps in the rectosigmoid colon, equivalent to that above for the whole colon, has, in essence, already been presented earlier in this assessment. This is because the only data available for this analysis come from NBI studies and, thus, the results presented in Figures 15 and 16 represent all the available data on high-confidence assessments of polyps in the rectosigmoid colon; there are no equivalent data for i-scan or FICE.
Assessment of test impact on recommended surveillance intervals
Narrow-band imaging
Thirteen studies55,57,58,60–65,67,68,70,76 reported results on the impact that the use of NBI would have on recommended surveillance intervals (in comparison to surveillance intervals calculated following histopathology of all polyps) (Table 21). The agreement between the surveillance interval allocated using a NBI-based strategy and using the results of histopathology for all polyps ranged from 84%63,76 to 99%.62 Eleven of the 13 studies reporting on this outcome achieved a level of agreement that was > 90%,55,57,58,61–65,67,68,70 although for three of these studies58,63,68 an agreement of > 90% was achieved by only one of the tested strategies (in two studies using a modified recommendation of colonoscopy in 10 years for patients with one or two small adenomas instead of 5 years,58,68 and in one study limiting the analysis to studies with high-confidence predictions for polyps ≤ 5 mm in size63). Where there were discrepancies between the surveillance interval assigned using the NBI-based strategy and the histopathology-only strategy, some studies reported whether the NBI strategy led to longer or shorter surveillance intervals being assigned. In the majority of studies in which a discrepancy in the surveillance interval was reported, the NBI-containing strategy led more often to shorter surveillance intervals being set (i.e. patients were recalled for a colonoscopy sooner than would have been the case with the histopathology-based surveillance interval) than to longer surveillance intervals. There were, however, some exceptions; in particular, in the study by Repici and colleagues,62 in the NBI-containing strategy, a difference between the surveillance intervals assigned was more likely to lead to the assignment of a longer interval (i.e. patients not recalled for repeat colonoscopy as early as they would have been with the histopathology-based surveillance interval) than to a shorter one.
TABLE 21
Surveillance interval prediction
Nine studies clearly calculated the concordance of surveillance intervals between VCE and histopathology in line with the PIVI requirements.57–59,61–64,67,76 The criterion of the PIVI statement, that agreement should be ≥ 90%, was met by all but one study,76 with one further study meeting the PIVI criterion in one of the two tested strategies.58 When the agreement was ≥ 90%, the lower limit of the 95% CI (when reported) fell below 90% in two instances.55,62
i-scan
Two studies79,82 examined the effect that the use of i-scan had on recommended surveillance intervals in comparison with those that were allocated based on histopathological assessment of all polyps (Table 22). Both studies79,82 used in vivo diagnosis of diminutive polyps to guide surveillance interval decisions in accordance with the PIVI requirements. Both studies79,82 also calculated agreement in surveillance intervals between i-scan and histopathology when using two different guidelines for determining the surveillance interval. Across these two studies, a surveillance interval agreement of > 90% was achieved regardless of the guideline used, with agreement ranging from 93.2%82 to 97.2%.79 In the study by Basford and colleagues,79 identical results (an agreement of 97.2%) were achieved when using both the guidelines assessed. Both studies reported whether using i-scan resulted in a longer or shorter surveillance interval being allocated than that allocated by histopathology. In the Basford and colleagues study,79 two patients were set a shorter interval with i-scan and one patient a longer interval. In the Rath and colleagues study,82 i-scan tended to results in longer intervals being allocated than with histopathology, except in one case.
TABLE 22
Surveillance interval prediction using i-scan
Flexible Spectral Imaging Colour Enhancement
Two studies83,84 reported results on the impact that the use of FICE would have on recommended surveillance intervals (in comparison with surveillance intervals calculated following histopathology of all polyps), although neither assessed this in accordance with the PIVI criteria. This analysis, in both of these studies, included polyps < 10 mm in size (i.e. neither was restricted to diminutive polyps). The agreement between the surveillance interval allocated using a FICE-based strategy and using the results of histopathology was 100% in one study83 and 97% in the other study,84 regardless of whether the BSG or ASGE guidelines were used to determine the surveillance intervals. In the single study for which there was a discrepancy for two participants between the surveillance interval assigned using the FICE-based strategy and the histopathology strategy, it is not known whether the FICE-based strategy led to a longer or a shorter surveillance interval being set (Table 23).
TABLE 23
Surveillance interval prediction using FICE
Assessment of other outcomes
In addition to the outcomes reported above on test accuracy and the impact on recommended surveillance intervals, the review also aimed to report data on the interpretability of the tests; interobserver agreement; intraobserver agreement; test acceptability (to patients and/or clinicians); adverse events; the number of polyps designated to be left in place; the number of polyps designated to be resected and discarded; the number of polyps designated for resection and histopathological examination; the length of time to perform the colonoscopy; the number of outpatient appointments; HRQoL; incidence of colorectal cancer; and mortality.
Narrow-band imaging
None of the studies reported on the interpretability of the test; test acceptability (to patients and/or clinicians), number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality.
One study, by Lee and colleagues,77 reported on interobserver agreement, although this was the agreement between the characterisation obtained during real-time assessment and that obtained by an independent reader who reviewed all recorded endoscopic images while blind to the real-time assessment and the histopathology results. The interobserver agreement was 86.5%, with a κ value of 0.730 (95% CI 0.623 to 0.837), which represents ‘substantial’ agreement. One other study, by Rogart and colleagues,74 reported interobserver agreement for 20 test images, but, as this did not include any real-time assessment these data were not extracted. Lee and colleagues77 were also the only researchers to report on intraobserver agreement. This was the agreement between the characterisation obtained during real-time assessment and that obtained by the same endoscopist who reviewed all recorded endoscopic images 1–3 months after the colonoscopy. The intraobserver agreement was 89.7%, with a κ value of 0.795 (95% CI 0.699 to 0.890), again representing ‘substantial’ agreement.
Adverse events were not reported by most studies.20,54–56,58–71,74,76,78 Of the three studies that did make mention of potential adverse events,57,75,77 all indicated that no events had occurred. Kaltenbach and colleagues57 reported no post-polypectomy bleeding, coagulation syndrome, perforation or optical misdiagnosis of advanced histopathology, Lee and colleagues77 stated that participants did not experience any procedure-related complications and Shahid and colleagues75 stated that none of the patients experienced any endoscopic complications.
Ignjatovic and colleagues70 reported on the number of diminutive polyps that would have been left in place if the management strategy was to leave diminutive hyperplastic polyps in situ in the rectosigmoid colon. The endoscopists in this study made a high-confidence optical diagnosis for 323 polyps (< 10 mm in this study) and, of these, 33 would have been left in situ. All 33 were correctly predicted to be hyperplastic polyps and all were located in the sigmoid colon or the rectum. Repici and colleagues62 made a statement indicating that, in their study, a discard-type strategy would have reduced the need for polypectomy by 48%.
Two studies reported on the number of polyps that would have been resected and discarded if a resect and discard type of management strategy had been in place. Gupta and colleagues68 reported a hypothetical strategy in which, if all the 884 diminutive polyps in their study (in which the total number of polyps of any size was 1254) were resected and discarded, this would represent a 70.5% reduction in histopathology. Using this strategy, 13 adenomas with advanced histopathological features would have been discarded. However, it must be noted that this study did not record whether characterisations were made with high or low confidence and did not report how many diminutive polyps were in the rectosigmoid colon. Ignjatovic and colleagues70 reported that a high-confidence optical diagnosis was made for 323 polyps (< 10 mm in size in this study) and, of these, 290 would have been resected and discarded. The Ignjatovic and colleagues study70 was the only NBI study to ask endoscopists to identify polyps that they would have sent electively to histopathology, even if a policy of optical diagnosis had been in place. These were polyps where the optical diagnosis was made with low confidence or where no optical diagnosis could be made. For the subgroup of diminutive polyps in this study, 7.5% (22 of 293 polyps) would have been sent for elective histopathology.
The length of time taken to perform the withdrawal phase of the colonoscopy was reported by three studies. Kaltenbach and colleagues57 reported a mean withdrawal time of 10.3 minutes [standard deviation (SD) 5.7 minutes, range 3.3–58.0 minutes]. A procedure time of 12 seconds is reported, but a definition of procedure time is not provided in the study publication, so it is not clear what this comprises. In the Kang and colleagues78 study, the mean withdrawal time in the NBI group was 13.5 minutes (SD 7.3 minutes), whereas in the Wallace and colleagues study63 it was 16.1 minutes (SD 7.3 minutes). A fourth study, Shahid and colleagues,75 reported that the average withdrawal time at their centre was typically 8–10 minutes, but withdrawal time was not reported specifically for their study. However, they did report that NBI inspection time was typically < 1 minute.
i-scan
None of the studies reported on the interpretability of the test, test acceptability (to patients and/or clinicians), number of polyps designated to be left in place, number of polyps designated to be resected and discarded, number of polyps designated for resection and histopathological examination, number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality.
One study, by Lee and colleagues,77 reported on interobserver agreement, although this was the agreement between the characterisation obtained during real-time assessment and that obtained by an independent reader who reviewed all recorded endoscopic images while blind to the real-time assessment and the histopathology results. The interobserver agreement was 87.9%, with a κ value of 0.751 (95% CI 0.640 to 0.861), which represents ‘substantial’ agreement. One other study, by Pigo and colleagues,81 reported interobserver agreement but this was based on endoscopists’ assessments of still images so, because this did not include any real-time assessment, these data were not extracted. Two studies, by Lee and colleagues77 and Rath and colleagues,82 reported on intraobserver agreement. In the Lee and colleagues study77 this was the agreement between the characterisation obtained during real-time assessment and that obtained by the same endoscopist who reviewed all recorded endoscopic images 1–3 months after the colonoscopy. The intraobserver agreement was 86.4%, with a κ value of 0.729 (95% CI 0.616 to 0.841), again representing ‘substantial’ agreement. In the Rath and colleagues’ study82 it is not clear how intraobserver agreement was assessed because no details are reported in the paper. The authors state that agreement was achieved in 113 out of 121 polyps (93.4%), with a κ coefficient of agreement of 0.867 (95% CI 0.799 to 0.967), which indicated almost perfect agreement. In the Pigo and colleagues study81 intraobserver agreement was assessed based on the endoscopists’ assessment of still images rather than real-time assessment. Furthermore, the intraobserver agreement for the evaluation of diminutive polyps was not reported, so these data were not extracted.
As already stated in Narrow-band imaging, Lee and colleagues77 reported that participants did not experience any procedure-related complications. The other four i-scan studies79–82 made no reports of adverse events.
The length of time taken to perform the withdrawal phase of the colonoscopy was not reported in any of the studies. Basford and colleagues,79 however, commented that the in vivo assessment was performed in the time between finding a polyp and preparing for polypectomy and so did not cause a significant delay. Hoffman and colleagues,80 who examined only the last 30 cm of colon, reported that with surface enhancement with i-scan the total examination time was 5 minutes.
Flexible Spectral Imaging Colour Enhancement
None of the studies reported on the interpretability of test, interobserver agreement, intraobserver agreement, test acceptability (to patients and/or clinicians), adverse events, number of polyps designated to be left in place, number of polyps designated to be resected and discarded, number of polyps designated for resection and histopathological examination, length of time to perform the colonoscopy, number of outpatient appointments, HRQoL, incidence of colorectal cancer or mortality.
Head-to-head comparisons
Head-to-head comparisons of NBI, i-scan and FICE were not within the scope of this assessment; nevertheless, two studies met the inclusion criteria for the systematic review that did compare two technologies against each other. When NBI was compared with i-scan in a prospective cohort study of the real-time histopathological prediction of diminutive colonic polyps, Lee and colleagues77 found no statistically significant differences between the two technologies (sensitivity: NBI, 88.8% vs. i-scan 94.6%; specificity: NBI 86.8% vs. i-scan 86.4%; and accuracy: NBI 87.8% vs. i-scan 90.7%; p > 0.05). In the RCT that compared NBI with FICE, Kang and colleagues78 found that for polyps < 5 mm in size there was no statistically significant difference (p > 0.05) in accuracy (NBI 74.9% vs. FICE 80.1%) or sensitivity (NBI 81.9% vs. FICE 74.5%), but there was a statistically significant difference in specificity (NBI 75.7% vs. FICE 88.4%; p = 0.006). The authors concluded that better results should be achieved for both technologies before either are used for real-time optical biopsy of colorectal polyps in colorectal screening of the general population.78 It is worth noting that in the study by Lee and colleagues77 a single endoscopist with experience of both NBI and i-scan undertook the study colonoscopies, whereas the four endoscopists in the Kang and colleagues study78 had no prior experience of either NBI or FICE.
Summary of diagnostic test performance evidence
- Thirty studies met the inclusion criteria for the systematic review of test accuracy. These assessed NBI (24 studies20,54–78), i-scan (five studies77,79-82) and FICE (three studies78,83,84). Two of the included studies assessed two of the included interventions (NBI and i-scan;77 and NBI and FICE78). The way studies reported test accuracy outcomes (in terms of the region of the colon and the level of confidence assigned to the polyp characterisation) varied.
- Most studies enrolled participants from more than one of the populations eligible for inclusion in this review (receiving colonoscopy for screening, surveillance or symptoms), but these studies did not report results separately for each participant type.
- The included studies were rated as being likely to be at a low risk of bias.
Narrow-band imaging
- In the whole colon, characterisations of diminutive polyps made with any level of confidence had a sensitivity ranging from 0.55 to 0.97 (17 studies55,56,58,62–71,74,75,77,78) and a specificity ranging from 0.62 to 0.95 (16 studies55,56.58,62–71,75,77,78). A bivariate meta-analysis (16 studies55,56.58,62–71,75,77,78) produced a summary sensitivity value of 0.88 (95% CI 0.83 to 0.92) and specificity of 0.81 (95% CI 0.75 to 0.85). For characterisations in the whole colon made with high confidence, summary sensitivity and specificity (11 studies55–57,59–65,77) were slightly higher [sensitivity 0.91 (95% CI 0.85 to 0.95) and specificity 0.82 (95% CI 0.76 to 0.87)] and limiting this analysis to studies in which the endoscopists were experienced in the use of NBI (four studies59,60,62,77) did not greatly alter these results [sensitivity 0.92 (95% CI 0.89 to 0.94) and specificity 0.82 (95% CI 0.72 to 0.89)].
- In the rectosigmoid colon, characterisations of diminutive polyps made with any level of confidence (four studies54,55,58,63) had a sensitivity ranging from 0.84 to 0.90 and a specificity ranging from 0.76 to 0.95. A bivariate meta-analysis (three studies54,58,63) produced a summary estimate for sensitivity of 0.85 (95% CI 0.75 to 0.91) and for specificity of 0.87 (95% CI 0.74 to 0.94). For characterisations in the rectosigmoid colon made with high confidence (five studies54,55,61–63), sensitivity ranged from 0.83 to 0.96 and specificity from 0.88% to 0.99%. A bivariate meta-analysis (four studies54,61–63) produced a summary estimate for sensitivity of 0.87 (95% CI 0.80 to 0.92) and for specificity of 0.95 (95% CI 0.87 to 0.98). Limiting the analysis of high-confidence characterisations in the rectosigmoid colon to the two studies54,62 in which the endoscopists were experienced in the use of NBI increased the summary values for sensitivity and specificity [sensitivity 0.90 (95% CI 0.71 to 0.97) and specificity 0.98 (95% CI 0.91 to 1.00)].
- Some studies that reported sensitivity and specificity were not included in meta-analysis because it was not possible to impute the required 2 × 2 table data. In two of three instances where this occurred, the sensitivity and specificity reported by the absent study lay within the 95% CI of the summary estimates of the meta-analysis. In one case (the meta-analysis of high-confidence polyp characterisations in the whole colon) the missing study, that by Ladabaum and colleagues,58 reported a sensitivity that lay within the 95% CI of the summary estimate but a specificity that lay outside the 95% CI of the summary estimate.
- The NPV of NBI for the characterisation of diminutive polyps in the whole colon (made with any level of confidence) ranges from 43% to 96% (16 studies55,56,58,62–71,75,77,78). Five studies55,64,66,67,69 reported NPVs of ≥ 90%, but the lower limit of the 95% CI fell below 90% in every study except one.55 When limited to high-confidence characterisations, NPVs ranged from 48% to 98% (13 studies20,55–65,77), with five studies20,55,57,64,77 reporting NPVs of ≥ 90%. However, the lower limit of the 95% CI remained above 90% in only two studies.55,64
- The NPVs of NBI for the characterisation of diminutive polyps in the rectosigmoid colon (made with any level of confidence) ranged from 87% to 98% and was > 90% in four out of the five studies that reported this outcome54,55,63,68 (but the lower limit of the 95% CI remained > 90% in only three studies54,55,68). When limited to high-confidence characterisations in the rectosigmoid colon (five studies54,55,61–63), the NPVs ranged from 92% to 99%, but the lower limit of the 95% CI fell below 90% in two studies.62,63
- Accuracy (the proportion of correctly classified polyps) of polyp characterisations in the whole colon was ≥ 90% in five studies and between 76% and 89% in 10 studies (16 studies reported this outcome55,56,58,62–71,75,77,78). High-confidence characterisations typically increased accuracy by 3–5% in studies reporting both overall and high-confidence data (eight studies55,56,58,62–65,77).
- Agreement between the surveillance interval allocated using a NBI-based strategy, and using the results of histopathology, was > 90% in 11 of the 13 studies that reported this outcome.55,57,58,61–65,67,68,70 When there was a discrepancy in surveillance intervals, the NBI-containing strategy more often led to an earlier recall for colonoscopy than would have occurred with the histopathology-based surveillance interval.
- No outcome data were reported (interpretability of the test, test acceptability, number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality) or sparse outcome data (interobserver agreement, adverse events, polyps designated as ‘left in place’, polyps designated resect and discard, time taken to perform colonoscopy) were reported for other outcomes of interest to this review.
i-scan
- Five studies77,79–82 provided sensitivity and specificity outcomes for the characterisation of diminutive polyps as adenomas or hyperplastic polyps using i-scan. Often only a single study provided data for a particular combination of the region of the colon and the level of confidence assigned to the polyp characterisation.
- In the whole colon, or in regions of the colon, characterisations of diminutive polyps made with any level of confidence ranged in sensitivity from 0.82 to 0.95 and in specificity from 0.83 to 0.96. It was not possible to meta-analyse any of these results. For high-confidence characterisations in the whole colon, or in regions of the colon, sensitivity ranged from 94% to 98% and specificity from 90% to 95%. The only meta-analysis possible, which was conducted to inform the economic model, was for high-confidence characterisations of diminutive polyps in the whole colon. The summary value for sensitivity was 0.96 (95% CI 0.92 to 0.98) and for specificity was 0.91 (95% CI 0.84 to 0.95).
- No outcome data were reported (interpretability of the test, test acceptability, polyps designated as ‘left in place’, polyps designated resect and discard, number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality) or sparse outcome data (interobserver agreement, adverse events, time taken to perform colonoscopy) were reported for other outcomes of interest to this review.
Flexible Spectral Imaging Colour Enhancement
- None of the studies provided evidence on the high-confidence characterisation of diminutive polyps or restricted their analysis to a part of the colon (e.g. the rectosigmoid colon).
- It was possible to run a bivariate meta-analysis that produced a summary estimate for sensitivity of 0.81 (95% CI 0.73 to 0.88) and for specificity of 0.85 (95% CI 0.79 to 0.90).
- None of the other outcomes of interest to this review was reported.
Pooled analysis of virtual chromoendoscopy technologies
- A pooled analysis of high-confidence decisions characterising diminutive polyps in the whole colon (NBI, 11 studies; and i-scan, two studies) was undertaken to inform a scenario analysis using the economic model.54–57,59–62,64,65,77,79 This produced a pooled summary estimate for sensitivity of 0.92 (95% CI 0.87 to 0.95) and for specificity of 0.83 (95% CI 0.78 to 0.87).
Head-to-head comparisons
- Head-to-head comparisons of the technologies were not within the scope for this assessment, but two of the included studies compared two technologies against each other. For the real-time histopathological prediction of diminutive colonic polyps, no statistically significant differences were found when a single endoscopist with experience of NBI and i-scan compared these technologies in a prospective cohort study.77 A RCT conducted by endoscopists without experience of either NBI or FICE78 found no statistically significant difference in accuracy or sensitivity, but a statistically significant difference in specificity.
Table 24 provides a summary of the pooled sensitivity and specificity values from our bivariate meta-analysis, when available.
TABLE 24
Summary of bivariate meta-analysis results
Ongoing studies
We identified 19 potentially relevant ongoing studies on the use of NBI, i-scan or FICE to characterise diminutive colorectal polyps. Two were identified from searches of clinical trials databases (see Chapter 3, Identification of studies for details of these searches) and 17 were identified from conference abstracts found by the clinical effectiveness searches. Until further details are available it is not clear whether or not all would meet the eligibility criteria for this review, but they have the potential to do so. These studies are listed in Appendix 5.
- Assessment of diagnostic studies - Virtual chromoendoscopy for the real-time ass...Assessment of diagnostic studies - Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation
- Background and introduction - Adenoidectomy with or without grommets for childre...Background and introduction - Adenoidectomy with or without grommets for children with otitis media: an individual patient data meta-analysis
- Methods - A cluster randomised trial, cost-effectiveness analysis and psychosoci...Methods - A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial
- Overall conclusions - Risk assessments and structured care interventions for pre...Overall conclusions - Risk assessments and structured care interventions for prevention of foot ulceration in diabetes: development and validation of a prognostic model
- Conclusions - Adalimumab, etanercept and ustekinumab for treating plaque psorias...Conclusions - Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...