NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Brazzelli M, Hernández R, Sharma P, et al. Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: a systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2018 Dec. (Health Technology Assessment, No. 22.72.)

Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: a systematic review and economic evaluation.
Show detailsThe evidence of the cost-effectiveness of using CDU or CEU alone or in conjunction with CTA for the surveillance of adults after EVAR was explored in the health economic component of this assessment. A two-step approach was used: (1) a systematic review of economic evaluations to retrieve any readily available evidence on cost-effectiveness, followed by (2) a de novo decision-analytic model to synthesise the available evidence on effectiveness, health-care resources used and costs. Review of the cost-effectiveness studies reports the systematic review of cost-effectiveness studies and Economic analysis with a newly developed decision model focuses on the economic model exercise.
Review of the cost-effectiveness studies
In order to summarise the available evidence on cost-effectiveness, we conducted a systematic literature review to identify studies that reported an economic evaluation of surveillance strategies for adult individuals after an EVAR intervention that included CDU and/or CEU compared with CTA.
Methods for review of the cost-effectiveness studies
Search strategy
Comprehensive search strategies were designed to identify economic evaluations of surveillance after EVAR (see Appendix 1). Searches were undertaken on 29 March 2016 and updated on 5 September 2016. The following databases were searched: NHS Economic Evaluation Database (from inception to 31 March 2015), the HTA Database (from inception to 5 September 2016) and MEDLINE In-Process and Epub Ahead of Print (from 1946 to 5 September 2016), EMBASE (from 1947 to week 36 2016) and Research Papers in Economics (from inception to 5 September 2016). The websites of HTA organisations were consulted for additional reports. The reference lists of all included studies were scanned, and appropriate experts were contacted for details of additional reports of cost-effectiveness.
The titles and abstracts of all citations identified by the search strategies for economic evaluations were screened for inclusion by a health economist (RH). The full-text papers of potentially relevant studies were retrieved and formally assessed for inclusion. Any uncertainty regarding study selection was discussed with the review team.
Inclusion and exclusion criteria
The inclusion criteria required the studies to be full economic evaluations (i.e. to consider the costs and effects of more than one strategy) in order to be included in the review. No restrictions were imposed on the way in which costs and/or effects were calculated. In addition, the study should compare post-EVAR surveillance strategies with at least one of the relevant diagnostic tests (e.g. CDU, CEU and CTA).
Either RCTs or decision-model economic evaluations were included. Studies that did not meet the inclusion criteria but reported relevant data that could inform the de novo economic model (e.g. costs, quality of life, model structure, probabilities) were retained for further consultation.
Data extraction
Data were extracted from the included studies using a prespecified data extraction form. The following information was sought:
- Background information, such as the research question, study design, intervention and comparator details.
- Characteristics of the study population (e.g. age, setting, inclusion criteria, exclusion criteria).
- Costing methodology, in particular the perspective, year, currency and the discount rate applied.
- Methodology used for the analysis of costs, effectiveness and uncertainty.
- Mean costs and outcomes, incremental costs and outcomes for the differences between groups and incremental cost-effectiveness ratios (ICERs). The results were reported when uncertainty was explored (e.g. 95% CI from the bootstrap analysis).
- Study strengths and limitations, as reported by the study authors.
- Conclusions and suggestions for further research, as reported by the study authors.
Quality assessment of the included studies
Cohort-based studies were assessed for quality against the British Medical Journal checklist for referees of economic evaluations.135 When possible, the results were assessed from the NHS perspective. No decision-modelling studies matching the inclusion criteria were identified and therefore no studies were appraised against the NICE reference case.60,136
Data synthesis
No data synthesis was attempted, and a summary of the study characteristics, the costing methods used and the quality assessment of each study is provided.
Results of the review of cost-effectiveness studies
After deduplication of the records, 278 abstracts were screened for suitability. Seven studies were selected for full-text assessment, with only five of these studies meeting the inclusion criteria.41,45,66,137,138 Two studies were conducted in the USA45,137 and three studies were conducted in Europe (in Italy,66 Ireland138 and the UK41). All of these studies considered the effects of crossing from CTA to CDU plus plain radiography as first-line surveillance test. The studies estimated the difference in the number of CTAs required by the new surveillance strategy and the replaced strategy.
Beeman et al.45 attempted to determine the cost savings and outcome differences of moving from both CTA and CDU imaging at 2 weeks, 6 months and 12 months after discharge and yearly thereafter (group 1 – before 1 July 2004) to CDU imaging as the sole surveillance test after the 2-week scans (group 2 – after 1 July 2004), with CTA being conducted only if any problem was detected by CDU. The authors analysed data on 82 patients for group 1 and on 117 patients for group 2. The clinical outcomes included the number of endoleaks detected and the measurement of the AAA sac diameter. The average length of the follow-up period was 3.5 years (range 0–9 years) and 1.6 years (range 6 months–4 years) for groups 1 and 2, respectively. The authors used hospital charges for CTA and CDU and noted that the decreased charges of US$1595 per patient per year (2008 prices) or US$198 per patient per year using Medicare reimbursements were realised by eliminating CTA surveillance in group 2. Moreover, the sensitivity of CDU for detecting endoleaks was 0.71 and the specificity was 0.99, whereas the sensitivity of CTA was 0.731 and the specificity was 0.991. The authors could not find any difference in aneurysm sac diameters measured by CDU and CTA when these scans were performed within 1 month of each other in group 1. Although the authors do not advocate eliminating CTA from the surveillance protocols, they state that its use should be limited to those circumstances in which it could provide other details about problems first detected by the ultrasound examination.
Bendick et al.137 evaluated the use of an ultrasound contrast agent to enhance imaging for stent–graft surveillance and compared the costs of this technique with those of CTA. Data on the first 40 patients received in their vascular laboratory were analysed. The follow-up examinations ranged from 1 to 24 months after graft placement, with a mean follow-up time of 13.7 ± 6.1 months. Clinical outcomes included type I or type II endoleaks and costs were calculated using hospital charges with average costs per study that were equal to US$2779 for CTA, US$525 for CDU (including contrast) and US$147 for plain film abdominal radiography. No details of the price year were stated. The authors reported a sensitivity to the presence of any endoleaks of 53% (8/15) for CDU, 93% (14/15) for CEU and 73% (11/15) for CTA. The average 3-year charges per patient were US$22,232 and US$5376 for CTA-based surveillance and surveillance using CDU (including contrast) plus radiography, respectively (a saving of US$16,856 per patient over 3 years). The authors concluded that the technique of duplex ultrasound with an ultrasound contrast agent should become the method of choice for stent–graft surveillance if the promising early results shown in their present series can be demonstrated in a larger patient population.
Chisci et al.66 evaluated whether or not the imaging modality of surveillance influenced the detection of these conditions affecting the rate of asymptomatic secondary interventions (i.e. endoleaks, AAA expansion, migration, graft infection, graft thrombosis, conversion to open repair, postoperative renal impairment, bowel ischaemia and myocardial infarction). The authors followed a cohort of individuals for whom the follow-up protocol was changed at a given date. Protocol I, performed from January 2003 to December 2006, consisted of CDU plus CTA at 1 month after the procedure and every 6 months thereafter. Protocol II, performed from January 2007 to June 2010, included CDU plus CTA at 1 month after operation, CDU plus plain radiography every 6 months thereafter and CTA carried out during follow-up only for specific conditions. The authors analysed data for 376 individuals in protocol I and 341 individuals in protocol II with a mean follow-up of 1148 days (range 1–3204 days) and 942 days (range 1–1512 days), respectively (p < 0.001). On the 3-year analysis, the authors reported that protocol I cost approximately €3000, whereas protocol II cost approximately €1000; this was a threefold reduction in overall costs for protocol II (p < 0.0001). However, there were no details of the costing method used or the cost categories included in this analysis. The authors concluded that the detection rate of asymptomatic secondary interventions following EVAR is not affected by the type of surveillance imaging and that a surveillance schedule based primarily on CDU and radiography appears to be justified.
Gray et al.138 retrospectively reviewed the CDU and CTA scans of all 145 patients who underwent EVAR at the Mater Misericordiae University Hospital, Ireland, from 1 June 2003 to 1 July 2010 and compared their results for endoleak detection and determination of residual sac size. The authors’ aim was to assess the cost savings obtained if CDU was employed as a first-line surveillance tool following EVAR and to compare CDU and CTA in terms of efficacy. A total of 484 scans (68%) from 114 patients (78.6%) were available for comparison. The hospital protocol for patients after EVAR included CDU and CTA scans of the aorta within 7 days of surgery. After discharge, all patients underwent CDU at 1 month and then CDU and CTA at 6 months, 12 months and annually thereafter, provided that there was no documented endoleak on either the CDU or CTA. The costs of CTA (€500 per scan) and of radiography (€85) were considered in the costing calculations (expressed in 2010 prices). However, no details of the unit cost sources were reported. The authors found that CDU was 100% sensitive and 95.7% specific in the detection of endoleaks, with a positive predictive value of 28.7% and a negative predictive value of 100%. Furthermore, no statistically significant difference between the two imaging modalities was detected for the determination of residual AAA sac diameter. The authors hypothesised that a reduction in costs resulted from a change in protocol for the year 2010. Adopting a protocol with CDU and abdominal radiography as the first-line surveillance tool would result in a reduction in the number of postoperative CTA scans from 235 to 36. This would equate to a reduction in expenditure from €117,500 to €34,915 (a saving of €82,585). The authors concluded that CDU combined with plain abdominal radiography could safely replace CTA as the primary long-term imaging modality, resulting in a significant cost saving without the loss of scan accuracy.
The only study conducted in the UK41 was a retrospective review of a prospectively maintained database of all patients undergoing elective, standard EVAR at a large tertiary referral centre (Royal Liverpool University Hospital). As with the other studies, the authors assessed the efficacy of a modified post-EVAR surveillance protocol based, primarily, on CDU and radiography, with CTA triggered only by significant findings on the CDU scan or radiography. The study included patients who had their EVAR operation between 1 August 2005 and April 2009, for whom at least 1 year’s post-surgical follow-up data were available. The primary outcome measure considered was aneurysm rupture, whereas the secondary outcome measures included the requirement for secondary intervention and the number of CTA scans avoided, from which the radiation dose reduction and cost savings were calculated. The costs were expressed in 2010–11 prices and NHS tariffs were used to cost the tests (radiography, €35.71; CDU, €187.47; CTA, €269.61; exchange rate: £1 = €1.18).41 The authors analysed data on 194 patients who underwent a total of 606 sets of surveillance imaging: 194 sets at 1 month (radiography, CDU and CTA) and 412 per protocol sets thereafter (radiography and CDU). No patient presented with ruptures or aneurysm-related complications that were not identified by the modified surveillance protocol. The authors obtained the number of tests performed in the modified protocol and compared this with those that would have been performed for the same group had the protocol not been modified: 412 tests would have been conducted for the follow-up period that would have costed €14,711 for radiography, €77,326 for CDU and €111,078 for CTA. With the modified protocol, the number of tests and costs for radiography and CDU remained the same. However, only 71 CTA scans were conducted, with a cost of €19,142, which was a saving of €91,936. The authors concluded that follow-up after EVAR primarily based on CDU and radiography was feasible and safe, and reduced the use of CTA substantially, with consequent reductions in exposure to ionising radiation or an intravascular contrast medium, and costs.
Summary
Five studies met the inclusion criteria. All of these compared a surveillance strategy based on CDU or CEU with a CTA-based strategy. All of the studies assessed the reduction in costs as a result of the smaller number of CTA scans performed in the modified protocol. Only cohort studies were identified in the searches. However, the studies by Beeman et al.45 and Chisci et al.66 compared cohorts before and after the protocol changes took place. In the other studies, an economic analysis was conducted on the basis of the resources required (i.e. the number of CTA scans performed) if a hypothetical alternative protocol were to be used. Although all of the studies41,45,66,137,138 fairly agree on the clinical outcomes of interest (i.e. endoleaks, AAA size and the need for secondary interventions), the reporting of costs and the cost method was disparate; although one study gave details of the cost categories, the unit cost used, the sources and the price year used, another study reported only the final cost calculations.
The only study from the UK that could inform NHS policy was the study conducted by Harrison et al.41 However, neither this nor any of the non-UK studies used a preference-based measure of effectiveness. Moreover, judging from the number of scans used in the authors’ cost calculations, the follow-up period considered was just over 2 years. This time horizon might not be long enough to consider all of the costs and consequences that are relevant for the question posed. As such, a new economic model was developed to assess the relative efficiency of CDU or CEU in the surveillance of individuals after EVAR. This is reported in the next section.
Economic analysis with a newly developed decision model
None of the available economic evaluations from the systematic review provided a definite answer on the cost-effectiveness of the use of CDU or CEU compared with that of CTA from the NHS perspective. Therefore, a de novo economic model was developed. The aim of the economic model was to assess the relative efficiency of surveillance strategies that used CEU or CDU alone or in combination with CTA.
Methods
Care pathways
Care pathways were discussed and developed within the project management group and the project advisory group meetings. It was agreed that surveillance involves the search for information about abnormalities that are relevant to the disease. It was also agreed that, once there is any indication of an abnormality, the patient status changes and the surveillance stops. After this, the following steps are then part of diagnostic investigations and/or eventual treatment. Hence, surveillance applies only to those individuals who are deemed to have no detected EVAR-related abnormalities and, as such, the model considers those patients who were regarded as not having an EVAR-related complication (e.g. at 6 months post surgery).
Five surveillance strategies were agreed:
- annual CTA scan plus plain radiography
- annual CDU scan plus plain radiography
- annual CEU scan plus plain radiography
- colour duplex ultrasound scan together with CTA scan and plain radiography at 1 year, followed by CDU scan and plain radiography on an annual basis
- contrast-enhanced ultrasound scan together with CTA scan and plain radiography at 1 year, followed by CEU scan and plain radiography on an annual basis.
A positive test result in any of the surveillance strategies would trigger either further diagnostic investigations or treatment. This part of the decision model was identical for all of the strategies.
The economic model
A Markov model approach was selected for the decision-analytic model exercise.135 Markov models have Markov states in which individuals spend a period of time, which is named a ‘cycle’. At the end of each cycle, the individuals can remain in their current Markov state or move to another state. The probabilities of moving to other Markov states or remaining in the current state are named ‘transition probabilities’. Individuals in the model would accrue costs and benefits (e.g. life-years) depending on the time spent in each Markov state and the interventions and/or events modelled within each Markov state. Markov models are particularly suitable to model recurrent issues and chronic diseases. They allow the incorporation of health states to reflect the movement of patients during surveillance, further diagnosis and treatment. In the current study, model states reflect the underlying condition (e.g. post-EVAR state with known or unknown complications), together with the decision on treatment (e.g. reintervention after EVAR). In all of these models, an absorbing state is included, in which all individuals would end up if the model was run for a sufficiently long period of time (e.g. Markov death state).
Description of the Markov model and the model structure
The model overall state-transition diagram is reported in Appendix 14. A simpler, schematic representation of the Markov model is shown in Figures 6–9. In these figures, circles represent the Markov states, whereas squares represent an event that occurs within a Markov state (e.g. an emergency procedure). Arrows show the direction of the possible transitions in the model. Unless specified, individuals can remain in a Markov state for more than one cycle. Eight Markov states are considered in the model:

FIGURE 6
Schematic diagram of the surveillance for EVAR Markov model; underlying condition.
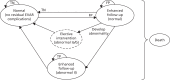
FIGURE 9
Schematic diagram of the surveillance for EVAR Markov model (from normal, no residual EVAR complications Markov state). FP, false positive; TN, true negative; TP, true positive.
- normal (no residual EVAR complication)
- abnormal Ia (intervention required)
- abnormal Ib (intervention required – endoleak)
- abnormal II (no intervention required)
- enhanced follow-up (normal)
- elective surgery (one cycle, temporary state)
- enhanced follow-up (abnormal II)
- death.
Figure 6 shows the four Markov states that reflect the underlying condition but are yet to be detected (‘undiagnosed’ states 2–4 above). Figures 7–9 show one of these four Markov states representing the underlying condition together with the Markov states that individuals can move to (e.g. those states that result from a diagnosis – the ’diagnosed’ side of the figure – correctly or not). The performance of a surveillance strategy in this model is given by the correct identification of those individuals with an abnormal condition and the corresponding transfer of those individuals into the true-positive states on the right sides of Figures 7–9.
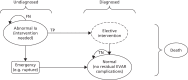
FIGURE 7
Schematic diagram of the surveillance for EVAR Markov model (from abnormal Ia, intervention needed Markov state). FN, false negative; TN, true negative; TP, true positive.
All individuals start in the ‘normal (no residual EVAR complication)’ health state and can develop abnormalities as the model runs (see Figure 6). The surveillance strategies aimed to detect a variety of conditions and complications . These were generically described as ‘abnormalities’ and were divided into two categories: conditions that trigger an elective intervention and conditions that, on clinical assessment, necessitate closer follow-up (e.g. additional 6-month CTA scans). The first category was subdivided into two: abnormal 1a includes non-endoleak-triggered interventions (e.g. limb occlusions, graft infections) and abnormal 1b counts for the endoleak-prompted interventions (e.g. type I, III and IV, type II or endotension with sac expansion > 5 mm). Examples of patients with conditions within the close follow-up group (abnormal II) are those with type II endoleaks with a sac expansion of < 5 mm in 6 months or with limbs with kinking or partial thrombosis.
These abnormal Markov states are tunnel states. In tunnel states, individuals go through the health states in a defined sequence. The rationale behind these tunnel states for individuals with an abnormality is to count the length of time for which the individuals have had the abnormality. Thus, the probability of experiencing a rupture as a result of, for instance, a type I or III endoleak will increase as length of time for which the individual has had the endoleak increases (i.e. the length of time that the individual remains in the undetected health state). Finally, individuals can move from any health state to the absorbing death state (presented, for simplicity, at the side of the figures).
Abnormal Ia
Once an abnormal condition has been identified, individuals move to another Markov state, in which they can be treated (see Figure 7) or followed up more closely (see Figure 8). Figure 7 shows the pathway of individuals who developed a non-endoleak abnormality that would require elective surgery. If the condition is undetected through surveillance (a false-negative result), the person remains in the abnormal Ia state. This person can experience an event and, as a result, go through an emergency intervention. If the situation is resolved, the person would move back to the original normal state. If the underlying condition is detected through surveillance (a true-positive result), the individual would move to the elective surgery state. This is a temporary state and individuals can remain within this state only for one cycle (to have surgery and subsequently move on to another health state). Again, once the surgical intervention is successful, the individual moves back to the original normal state. Moreover, individuals can move to the absorbing death state from any of the other health states as a result of an emergency (e.g. rupture), surgery (e.g. hospital mortality) or other comorbidities (general population mortality). A similar structure was followed for individuals who developed an endoleak abnormality that required an elective intervention (abnormal Ib Markov health state).

FIGURE 8
Schematic diagram of the surveillance for EVAR Markov model (from abnormal II, no intervention needed Markov state). FN, false negative; TN, true negative; TP, true positive.
Figure 8 shows the pathway for the abnormal II health state. Undetected individuals (a false-negative result) will remain in this state (i.e. represented by the back arrow from the state). These people have the risk of experiencing an event that would trigger an emergency intervention. If the abnormality is detected (a true-positive result), the individuals will move to enhanced follow-up, which is defined as 6-month visits at which a CTA-based assessment is conducted. If the patient is stable and no further interventions (e.g. elective surgery) are decided after 2 years (four model cycles), the patient returns to the surveillance pathway (e.g. annual check-ups based on the original surveillance test – CDU, CEU or CTA).
Abnormal II
The model also includes a false-positive state for those individuals without an abnormal condition (see Figure 9) but with a positive test result [e.g. enhanced follow-up (normal)]. If the individual developed an abnormality while under enhanced follow-up, they would either move straight to elective intervention (e.g. for abnormal Ia or Ib) or remain under enhanced follow-up but within a different Markov state (e.g. for abnormal II).
Normal state
Individuals can suffer an event between surveillance visits. This is considered in the model to be an event within a Markov state and is shown in Figures 6–9 as a square (e.g. emergency procedure). The model assumes that individuals who survive an unsuccessful second intervention could undergo a third intervention. However, a pragmatic assumption based on small probabilities was made and individuals can either have a successful third intervention or die.
Parameter estimates used in the economic model
The parameter estimates required to populate the economic model were obtained from the results of the clinical effectiveness search, which was supplemented by structured and focused searches (e.g. of EVAR trials with a longer follow-up period). When no suitable data resulted from these searches, expert opinion was sought. Probabilities gives details of the probabilities, unit costs and utility weights used in the model. Also provided within this section are details of the probability distributions used for the probabilistic sensitivity analysis.
Probabilities
The model starts with the whole cohort in the normal state, and therefore no prevalence data were necessary. The annual incidence of abnormalities was developed from data reported by Tang et al.,93 based on ENGAGE (Table 15). The authors report 1-year data, excluding the first 31 days after EVAR. From a total of 325 patients, 25 abnormalities were reported (20 endoleaks, one stent–graft occlusion, three stent–graft stenoses and one other event related to the stent–graft). These data were initially used to obtain the proportions of cases within each of the model abnormal subgroups (graft occlusion for abnormal Ia, types I and III endoleaks for abnormal Ib, and type II endoleak for abnormal II). However, in consultation with the experts in the project advisory group, these figures were revised, as it was believed that a higher proportion of abnormalities Ia and Ib are currently seen in UK practice (Professor Srinivasa Rao Vallabhaneni, and Dr Russell Jamieson, NHS Grampian, 2017, personal communication). Beta distributions were used to assess the uncertainty around the central point estimates.
TABLE 15
Incidence and mortality
Unfortunately, the systematic review of clinical effectiveness could not identify any studies that directly compared the performance of alternative CDU and CEU surveillance strategies. Therefore, test performance data were used to feed the model. Test sensitivity and specificity (Table 16) were obtained from the systematic review by Karthikesalingam et al.132 Alternative data were available (see Diagnostic performance of imaging modalities for surveillance after endovascular abdominal aortic aneurysm repair in Chapter 2). However, Karthikesalingam et al.132 is the only review reporting sensitivity and specificity for all three tests (CDU, CEU and CTA), and the quality assessment of all of these diagnostic review studies resulted in the Karthikesalingam et al.132 review being deemed to be of higher quality. Beta distributions were used to address uncertainty around the central parameter values.
TABLE 16
Test sensitivity and specificity
The probability of having a reintervention and the risk of rupture and mortality are reported in Table 17. The probability of having a reintervention was developed from Tang et al.93 Disregarding the first month post EVAR, 13 individuals out of 319 had a secondary procedure. The proportion of successful secondary procedures was based on the proportion of individuals free of a secondary intervention in the EVAR 1 trial – 15-year follow-up data.48 The model allows for individuals with an unsuccessful surgery to go to a third procedure. We found no data to inform the model parameters related to the third intervention (e.g. the probability of having a third intervention and the proportion of successful interventions) and therefore the data as for the second intervention were applied.
TABLE 17
Reintervention, rupture and mortality
The risk of rupture for undetected endoleaks was based on an analysis of early data from the EUROSTAR registry. Buth et al.91 conducted two cohort analyses comparing a cohort of people with type I and type III endoleaks (n = 297) with those who had never experienced an endoleak (n = 1975), and a cohort of people with type II endoleaks (n = 320) with those who had never experienced an endoleak (n = 3275). The authors state that the cumulative rate of rupture from type I and type III endoleaks was 4% at 2 years compared with 0.7% for those who had never experienced an endoleak. Moreover, the number of late ruptures in patients with type II endoleaks was not significantly different from the number of late ruptures in those who had never experienced an endoleak. The risk of rupture for individuals with type I and type III endoleaks after 1 year was adjusted based on data reported by Moll et al.31 The authors report the risk of rupture according to aneurysm size, based on population studies. To calculate the risk in the model, an aneurysm size of 55 mm at baseline was assumed, together with a growth rate of 5 mm per cycle. These rupture risks were applied to the undetected abnormal Ib group in the model only.
Table 17 also reports the mortality data assumed in the model. Age- and gender-specific general population mortality rates were applied to the cohort. The risk of surgical death in an elective setting was based on expert opinion (Professor Srinivasa Rao Vallabhaneni and Dr Russell Jamieson, personal communication). The main event that surveillance is trying to avoid is the aneurysm rupture and its associated high mortality rate. The risk of death from a rupture was calculated based on the systematic review and meta-analysis of late ruptures by Antoniou et al.139 The authors identified 11 studies (case series) that reported a total of 190 ruptures: 30 patients were managed with palliative care or died before surgery. Moreover, the authors reported a perioperative mortality rate of 32% (95% CI 24% to 41%).
Costs
Unit costs were obtained from NHS Reference Costs 2015 to 2016140 (Table 18). The unit costs for ultrasound tests (with and without contrast) of < 20 minutes’ duration reported in the NHS reference costs are surprisingly similar. Moreover, the stated average unit cost for an ultrasound with contrast was lower than an ultrasound without contrast. Therefore, the unit cost for a vascular ultrasound scan was used to cost CDU and CEU tests. The clinical experts noted that clinical staff (i.e. a consultant radiologist) should be present to administer the contrast agent for CEU. In addition, CEU includes a contrast agent [i.e. sulfur hexafluoride or perfluorocarbon encapsulated by a phospholipid shell (SonoVue)] with an associated cost of £46 for a 10-vial box (Mr Craig Rore, Grampian Medicines Information Centre, 2017, personal communication). Therefore, the unit cost of CEU was adjusted to add the cost of 30 minutes (Professor Srinivasa Rao Vallabhaneni, personal communication) of a medical consultation (based on a cost per hour of £135)141 plus £4.60 for the cost of the contrast. Furthermore, the cost of a CT scan of one area, with pre and post contrast, was used for CTA. Notably, NHS Reference Costs 2015 to 2016140 does not report the unit cost for a plain radiography. The cost of a plain radiography is absorbed within other cost categories (e.g. outpatient visit) because of its relatively high volume and low cost. As plain radiography was considered in all of the strategies in a similar manner (on an annual basis), an executive decision was made and the unit cost of plain radiography was not incorporated in the model. If a surveillance test outcome was indeterminate, a further assessment was assumed (i.e. with CTA) and the cost of a visit was added to the cost of the subsequent test (i.e. non-admitted face-to-face attendance, follow-up – vascular surgery).
TABLE 18
Unit costs
Endovascular AAA repair reintervention was costed as a weighted average of the unit costs for elective EVAR repair (complex and non-complex). The cost of percutaneous transluminal embolectomy or thrombolysis was used as the cost of other procedures for the abnormal Ia group. Emergency procedures were costed assuming non-elective categories plus the cost of emergency medicine (i.e. any investigation with category 5 treatment) and ambulance service (i.e. see and treat and convey).
Utility weights
Population-based utility weights for patients aged ≥ 74 years were assumed for individuals after EVAR (Table 19). These utility weights were calculated using the equation provided by Ara and Brazier142 [i.e. EuroQol-5 Dimensions (EQ-5D) = 0.9508566 + 0.0212126 × (1, if males, or 0, if females) – 0.0002587 × age – 0.0000332 × age2].142 The rationale behind this is that the condition is mostly asymptomatic and, as such, would have no effect on the individual’s quality of life. Interestingly, this utility weight is of a similar value to the one reported by Brown et al.143 on the EVAR 1 RCT for baseline EQ-5D score [mean 0.75 (SD 0.22); mean age 74 years]. The utility decrement for those going into any reintervention was developed from the EVAR 1 trial.143 This decrement was calculated as a proportional reduction from baseline until the first year post EVAR, when patients are assumed to be back to the population-based utility weight.
TABLE 19
Quality-of-life weights
Base-case analyses
The model base-case analysis was run for a cohort of 74-year-old men for a lifetime time horizon. A 6-month cycle length was defined. The analysis was conducted from the NHS and Personal Social Services perspective. Costs were expressed in 2015–16 Great British pounds and effectiveness was expressed in quality-adjusted life-years (QALYs). Costs and QALYs were discounted at an annual rate of 3.5%.60 The cost-effectiveness analysis results are reported using ICERs.60 ICERs are calculated as the ratio of the difference in expected costs between two alternative strategies to the difference in expected QALYs. This ratio measures the additional costs that would have to be paid in order to obtain an extra unit of effectiveness (i.e. an extra QALY). A probabilistic sensitivity analysis was conducted, in which 10,000 iterations were run. The stability of results was verified by examining the probabilistic results for a lower number of iterations (e.g. 1000). The probabilistic analysis results are reported using cost-effectiveness acceptability curves (CEACs).144,145 These curves show the probability that a particular strategy is cost-effective at alternative values of willingness to pay for an extra QALY.
Assessment of uncertainty (sensitivity analysis)
A number of sensitivity analyses were conducted to address the uncertainty in this economic evaluation (one-way, two-way, threshold, scenario and probabilistic sensitivity analyses).
Approximately 90% of EVAR procedures in the UK are conducted in males (see Epidemiology of abdominal aortic aneurysm). Therefore, the base-case analysis was run for a male cohort. Gender-specific data were not available and the only differing data for men and women were general population mortality rates and utility weight. Female utility weight for 74-year-olds is lower (0.75) than that for males (mean 0.77), but mortality data show a longer life expectancy that could result in a longer time for benefits, but also costs. Therefore, a further analysis was conducted using these data, to observe the effect of longer life expectancy in the model results. In addition, one-way sensitivity analyses were conducted on all cost categories (e.g. cost of tests, visits and surgery), test diagnosis sensitivity and specificity, incidence of abnormalities, adherence to surveillance and mortality as a result of an unexpected event (rupture) and emergency intervention. Ranges to run these analyses were informed by the lower- and upper-unit cost quartiles published in NHS Reference Costs 2015 to 2016140 (cost variables), the 95% CI reported by Karthikesalingam et al.132 (sensitivity and specificity) and, for those variables for which there were no published data available, by expert opinion (e.g. adherence to surveillance).
Given the base-case and sensitivity analyses results, a threshold analysis was conducted for the cost of CEU, which explored the value that would make CEU cost-effective. Two scenario analyses were developed; the first assumes that the information from the CEU test is perfect, that is, sensitivity and specificity are equal to 1, plus no indeterminate or inconclusive results. This scenario corresponds to the notion that CEU could be the present reference standard.
A further scenario analysis was implemented, which assumed a cohort with a higher proportion (50%) of individuals belonging to abnormal Ib group together with a higher overall incidence for any abnormality (up to 10% per 6-month cycle). This scenario explored the effects of monitoring only those individuals at high risk of developing abnormalities.
The base-case and selected sensitivity analyses results are presented in Results. The full sensitivity analysis results are reported in Appendix 15.
Results
In Table 20, the base-case analysis results are reported. Annual follow-up with CDU only is the strategy with the lowest expected cost, followed by CTA only and CEU only. The strategies with higher expected costs are those that use CDU or CEU in conjunction with CTA at the start. In Table 21, the strategy expected costs are disaggregated into three cost categories: costs of surgical procedures, costs of surveillance visits and costs of tests. Consistently throughout the alternative strategies, surgical costs represent a higher proportion of the total costs. For the strategies involving CTA, the costs of the test represent over 30% of the total costs. The costs of visits were considered only in the case of a reassessment, and therefore these represent the lowest proportion in all of the surveillance strategies (i.e. between 6% and 13%).
TABLE 20
Base-case cost-effectiveness results: men
TABLE 21
Base-case cost results: disaggregated
The CTA-only strategy produces the lowest number of expected QALYs (see Table 20). This can be explained by the relatively low sensitivity of CTA that was assumed in the model. As such, the CDU-only strategy dominates the CTA-only strategy (i.e. CDU has lower expected costs and a higher number of expected QALYs than CTA only). Moreover, adding CTA to CDU or CEU at the start results in more QALYs than using only one imaging modality. However, either these strategies are dominated (i.e. CDU and CTA, then CDU) or the incremental cost for an additional QALY is well above the often-accepted cost-effectiveness threshold [£30,00060 (i.e. CEU and CTA, then CEU)]. Furthermore, CEU-based strategies result in a higher number of expected QALYs than all of the other strategies, although the ICER to adopt any of the CEU-based strategies is well above the £30,000 threshold.60
Figure 10 shows the cost-effectiveness plane for the base-case analysis. For ease of interpretation, square data markers were used for CDU-based strategies, triangle data markers were used for CEU-based strategies and dots were used for CTA-only strategies. It can be clearly observed that the CEU-based strategies produce more QALYs, but at higher expected costs, than the CDU-based strategies. Furthermore, it is of note that the CDU plus CTA and then annual CDU strategy is dominated by the CEU-only strategy. However, the ICERs to move to any of these strategies (either CDU and CTA, then CDU or CEU only) from CDU only are well above the usual cost-effectiveness threshold.
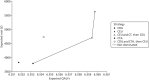
FIGURE 10
Base-case cost-effectiveness results: men.
Table 22 shows the probabilistic sensitivity analysis results for the base case. Annual follow-up with CDU only has the highest probability of being cost-effective for any value of willingness to pay for an extra QALY below £50,000 (i.e. a probability of > 58%). Surveillance with CTA only has a probability of between 32% and 42% of being cost-effective at values of willingness to pay for an extra QALY of between £10,000 and £50,000. Adding CTA to CDU or CEU has a zero probability of being cost-effective at any willingness-to-pay values. Finally, as surveillance with CEU only produces more expected QALYs, this strategy has a growing probability of being cost-effective at increasing willingness-to-pay values. However, at £50,000, its chance of being cost-effective is just 4.1%. Figure 11 presents the CEACs. It is worth noting that the probability of CDU being cost-effective stabilises at around 60% for high values of willingness to pay for a QALY. At higher values than those reported in the figure, surveillance with CEU increases its chances of being cost-effective compared with CDU- and CTA-only strategies (i.e. 27% at £100,000 – data not shown). Finally, the ICERs calculated with the probabilistic analysis were lower than the deterministic base-case analysis reported in Table 20 (i.e. ICER for CEU with respect to CDU: £129,700 and for CEU and CTA; then CEU strategy with respect to CEU only: £2,479,000). However, these ICERs are all well above the usual threshold used in the UK (e.g. £30,000).
TABLE 22
Probabilistic analysis results

FIGURE 11
Cost-effectiveness acceptability curves, base case: men.
Sensitivity analysis results
Base-case analysis for women
Table 23 shows the results of the base-case analysis for women. General population mortality data146 for women were used for this analysis, as well as the utility weight for women > 74 years of age (i.e. mean 0.75), using the methods provided by Ara and Brazier 2010.142 Expected costs and QALYs for women are generally higher than for males, reflecting the longer life expectancy of women. Overall, the results are very similar to those of the base-case analysis for men, with CDU having the lowest expected cost, followed by CTA only and surveillance with CTA only being dominated by surveillance with CDU only. CEU-based strategies have ICERs that are well above the often-used willingness to pay for an extra QALY threshold.60 Owing to the similarity of these results to those for the males model run, all other sensitivity analyses were conducted using data for only males.
TABLE 23
Base-case analysis: women
One-way sensitivity analysis results for the unit cost of the CDU test are reported in Table 24. The upper quartile for a vascular ultrasound in NHS Reference Costs 2015–16140 is £70. For this reason, a range up to £80 was used in an attempt to include other plausible values. The base-case unit cost for a CDU test was £58; thus, for any values below this, the base-case results hold. At a CDU unit cost of £80, CDU is more costly than CTA, and, therefore, CTA becomes cost-effective. CEU only improves its cost-effectiveness compared with CDU as the unit cost for CDU increases. However, the ICERs for CEU-based strategies are still above the £30,000 threshold, at a unit cost of £80 for a CDU test.
TABLE 24
One-way sensitivity analysis for the unit cost of a CDU test
Table 25 presents the results for the one-way sensitivity analysis for the unit cost of CEU. The lower quartile reported in NHS Reference Costs 2015 to 2016140 for a vascular ultrasound was £39; thus, a lower value was used in order to consider other lower plausible values. In addition to the £72.10 for the contrast agent and the extra staff involved (who remained unchanged for the present one-way sensitivity analysis), the ultrasound cost for the CEU base-case analysis was £58, so any values above this would result in CEU being less cost-effective. The base-case results are robust to changes in the cost of the CEU test. A threshold analysis was conducted, and, owing to CEU being more sensitive but less specific, CEU needs to be slightly cheaper than CDU in order to become cost-effective.
TABLE 25
One-way sensitivity analysis for the unit cost of a CEU test
Test performance
Two-way sensitivity analyses were conducted for sensitivity and specificity for each compared test. Figure 12 presentd the results for CDU. The figure shows the strategy with the highest net benefit at £30,000 per QALY according to alternative values of sensitivity and specificity for CDU. The value ranges used were broader than the 95% CIs reported by Karthikesalingam et al.132 (i.e. sensitivity, 95% CI 0.62 to 0.83; specificity, 95% CI 0.90 to 0.97), in order to explore the effects of alternative plausible figures. At low levels of sensitivity and specificity for CDU, the imaging strategy that has the highest net benefit is CTA. Surveillance with CDU only has the highest net benefit at 93% specificity (or higher), regardless of the CDU sensitivity.
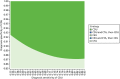
FIGURE 12
Two-way sensitivity analysis: CDU test sensitivity and specificity (net benefit, willingness-to-pay threshold of 30,000).
Perfect information from contrast-enhanced ultrasound
There was an indication from the clinical experts that the CEU test might have become the reference standard. A scenario analysis was conducted, assuming perfect information from CEU. That is, it was assumed that sensitivity and specificity were equal to 100% and that no indeterminate or inconclusive results were possible. Moreover, a threshold analysis was conducted to explore the difference in cost between CEU and CDU that would make CEU cost-effective. The results of this analysis are reported in Table 26 and show that, if the test performance from CEU is assumed to be perfect, a cost difference of up to £55 between CEU and CDU could make CEU cost-effective at a threshold value of willingness to pay for a QALY of £30,000. Larger cost differences would shift the ICER above the frequently used cost-effectiveness threshold (i.e. £30,000).
TABLE 26
Scenario analysis assuming perfect information from CEU. Value refers to the cost difference between CEU and CDU
High-risk patient group
A relatively more sensitive test can be beneficial when a higher proportion of individuals have the condition under study. A scenario analysis was considered in which half of the abnormal individuals belonged to the abnormal Ib group (e.g. types I and III endoleaks, plus other conditions necessitating elective intervention). Table 27 presents the results from a one-way sensitivity analysis for the incidence of any abnormality. The base-case analysis assumed circa 4% incidence per 6-month cycle. In the present analysis, this incidence of abnormalities implies that 2% of abnormalities are type Ib. Alternatively, the percentages in Table 27 could be broadly interpreted as the annual incidence of Ib abnormalities. To facilitate the comparison between CDU- and CEU-only strategies, in Table 27 the ICER for the CEU-only strategy has been calculated with respect to the CDU-only strategy and not the strategy with an immediately lower cost (i.e. CDU and CTA, then CDU). The results show that CEU is more cost-effective than CDU when the incidence of group Ib abnormalites is > 6% per year (3% incidence per 6-month cycle, which corresponds to 6% in Table 27).
TABLE 27
Scenario analysis assuming that 50% of abnormalities are Ib (e.g. types I and III endoleaks)
Summary of cost-effectiveness
This chapter reported on a systematic review of economic evaluations and a model-based economic evaluation of alternative strategies to monitor individuals after an EVAR intervention. Similarly to the systematic review of clinical effectiveness, only cohort studies were identified in the systematic review of economic evaluations. Five studies met the inclusion criteria. Although two studies45,66 compared outcomes before and after a surveillance protocol took place, the three remaining studies hypothesised the resource use implications (i.e. the number of CTA scans performed) of moving to surveillance strategies, using ultrasound as first-line test. Only one study gave full details of the cost calculations. Moreover, none of the studies assessed the relative efficiency of CDU and CEU, which is addressed by the current assessment. As such, the studies identified were unsuitable to fully inform the study question posed and therefore unlikely to help decision-making in the UK. Thus, a new model was developed following UK methodological guidelines.60
The developed model included five strategies. Three of these were CTA, CDU or CEU used on an annual basis, and the two other strategies considered CTA in addition to CDU or CEU for the first surveillance visit, with CTA scans being conducted afterwards only if further investigations were needed. Plain radiography was included in all of the strategies as part of the surveillance assessment.
The model base-case results show that, once the primary EVAR surgical complications have been discarded, surveillance with CDU as a first-line test becomes the less expensive option. This strategy is less expensive and produces more expected QALYs than a strategy that uses CTA only. Adding CTA to CDU in the first surveillance visits is not worthwhile. Moreover, surveillance strategies based on CEU result in more expected QALYs, but are also more expensive, and the ICERs are well above the usual threshold used in the UK (i.e. £30,000). The our base-case probabilistic analyses show that the CDU-only strategy has a probability of being cost-effective of between 57% and 64%, depending on the cost-effectiveness threshold (e.g. 62% at £30,000).
Extensive sensitivity analyses were conducted (see Appendix 15), with the base-case results being robust for the great majority of these. The sensitivity analysis showed that a CEU-only strategy could become cost-effective at very high rates of test sensitivity and specificity (e.g. when it was assumed to produce perfect information – sensitivity and specificity of 100% and no indeterminate results). Even in this case, the cost difference between CDU and CEU should not be above £55 for CEU to be cost-effective at a £30,000 threshold of willingness to pay for an additional QALY.
As risk stratification of patients might become a feasible option, a further sensitivity analysis was conducted to explore the effect of using these surveillance strategies in a very high-risk group only. Incidence rates of > 2% per 6-month cycle were considered for the abnormal Ib group (i.e. types I and III endoleaks, together with type II endoleaks with sac expansion > 5 mm and other conditions commonly detected by non-radiography imaging modalities). At an annual incidence rate of 7% for this group, CEU-only surveillance becomes cost-effective with an ICER of £29,756 with respect to CDU-only surveillance. It is worth noting that, although an incidence of 7% for type I or type III endoleaks is unlikely to be observed in clinical practice, an incidence of 6% for type II endoleaks with sac expansion is perhaps possible.
We can conclude that the use of CDU as a first-line test for the surveillance of individuals after EVAR is cost-effective, with a probability of > 58% at the usual cost-effectiveness threshold used in the UK.60 The analysis results are driven by the interplay of the test performance data (i.e. sensitivity and specificity) and the cost of the test. Lower unit costs together with higher specificity are needed for CEU to become cost-effective.
- Assessment of cost-effectiveness - Contrast-enhanced ultrasound and/or colour du...Assessment of cost-effectiveness - Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: a systematic review and economic evaluation
- Discussion - Clinical effectiveness and cost-effectiveness of a multifaceted pod...Discussion - Clinical effectiveness and cost-effectiveness of a multifaceted podiatry intervention for falls prevention in older people: a multicentre cohort randomised controlled trial (the REducing Falls with ORthoses and a Multifaceted podiatry intervention trial)
- List of abbreviations - A randomised controlled trial of adjunctive triamcinolon...List of abbreviations - A randomised controlled trial of adjunctive triamcinolone acetonide in eyes undergoing vitreoretinal surgery for open globe trauma – the ASCOT study
- Randomised pilot trial design and methods - Carer administration of as-needed su...Randomised pilot trial design and methods - Carer administration of as-needed subcutaneous medication for breakthrough symptoms in people dying at home: the CARiAD feasibility RCT
- Clinical effectiveness results - Home environmental assessments and modification...Clinical effectiveness results - Home environmental assessments and modification delivered by occupational therapists to reduce falls in people aged 65 years and over: the OTIS RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...