NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Peters MJ, Khan I, Woolfall K, et al. Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT. Southampton (UK): NIHR Journals Library; 2019 Feb. (Health Technology Assessment, No. 23.5.)

Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT.
Show detailsAim and objectives
The aim of the FEVER observational study was to inform the feasibility of conducting a definitive RCT by addressing the following specific objectives:
- Estimate the size of the potentially eligible population for the definitive FEVER RCT, both overall and in subgroups defined by confirmed versus suspected infection and by site/type of infection.
- Confirm, using empirical data, the temperature threshold(s) currently employed for a standard approach for antipyretic intervention in UK PICUs, and whether or not these vary in accordance with confirmed versus suspected infection and site/type of infection.
- Estimate the characteristics (e.g. mean and SD) of selected important, relevant, patient-centred primary outcome measure(s) to inform both the selection of an ultimate primary outcome and sample size calculation.
Methods
Study design
The FEVER observational study was an epidemiological cohort study of fever attributable to infection in critically ill children following an unplanned admission to a PICU.
Research governance
Approval was granted by the Greater Manchester West Research Ethics Committee on 11 January 2017 (reference number 17/NW/0026) and the HRA on 17 January 2017 (Integrated Research Application System reference number 209929). The protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/ (accessed 16 November 2018).
The FEVER observational study was registered on the ClinicalTrials.gov database. Registration was confirmed on 20 January 2017 (reference number NCT03028818). The study was adopted in the National Institute for Health Research (NIHR) Clinical Research Network (CRN) Portfolio as a non-consenting study on 15 February 2017 and issued the NIHR CRN Portfolio number 33374.
Local confirmation of capacity and capability was obtained from each participating hospital trust to collect data for the study. For NHS England sites, the statement of activities was used as the study agreement. For sites in Northern Ireland, Wales and Scotland, an adapted clinical trial site agreement, based on the model agreement for non-commercial research in the health service, was signed by each participating hospital trust and the sponsor (ICNARC).
Data collection was embedded in the Paediatric Intensive Care Audit Network (PICANet), which has approval to collect patient-identifiable and personal data without consent. No patient-identifiable data were collected or used for the FEVER observational study. A release of data and a customised data collection request was made to the Healthcare Quality Improvement Partnership for access to unidentifiable routine PICANet data and to collect the additional data required for this study. Healthcare Quality Improvement Partnership approval was received for this purpose on 1 March 2017.
Study management
The study co-ordinator was responsible for day-to-day management of the FEVER observational study, with support from the ICNARC CTU and the SMG.
Amendments to the protocol
After receiving approval of the protocol from the HRA on 17 January 2017, three non-substantial amendments were submitted and categorised:
- Minor amendment 1 (approved 3 February 2017) consisted of the addition of 16 research sites.
- Minor amendment 2 (approved 21 February 2017) consisted of the addition of two research sites, and changes in principal investigator (PI) responsibilities in four participating sites.
- Minor amendment 3 (approved 17 March 2017) consisted of changes in PI responsibilities for a participating site.
NHS support costs
In order to enable screening and data collection for the FEVER observational study, resources equivalent to 0.09 whole-time equivalent of a Band 6 clinical/research nurse per PICU for a period of 6 months were agreed prior to the submission of the research grant for the FEVER feasibility study.
Participants: sites
Twenty-two PICUs (Table 3) were selected from those actively participating in PICANet. These sites represented a variety of UK PICU configurations, including general or combined intensive care units in general academic medical centres or within stand-alone children’s hospitals. In order to be selected to participate in the FEVER observational study, sites had to meet the following inclusion criteria:
TABLE 3
Final sites included in the study
- Meet all responsibilities as stated in the statement of activities (for sites in England) or the adapted clinical trial site agreement (for sites in Scotland, Wales and Northern Ireland).
- Identify and sign up a local PI.
- Identify a responsible FEVER observational study research nurse (to be funded or part-funded, centrally).
- Agree to collect additional FEVER data on all patients eligible for data collection.
- Agree to enter study data for all eligible patients via data collection screens and send securely to ICNARC.
Site initiation
Site initiation meetings were held via teleconference with all participating sites prior to the commencement of data collection (between 1 and 8 March 2017). The purpose of these meetings was to present the background and rationale for the study and to discuss data collection and submission. The PI from each participating site attended the meeting.
Following participation in the site initiation meetings, screening and data collection was commenced at participating sites once the statement of activities (sites in England) or clinical trial site agreement (sites in Scotland, Wales and Northern Ireland) had been signed and necessary approvals were in place. The scheduled start of data collection for the FEVER observational study was 1 March 2017. Sites initiated after this date collected data retrospectively on participants admitted from this date.
Investigator site file
An electronic investigator site file was provided to all participating sites. This contained all essential documents for the conduct of the study and included the approved protocol, all relevant approvals (e.g. local confirmation of capacity and capability), a copy of the statement of activities/clinical trial site agreement, copies of the approved participant information leaflet and family poster and all standard operating procedures (e.g. for entering data onto the customised data collection screen).
Communication and motivation
The study co-ordinator, with support from the research assistant, maintained close contact with the research team at participating sites by e-mail and telephone throughout the study. A teleconference was held mid-way through data collection, on 14 June 2017, with research teams at participating sites. The purpose of this was to provide a forum for site teams to ask questions and to discuss interim data submission at 3 months to allow analysis to inform the pilot RCT. PICANet provided regular status reports, which displayed the number of participants eligible for data collection, the data collection completion and the outstanding number of associated validation queries. These reports were used to identify specific sites that were falling behind on data collection.
Study population
Eligibility criteria
All infants and children admitted to a PICU were recorded in routine PICANet data collection. Participants who were recorded as an ‘unplanned PICU admission’ were deemed eligible for data collection for the FEVER observational study. There were no exclusion criteria.
Consent
Informed consent was not required as there was no intervention in the patients’ care and no patient-identifiable data were collected. Patient information sheets (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 16 November 2018) and posters were displayed in participating PICUs to make parents/guardians aware that the unit was taking part in the study. Patients or parents/legal representatives were able to opt out from participation and have their data removed from the study.
Outcomes
- Potentially eligible population for a definitive FEVER trial.
- Temperature thresholds employed in usual care.
- The distribution of potential outcome measures for a definitive FEVER trial.
Data collection
Data entry
The FEVER observational study was nested within routine data collection for PICANet. PICANet is the national clinical audit network of paediatric critical care units in England, Wales, Northern Ireland and Scotland and was established in 2002 by the Universities of Leeds, Leicester and Sheffield with the support of the paediatric intensive care community. Nesting the study in PICANet ensured efficient design (with respect to data collection to address the study objectives) and facilitated effective study management through the ability to monitor data entry. Data were collected for the FEVER observational study through a mixture of routinely collected audit data and additional data collection through an additional screen on PICANet. Paper case report forms (CRFs) were provided to facilitate data collection and entry. Collection of data was delegated by the site PI to qualified members of the research team and recorded on the delegation log. Data entry was monitored via status reports generated by PICANet and was subject to ongoing validation to ensure that data were as complete and accurate as possible.
Data stages
Participants who were recorded as ‘unplanned PICU admissions’ were eligible for the collection of additional data. These data were split into three stages. Stages 1 and 2 aimed to identify potentially eligible participants for the definitive FEVER trial, and stage 3 provided infection data alongside temperature and antipyretic management.
Stage 1
Exclusion criteria:
- acute encephalopathy, including convulsive status epilepticus
- postcardiopulmonary bypass
- known/suspected myocardial disease
- severe rhabdomyolysis
- malignant hyperthermia
- neuroleptic malignant syndrome
- drug-induced hyperthermia
- receiving palliative care, or death perceived as imminent.
If the patient met one or more of the stage 1 exclusion criteria, no additional data were collected. If the patient did not meet any of the stage 1 exclusion criteria, data collection moved on to stage 2.
Stage 2
Inclusion criterion:
- suspected or confirmed infection on admission.
If the patient did not have a suspected or confirmed infection, no additional data were collected. If the patient had a suspected or confirmed infection, the patient was identified as ‘potentially eligible’ for the definitive FEVER trial and data collection moved on to stage 3.
Stage 3
- Data were collected on site and type of infection.
- Daily data (for first 5 calendar days in PICU) were collected for the highest daily temperature and any antipyretic and opioid analgesic interventions given.
Paediatric Intensive Care Audit Network data
For all unplanned admissions, routine PICANet data were provided on patient characteristics, daily interventions and status at PICU discharge.
Data submission
Participating sites downloaded a data extract from PICANet, consisting of the data collected specifically for the FEVER observational study and the specified PICANet routine data. The study data set was anonymous and submitted to ICNARC for analysis (Figure 4).
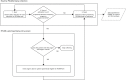
FIGURE 4
The PICANet processes.
Sample size
Based on PICANet data, a sample size of approximately 1100 children was deemed to be sufficient to permit the calculation of 30-day mortality (anticipated to be 6% based on data from PICANet) with a 95% CI of ±1.4% based on the following assumptions:
- There were 21,326 emergency/unplanned admissions to the 27 UK PICUs during the period of 1 January 2011 to 31 December 2012. This corresponded to an anticipated overall sample size for the FEVER observational study in a target number of 20 UK PICUs over a 6-month period of approximately 3960 admissions (33 per PICU per month).
- There were 11,007 (51.6%) emergency/unplanned admissions to the 27 UK PICUs during the same period for patients who were admitted with a primary or secondary diagnosis consistent with a probable infection. Of these, 946 admissions (8.6%) met one or more of the proposed FEVER exclusion criteria, corresponding to approximately 1900 admissions (16 per PICU per month) who required the additional FEVER data collection on temperature and FEVER management.
- Of admissions with probable infection, 1583 (14.4%) were re-admissions of the same child. Based on data from the UK Paediatric Intensive Care Outcome Study (UK PICOS),29 approximately 70% of children admitted with infection have a peak temperature of ≥ 37.5 °C (estimated to be the likely temperature threshold).
Data analysis
Interim analysis
An interim analysis of data from the FEVER observational study was undertaken in July 2017 to inform the final design of the pilot RCT.
Study populations
All potentially eligible participants for the definitive FEVER trial were included in the analyses. Analyses were carried out based on the following populations:
- All patients not meeting any stage 1 exclusion criteria and with confirmed or suspected infection (stage 2 criteria).
- As population 1, restricted to patients receiving any mode of mechanical ventilation (invasive, non-invasive or high-flow oxygen) on days 1–2 of PICU admission.
- As population 1, restricted to patients receiving invasive ventilation on days 1–2 of PICU admission.
Analysis methods
Potentially eligible populations
The number of potentially eligible participants for a definitive FEVER trial (per site per month) was estimated based on the number of recruited participants divided by the total duration of recruitment in months for each population cohort, considering the temperature thresholds employed on days 1–2 of PICU admission. The potentially eligible population is reported overall and as the median (IQR) across sites, with 95% CIs based on a Poisson distribution for each population.
Subgroup analyses
Subgroup analyses were performed for maximum temperature and current temperature thresholds by confirmed versus suspected infection and site/type of infection.
Temperature thresholds
The temperature thresholds employed in usual care were evaluated in each of the three populations by plotting:
- the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of ≥ X, with X varying from 36.0 °C to 39.5 °C
- the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of < X, with X varying from 36.0 °C to 39.5 °C
- the variation across sites in the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of ≥ 37.5 °C
- the variation across sites in the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of ≥ 38.0 °C
- for patients not receiving any antipyretic intervention on day 1 and receiving an antipyretic intervention subsequently, the cumulative distribution function of the maximum temperature on the first day on which they received any antipyretic intervention and on the preceding day
- for patients not receiving any antipyretic intervention on day 1 and receiving an antipyretic intervention subsequently, the variation across sites in the median value of the maximum temperature on the first day on which they received any antipyretic intervention and on the preceding day.
Potential outcome measures
The distribution of potential outcome measures for a definitive FEVER RCT were evaluated for the following potential outcome measures for each of the cohorts of potentially eligible participants for a definitive RCT, as defined in Study populations. Length of stay in PICU is reported as the mean (SD) and median (IQR). Duration of organ support is reported as the number (%) receiving the organ support, the median (IQR) duration of organ support among patients receiving the organ support and the mean (SD) duration of organ support among all patients (with patients not receiving the support assigned a value of 0). PICU mortality is reported as the number and percentage and the 95% CI for the percentage. The number of stays alive and free from a PICU and from mechanical ventilation are reported as the mean (SD).
Statistical analysis
Statistical analyses were conducted in accordance with a prespecified statistical analysis plan written prior to the final data lock. The final analyses were conducted using Stata®/SE version 14.2 (StataCorp LP, College Station, TX, USA) and R version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Missing data
All analyses were undertaken in the complete-case population. There was no imputation of missing data.
Results
Participants
From 1 March 2017 to 31 August 2017, a total of 4126 children with unplanned PICU admissions were recorded from 22 hospitals. A total of 4008 patients (97.1%) had completed data entry relating to stage 1 criteria, and, of these, 737 (18.4%) met one or more of the stage 1 exclusions. Of the 3141 patients (78%) meeting stage 1 criteria, 1263 (40%) had no infection and a further 25 (0.8%) had missing data. The remaining 1853 patients (59.3%) meeting stage 2 criteria were identified as the potentially eligible population for a definitive FEVER RCT. We were unable to successfully collect PICANet data from 12 patients (0.6%); therefore, 1841 patients (99.4%) were included in the analysis for population 1, 1532 patients (82.7%) for population 2 and 1156 patients (62.4%) for population 3 (Figure 5). The variation across sites in the number and percentage of patients meeting stage 1 and 2 criteria is presented in Appendix 1.

FIGURE 5
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram for the study.
Baseline characteristics
Participant demographics and case mix are presented in Table 4. Age distribution, temperature at first face-to-face contact and highest temperature recorded on day 1 appear similar across the three populations. The proportions of antipyretic interventions on day 1 also appear consistent across groups; however, the use of intravenous opiates on day 1 is greatest in those receiving invasive mechanical ventilation (population 3) (74% of invasively mechanically ventilated patients received intravenous opiates). Infection was confirmed during the PICU stay for approximately two-thirds of participants in each of the populations, with viral infections and the lungs being the most common type and site of infection, respectively. Again, this was consistent across groups, accounting for > 60% (both type and site) of infections in each of the three populations.
TABLE 4
Summary of participant characteristics for each population at baseline
Outcomes
Potentially eligible populations
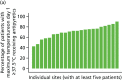
The rates of potentially eligible participants with temperatures of ≥ 37.5 °C and ≥ 38 °C by site and overall for each analysis population are presented in Table 5. Overall rates of potentially eligible participants in population 1 were 10.7 (95% CI 10.2 to 11.3) participants per site per month with a temperature of ≥ 37.5 °C and 7.0 (95% CI 6.5 to 7.5) participants per site per month with a temperature of ≥ 38 °C. The inclusion of mechanical ventilation (population 2) reduced the overall rates to 9.2 (95% CI 8.6 to 9.7) participants per site per month for a temperature of ≥ 37.5 °C and 6.1 (95% CI 5.6 to 6.5) participants per site per month with a temperature of ≥ 38 °C. Recruitment rates were further reduced in population 3 (invasive mechanical ventilation) to 7.3 (95% CI 6.8 to 7.7) participants per site per month and 5.0 (95% CI 4.6 to 5.4) participants per site per month for temperatures of ≥ 37.5 °C and ≥ 38 °C, respectively.
TABLE 5
Recruitment rate (number of participants per site per month), by site and overall for each potential trial population
Temperature thresholds
Figure 6 shows the proportion of participants in each population receiving an antipyretic intervention on day 1 with a maximum temperature above or below a given temperature ranging from 36.0 °C to 39.5 °C. In all participants (population 1), 55% of participants with a maximum temperature of > 36 °C received antipyretics, and this increased to 62% of participants at > 37 °C, 82% of participants at > 38 °C and 91% of participants at > 39 °C. The proportion of participants receiving antipyretics at the given temperatures on day 1 remained consistent when narrowing the inclusion criteria across populations 2 and 3 (Figure 6); however, there was variability across sites in temperature thresholds employed for antipyretic interventions on day 1 (Figure 7).
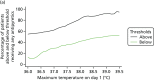
FIGURE 6
The proportion of participants receiving antipyretic intervention on day 1 with a temperature above or below a given threshold. (a) Population 1; (b) population 2; and (c) population 3.
For all participants not receiving an antipyretic intervention on day 1 but receiving subsequent antipyretic intervention, around 15% had a maximum temperature of ≤ 37 °C on the first day they received antipyretics (Figure 8). This increased to 68% of participants with a maximum temperature of ≤ 38 °C and 94% of participants with a temperature of ≤ 39 °C. The proportion of participants treated with antipyretics at these thresholds appeared to remain consistent across populations 2 and 3, with the mean temperature on the first day of receiving antipyretics being 37.8 °C (SD 0.8 °C) in population 1, 37.8 °C (SD 0.8 °C) in population 2 and 37.8 °C (SD 0.7 °C) in population 3. Again, there was considerable variability across sites in terms of temperature thresholds for antipyretic intervention on days 2–5 (Figure 9); however, the majority of participants in populations 1–3 with a temperature of ≥ 37.0 °C received an antipyretic intervention. The subgroup analyses of temperature thresholds employed by confirmed versus suspected and site/type of infection can be found in Appendix 2.
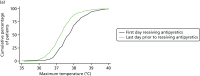
FIGURE 8
Cumulative distribution of maximum daily temperatures of participants not receiving any antipyretics on day 1 and receiving subsequent antipyretic intervention. (a) Population 1; (b) population 2; and (c) population 3.
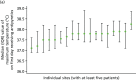
FIGURE 9
Variation across sites in the median value of maximum temperatures on the first day on which antipyretics were received. (a) Population 1; (b) population 2; and (c) population 3.
Potential outcome measures
The distributions of potential outcome measures were consistent across the ≥ 37.5 °C and ≥ 38 °C temperature thresholds (Table 6). Overall, PICU mortality (population 1) was around 5%, and this was higher among mechanically ventilated patients, at around 5.5%, and even higher among invasively ventilated patients, at around 6.5%. Similarly, PICU length of stay increased with narrowing inclusion criteria for all participants and those who survived to PICU discharge. Although the median duration of organ support among those receiving the support was similar across all populations, the proportions of patients receiving mechanical ventilation and cardiovascular support and the mean duration across all patients (including those not receiving the support) increased with narrowing inclusion criteria. Use of renal support was low in all populations. Consistent with the results on mortality, length of stay and organ support, the mean number of days alive and free from PICU and mechanical ventilation reduced across populations 1–3 with the narrowing of inclusion criteria.
TABLE 6
Potential outcome measures for each population
Summary of findings informing the FEVER pilot randomised controlled trial
An interim analysis was conducted in July 2017 on data collected from 21 out of 22 sites from between March and May 2017 to inform the design of the pilot RCT. This showed that the proposal to narrow the inclusion criteria to mechanically ventilated patients from the FEVER qualitative study was feasible in terms of recruitment. Data from the interim and full analyses also confirmed the lower temperature threshold for the usual care group of the pilot RCT at ≥ 37.5 °C, as this temperature falls within usual care across UK PICUs.
- The FEVER observational study - Different temperature thresholds for antipyretic...The FEVER observational study - Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...