NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Takwoingi Y, Whitworth H, Rees-Roberts M, et al. Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis (IDEA): test accuracy study and economic evaluation. Southampton (UK): NIHR Journals Library; 2019 May. (Health Technology Assessment, No. 23.23.)

Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis (IDEA): test accuracy study and economic evaluation.
Show detailsRecruitment of human immunodeficiency virus-positive patients
Following the closure of the main study to recruitment, two additional NHS trusts (King’s College Healthcare NHS Foundation Trust and Barts Health NHS Trust) were invited to participate in the study to facilitate the recruitment of HIV-positive patients. A total of 263 patients were recruited from 12 NHS trusts between 25 November 2011 and 19 December 2014. The number of patients recruited at each centre is shown in Table 27. Over half of the patients were recruited from two trusts: Royal Free London NHS Foundation Trust (29.3%) and Imperial College Healthcare NHS Trust (25.9%).
TABLE 27
Recruitment of HIV-positive patients by centre
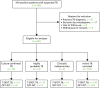
The flow of patients through the study is shown in Figure 6. Of the 263 HIV-positive patients recruited, 201 were included in the analyses. Reasons for exclusion are shown in Figure 6. Two patients from Frimley Health NHS Foundation Trust were excluded, as mentioned earlier in Chapter 3 (see Recruitment of participants into main study cohort).
The final diagnosis of four (2.0%) patients was clinically indeterminate (Figure 6 and Table 28). Among the remaining 197 patients, there were 32 (16.2%) cases of active TB and 165 (83.8%) cases of non-active TB. Of the 165 non-active TB cases, 68 (41.2%) were category 4D.
TABLE 28
Reference standard categories for HIV-positive patients
Baseline characteristics of the human immunodeficiency virus-positive cohort
The demographic characteristics of the 201 patients are given in Table 29. The median age of patients was 43 years (range 18–79 years) and the majority (67.7%) were male. A substantial number of HIV-positive patients were of black (45.3%) or white (37.8%) ethnicity. A total of 53 countries of birth were represented; countries with at least three participants are shown in Table 29. Many patients (97/201, 48.3%) were in paid employment.
TABLE 29
Demographic characteristics of HIV-positive cohort
The clinical characteristics of the patients are presented in Table 30. Of the 201 patients, 71 (35.3%) had no other comorbidities. There were 10 (5.0%) patients with pre-existing diabetes mellitus, four (2.0%) with chronic/end-stage renal failure and none was on immunosuppressive therapy. Vitamin D measurement was unknown for most (85.1%) patients (see Appendix 5, Table 55, for thresholds used to categorise vitamin D status). Table 30 shows CD4 (cluster of differentiation 4) count grouped into four categories. CD4 count was missing for eight patients. Most of the 193 patients had a CD4 count of ≥ 200 cells/µl (46.8%). The median CD4 count was 285 cells/µl (range 0–1228 cells/µl). The distribution of CD4 count in the cohort is shown in Figure 7.
TABLE 30
Clinical characteristics of HIV-positive cohort

FIGURE 7
Distribution of CD4 counts in HIV-positive substudy cohort.
Table 31 gives the social history of patients. Smoking history was missing for one patient; almost half (48.8%) of the patients had never smoked, whereas the remaining were current (31.3%) or ex-smokers (19.4%). Fourteen (7.0%) patients had a history of alcohol misuse and the history of recreational drug use was unknown for many (64.7%) patients. Few patients (6.0%) had a history of homelessness and 7.5% had a history of imprisonment.
TABLE 31
Social history of the HIV-positive cohort
The frequency of symptoms is shown in Table 32 based on data from 197 patients. The four other patients were recruited on the basis of abnormal clinical signs rather than symptoms. The main symptoms were cough, fever, night sweats, weight loss and lethargy. Patients generally presented with multiple symptoms; the median number of symptoms was three (range 1–10). Cough, as a symptom, was often present (68.5%) in patients.
TABLE 32
Symptoms at presentation for the HIV-positive cohort
Final diagnosis in human immunodeficiency virus-positive patients
The diagnostic tests performed during the diagnostic workup of patients are shown in Table 33. Chest radiography and culture were the most common tests performed (with each performed in 90.6% of patients). The number of T-SPOT.TB, QFT-GIT and TST tests performed as part of routine care, at each centre, is shown in Appendix 14 (see Table 78). These routine IGRA results were not used in the final diagnosis of patients in the IDEA study. TSTs were performed in only 12 patients at 3 of the 11 centres; TST results were available for all 12 patients.
TABLE 33
Diagnostic tests performed in the diagnostic workup of active TB in HIV-positive patients
The final diagnoses of active TB patients are summarised in Table 34. Of the 32 patients with active TB, 19 (59.4%) had smear-negative TB. A total of 13 patients (40.6%) had pulmonary TB, 15 (46.9%) patients had extrapulmonary TB, and the remaining four (12.5%) patients had both forms of TB. The most common sites of infection were the lungs (53.1%) and lymph nodes (31.1%). Of the 31 culture tests performed, 20 (64.5%) had no drug resistance. Table 35 shows the final diagnosis of non-active TB patients. A patient may have multiple conditions, but pneumonia was the most frequent diagnosis (40.0%).
TABLE 34
Final diagnosis of TB in HIV-positive patients
TABLE 35
Final diagnosis of non-TB in HIV-positive patients
Test results for human immunodeficiency virus-positive patients
Of the 201 patients, 194 (96.5%) had results for both T-SPOT.TB and QFT-GIT; reasons for missing T-SPOT.TB and QFT-GIT results are shown in Table 36. The cross-classified results of the two tests are given in Appendix 14, Table 79. Table 37 shows the results for T-SPOT.TB, QFT-GIT and the TST according to the four diagnostic categories. Table 38 shows the cross-classification of the two tests for those with (categories 1 and 2) and without active TB (category 4).
TABLE 36
Reasons for missing IGRA results for HIV-positive patients
TABLE 37
Results for T-SPOT.TB and QFT-GIT, by final diagnosis, in the cohort of HIV-positive patients
TABLE 38
Cross-tabulation of T-SPOT.TB and QFT-GIT against final diagnosis in the cohort of HIV-positive patients
Diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube in human immunodeficiency virus-positive cohort
The proportion of indeterminate test results was 23.1% (45/195) for T-SPOT.TB and 19.5% (39/200) for QFT-GIT. The difference between the two proportions was 3.6% (95% CI –4.5% to 11.6%; p = 0.4). Sensitivity was 68.0% (95% CI 48.4% to 82.8%) for T-SPOT.TB and 56.7% (95% CI 39.2% to 72.6%) for QFT-GIT (Table 39). The specificities were 83.5% (95% CI 75.8% to 89.0%) and 91.4% (95% CI 85.3% to 95.1%). The PPVs for T-SPOT.TB and QFT-GIT were 46.0% (95% CI 31.0% to 61.6%) and 60.7% (95% CI 42.4% to 76.4%), respectively, and the NPVs were 92.7% (95% CI 86.2% to 96.2%) and 90.0% (95% CI 83.6% to 94.1%), respectively.
TABLE 39
Diagnostic accuracy of T-SPOT.TB and QFT-GIT for diagnosis of active TB in the HIV-positive cohort
Using only culture-confirmed active TB cases, the sensitivity of T-SPOT.TB decreased slightly to 66.7% (95% CI 41.7% to 84.8%), whereas that of QFT-GIT increased to 61.1% (95% CI 38.6% to 79.7%). When the analyses were restricted to category 4D patients without active TB, specificities were higher than using all patients without active TB (see Table 39). In sensitivity analyses with indeterminate test results included as test positives, the sensitivity of T-SPOT.TB was 74.2% (95% CI 56.8% to 86.3%) and 59.4% (95% CI 42.3% to 74.5%) for QFT-GIT. The specificity of T-SPOT.TB was 63.1% (95% CI 55.4% to 70.2%) and 71.3% (95% CI 64.0% to 77.7%) for QFT-GIT. See Appendix 14, Table 80, for full results.
The diagnostic performance of the two IGRAs is shown stratified by CD4 count in Table 40. The estimates within each stratum give results comparable to the entire cohort in terms of the higher sensitivity of T-SPOT.TB and higher specificity of QFT-GIT. However, the estimates are based on very small numbers of active TB and non-active TB cases and are presented solely for illustrative purposes.
TABLE 40
Diagnostic accuracy of T-SPOT.TB and QFT-GIT stratified by CD4 count in the HIV-positive substudy cohort
Comparison of diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube in human immunodeficiency virus-positive cohort
Excluding indeterminate IGRA results, there were 146 T-SPOT.TB results and 158 QFT-GIT results. The sensitivity of T-SPOT.TB was higher than that of QFT-GIT, with a relative sensitivity of 1.12 (95% CI 0.87 to 1.44). There was no statistical evidence of a difference (p = 0.4). In contrast, the specificity of T-SPOT.TB was significantly lower than that of QFT-GIT, with a relative specificity of 0.91 (95% CI 0.84 to 0.99) (Table 41). When indeterminate IGRA results were included as test positives in a sensitivity analysis, the analysis included 191 T-SPOT.TB results and 196 QFT-GIT results. Unlike the primary analysis, there was no statistical evidence of a difference in specificity (see Appendix 14, Table 81).
TABLE 41
Comparison of diagnostic accuracy of T-SPOT.TB and QFT-GIT in HIV-positive patients
Discussion
Of the 263 patients recruited, 201 patients were included in the analyses. This is well below the target sample size of 390. Although T-SPOT.TB showed higher sensitivity than QFT-GIT, a relative increase of 12%, there was no statistical evidence of a difference in sensitivity. The sensitivity of QFT-GIT in the IDEA study was lower than that in other recent studies29–31 (see Appendix 11, Table 74).
The indeterminate rate was lower for QFT-GIT (19.5%) than for T-SPOT.TB (23.1%). The indeterminate results were mainly in those without active TB, with 92.3% (36/39) for QFT-GIT and 86.7% (39/45) for T-SPOT.TB. The impact of indeterminate results was explored in sensitivity analyses by including indeterminate results as test positives. There was a small increase in the sensitivity of QFT-GIT but a large increase in the sensitivity of T-SPOT.TB. This is because of the higher indeterminate rate for T-SPOT.TB (18.2%) among category 1 and 2 active TB cases than for QFT-GIT (5.9%).
- Recruitment of human immunodeficiency virus-positive patients
- Baseline characteristics of the human immunodeficiency virus-positive cohort
- Final diagnosis in human immunodeficiency virus-positive patients
- Test results for human immunodeficiency virus-positive patients
- Diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube in human immunodeficiency virus-positive cohort
- Comparison of diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube in human immunodeficiency virus-positive cohort
- Discussion
- Substudy of human immunodeficiency virus-positive participants - Interferon gamm...Substudy of human immunodeficiency virus-positive participants - Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis (IDEA): test accuracy study and economic evaluation
- Background - The effectiveness and cost-effectiveness of erythropoiesis-stimulat...Background - The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of technology appraisal no. 142): a systematic review and economic model
- Trial results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hyper...Trial results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review
- Comparison of the REFLUX trial with other randomised trials of laparoscopic surg...Comparison of the REFLUX trial with other randomised trials of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease - Clinical and economic evaluation of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease: 5-year follow-up of multicentre randomised trial (the REFLUX trial)
- List of abbreviations - SeHCAT (tauroselcholic [75selenium] acid) for the invest...List of abbreviations - SeHCAT (tauroselcholic [75selenium] acid) for the investigation of bile acid diarrhoea in adults: a systematic review and cost-effectiveness analysis
Your browsing activity is empty.
Activity recording is turned off.
See more...