NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Pandor A, Horner D, Davis S, et al. Different strategies for pharmacological thromboprophylaxis for lower-limb immobilisation after injury: systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2019 Dec. (Health Technology Assessment, No. 23.63.)

Different strategies for pharmacological thromboprophylaxis for lower-limb immobilisation after injury: systematic review and economic evaluation.
Show detailsA series of systematic reviews of the literature and (network) meta-analysis (when appropriate) were undertaken to (1) assess the effectiveness of pharmacological thromboprophylaxis for preventing VTE, (2) identify individual risk factors associated with VTE risk and (3) identify RAMs for the prediction of VTE risk in people with temporary lower-limb immobilisation due to injury.
All reviews of the evidence were undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement37 and were registered on the PROSPERO international prospective register of systematic reviews (CRD42017058688).38 The full protocol is available on the project web page [URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1518706/#/ (accessed 3 December 2018)].
Review of pharmacological thromboprophylaxis for preventing venous thromboembolism
Objective
The objective was to assess the effectiveness of pharmacological thromboprophylaxis for preventing any VTE, clinically detected (symptomatic) DVT, clinically relevant (symptomatic, proximal or extensive) DVT, PE and asymptomatic DVT in patients with temporary lower-limb immobilisation due to injury. In this study, proximal DVT is defined as disease at or above the level of the popliteal trifurcation. Distal DVT is defined as disease below the popliteal trifurcation, confined to the calf veins (e.g. peroneal, posterior, anterior tibial and muscular veins).
Methods of reviewing effectiveness
Identification of studies
Studies were identified by searching the following electronic databases and research registers:
- Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, MEDLINE and Versions(R) (OvidSP), 1946 to April 2017.
- EMBASE (OvidSP), 1974 to April 2017.
- Cochrane Database of Systematic Reviews (Wiley Online Library), 1996 to April 2017.
- Database of Abstracts of Review of Effects (Wiley Online Library), 1995 to March 2015.
- Cochrane Central Register of Controlled Trials (Wiley Online Library), 1898 to April 2017.
- Health Technology Assessment (HTA) database (Wiley Online Library), 1995 to April 2017.
- NHS Economic Evaluation Database NHS EED (Wiley Online Library), 1995 to March 2015.
- Science Citation Index Expanded (Web of Science), 1900 to April 2017.
- ClinicalTrials.gov (US National Institutes of Health), 2000 to April 2017.
- International Clinical Trials Registry Platform (World Health Organization), 1990 to April 2017.
The search strategy used free text and thesaurus terms and combined synonyms relating to the condition (i.e. VTE in people with lower-limb immobilisation) with synonyms relating to the interventions (e.g. LMWH, aspirin and oral anticoagulants). No language restrictions were used. However, as the search strategy of the current review updated the search strategy of an existing review on LMWH,15 searches were limited by date from 2013 (the last search date from the earlier review) to April 2017 for this intervention. For the other interventions, the search strategy was amended to include terms for aspirin and oral anticoagulants and searched from inception to April 2017. Further details of the search strategy can be found in Appendix 1. Searches were supplemented by hand-searching the reference lists of all relevant studies (including existing systematic reviews), performing a citation search of relevant articles, contacting key experts in the field and undertaking systematic keyword searches of the internet using the Google search engine (Google Inc., Mountain View, CA, USA).
All identified citations from the electronic searches and other resources were imported into and managed using EndNote bibliographic software version X8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA].
Inclusion and exclusion criteria
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer (AP) and any citations that clearly did not meet the inclusion criteria (e.g. non-human, unrelated to VTE) were excluded. Second, all abstracts and full-text articles were examined independently by two reviewers (AP and DH). When necessary, non-English-language studies were translated using Google Translate (Google Inc., Mountain View, CA, USA) to facilitate study selection and subsequent data extraction. Any disagreements in the selection process were resolved through discussion or, if necessary, arbitration by a third reviewer (SG), and articles were included by consensus.
Studies were considered eligible for inclusion if they met the following criteria: (1) study design – RCTs and controlled clinical trials; (2) population – adults (aged > 16 years) requiring temporary immobilisation (e.g. leg cast or brace in an ambulatory setting) for an isolated lower-limb injury; (3) interventions – chemical thromboprophylaxis with LMWH (e.g. dalteparin, enoxaparin, tinzaparin), fondaparinux or oral anticoagulants (e.g. apixaban, dabigatran etexilate, rivaroxaban); (4) comparators – these included placebo, no treatment, aspirin or alternative treatment (although the original protocol considered aspirin to be an option for VTE prophylaxis, NICE guidelines on venous thromboembolism (CG92)24 do not consider aspirin or other antiplatelet agents to be appropriate for VTE prophylaxis; in addition, aspirin is not indicated as a treatment for VTE prophylaxis in lower-limb immobilisation);21,25,26 and (5) outcomes – these included symptomatic or asymptomatic DVT, PE, major bleeding or mortality. Exclusion criteria for selection included studies that had not been designed as experimental studies (e.g. cohort studies and case–control studies), studies that had involved hospital inpatient care or any patient requiring hospital admission of > 5 days and studies in which patients received mechanical thromboprophylaxis or underwent ambulant orthopaedic surgery (e.g. arthroscopy, arthroscopic surgery).
Data abstraction and quality assessment strategy
Data relating to study design, methodological quality and outcomes were extracted by one reviewer (AP) into a standardised data extraction form and independently checked for accuracy by a second reviewer (DH). Any discrepancies were resolved through discussion or, if necessary, arbitration by a third reviewer (SG), and articles were included by consensus. If required, authors of primary studies were contacted to obtain additional data, clarify uncertainties and/or confirm data that had been extracted. When multiple publications of the same study were identified, data were extracted and reported as a single study.
The methodological quality of each included study was evaluated using a revised Cochrane Risk of Bias tool for randomised trials (RoB 2.0).39 The original tool40 was updated because of questionable inter-rater agreement, subjectivity in assigning risk-of-bias judgements and bias judgements assigned at the trial level.41–44 In general, RoB 2.0 redefined the potential for bias to five domains: (1) bias arising from the randomisation process, (2) bias as a result of deviations from intended interventions, (3) bias as a result of missing outcome data, (4) bias in the measurement of the outcome and (5) bias in the selection of the reported result. To limit subjectivity in assigning bias judgements, the RoB 2.0 tool provides detailed guidance and contains decision algorithms. An overall judgement of bias was assigned as ‘low risk’ if all domains were judged as being at a low risk of bias, a judgement of bias was assigned as ‘high risk’ if at least one domain was judged to be at a high risk of bias (or if the study had some concerns for multiple domains in a way that substantially lowers confidence in the result) and as ‘some concerns’ if some concerns of bias were noted in at least one domain.39 The methodological quality of each included study was independently evaluated by two reviewers (AP and DH). Any discrepancies were resolved through discussion or, if necessary, the involvement of a third reviewer (SG). Blinding of the quality assessor to author, institution or journal was not considered necessary.
Methods of data synthesis and analysis
The extracted data and quality assessment variables were presented for each included study, both in structured tables and as a narrative description. For each outcome of interest, a network meta-analysis (NMA) was performed to allow a simultaneous comparison between interventions using all available studies. The data were the number of events out of the number of patients randomised to each intervention, which were assumed to arise from an underlying binomial distribution. The probabilities of an event for each intervention were modelled using a logistic model to estimate odds ratios (ORs). The control intervention was defined as placebo, no treatment or aspirin, and the reference intervention defined in the NMA was the control intervention. Aspirin was grouped with placebo and no treatment on the basis that aspirin is not indicated as a treatment for VTE prophylaxis in lower-limb immobilisation21,25 and NICE guidelines on VTE (CG9224 and NG891) do not consider aspirin or other antiplatelet agents to be appropriate for VTE prophylaxis. It was planned to analyse different types of thromboprophylaxis drugs as separate interventions (i.e. LMWH, DOACs and fondaparinux) in the NMA on the basis of having different mechanisms of action and, therefore, potentially different effects.
The analysis was implemented using a Markov chain Monte Carlo simulation using WinBUGS software version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK).45 A fixed-effect model was used to estimate the effects of LMWH and fondaparinux relative to control in the available studies, namely a conditional inference. In addition, a random-effects model was used to allow for heterogeneity in the effects of interventions between studies and to estimate whether or not the interventions can have an effect in future studies. The random-effects model was the primary analysis. The baseline log odds in each study were given normally distributed prior distributions with mean 0 and variance 1000, namely N(0, 1000). The log-odds ratios for LMWH and fondaparinux versus control were given normally distributed prior distributions with a mean of 0 and variance of 1000, namely N(0, 1000). The between-study standard deviation (SD) was given a half-normal prior distribution with a mean of 0 and precision of 1.82, namely HN(0, 0.5495); this prior distribution was chosen to have, a priori, 95% of the study-specific odds ratios lie within a factor of 5 from the median odds ratio for each comparison. Convergence of the Markov chains to their stationary distributions was assessed using the Gelman–Rubin convergence statistic.46 For all outcomes other than major bleeding, convergence occurred within 30,000 iterations of the Markov chain and within 100,000 samples for major bleeding; a burn-in of 100,000 iterations was used in all analyses. There was some evidence of high autocorrelation between successive iterations of the Markov chain; parameters were estimated after retaining every 10th sample of the Markov chain to limit the number of unnecessary runs of the decision model that are informed by the results of the NMA. Results were presented using ORs, 95% credible intervals (CrIs) and the 95% predictive intervals47 for the OR in a randomly chosen study relative to the control, and the probability of each intervention being the best.48
It was planned to assess the following potential treatment effect modifiers in a series of meta-regressions: (1) population characteristics (e.g. proportion who were male, baseline risk of VTE), (2) type of injury (i.e. fractures, Achilles tendon rupture, other soft-tissue injury), (3) treatment of injury (surgical vs. conservative, above- vs. below-knee immobilisation), (4) thromboprophylactic agent used and (5) duration of thromboprophylaxis.
Results
Quantity and quality of research available
The literature searches identified 1105 citations. Of these, 13 studies (all RCTs) met the inclusion criteria.23,49–60 A flow chart describing the process of identifying relevant literature can be found in Figure 1. A total of 23 full-text articles were excluded as they did not meet all of the prespecified inclusion criteria. The majority of the articles were excluded primarily on the basis of inappropriate study design (i.e. non-randomised controlled trial or controlled clinical trial), wrong target population (i.e. not isolated lower-limb injury requiring temporary immobilisation) or unsuitable publication type (i.e. reviews, commentaries, editorials or multiple publications of the same study). A full list of excluded studies with reasons for exclusion is presented in Appendix 2.
Description of included studies (design and participant characteristics)
The design and participant characteristics of the 13 included studies23,49–60 that evaluated the effectiveness of pharmacological thromboprophylaxis for preventing VTE in ambulatory trauma patients with temporary lower-limb immobilisation are summarised in Table 1.
TABLE 1
Summary of design and participant characteristics: review of pharmacological thromboprophylaxis for preventing VTE
All studies were published between 1993 and 2017. In total, 6857 patients were included and randomised across 10 countries (i.e. Canada,50,58 China,60 Denmark,51,56 France,57 Germany,23,52,53,57 Italy,57 the Netherlands,49,57,59 Russia,57 Spain57 and Sweden54,55) to receive either intervention or control treatment. LMWH injections were the primary intervention, using variable agents (i.e. certoparin,52 dalteparin,50,54,55,58 nadroparin,49,53,57,59 reviparin23,56 and tinzaparin51) and dosing regimes (e.g. administered once daily without dose adjustment for bodyweight), but two studies used fondaparinux.49,57 One study used aspirin as a control group treatment,23 with others using placebo injections or nothing dependent on design.50–56,58–60 In general, most studies excluded patients at highest risk of VTE, namely those with active cancer,49,54,55,58,60 previous VTE49,50,52–56,58–60 or first-degree family history of VTE.59,60
Five identified studies used open-label methodology with subjective screening outcomes (duplex sonography or phlebography on cast removal).51–53,57,59 Six studies used double blinding within the design.50,54–56,58,60 The single largest study had symptomatic VTE only as an identified primary outcome, confirmed with imaging.59 Although all studies included adult patients with an isolated lower-limb injury requiring temporary immobilisation, there was wide variation in terms of injury type. Five studies focused on patients with fractures,49,50,54,58,60 one focused on Achilles tendon ruptures55 and the remaining seven studies included patients with mixed pathology.23,51–53,56,57,59 Depending on the type of injury, the management of lower-limb injury included conservative treatment,49,52,53,57 surgical management50,54,55,58,60 or both.51,56,59 In eight studies,49,50,54–58,60 patients were recruited within 4 days of injury, whereas, in the remaining studies,23,51–53,59 the time to recruitment was not stated. The duration of immobilisation ranged from 14 days50 to 44 days.54,56 In two studies, all54 or some (approximately one-third)56 patients first received prophylaxis prior to randomisation; these studies were included, as any final impact on outcome would be likely to take the form of a reduction in VTE outcome events. In addition, the results of these trials remain relevant to the study question in the light of current regimes, suggesting that prophylaxis continue for the duration of immobilisation (usually 4–6 weeks). The sample sizes of the included studies ranged from 10555 to 151959 patients, with the mean age of participants ranging from 34 years52,53 to 49 years.58 The number of male participants ranged from 42%58 to 79%.55
Quality characteristics
The overall methodological quality of the 13 included studies is summarised in Figure 2 and Table 2. Overall, risk of bias was present in all studies. Ten studies raised some concerns of bias.49–51,54–60 The potential sources of bias most frequently identified included concerns about the randomisation process (allocation concealment was not reported in nine studies),23,50–56,60 blinding (open-label design)23,49,51–53,57,59 and analyses intentions (only one study provided sufficient information on the selection of the reported result).59 A high risk of bias was noted in three studies.23,52,53 High risk of bias was principally attributable to outcome assessment; in three open-label studies,23,52,53 outcome assessment was performed on all patients with routine screening compression ultrasonography and phlebography for confirmation of positive findings. Finally, all of the included studies were conducted outside the UK, making generalisability of the findings to the UK setting uncertain.

FIGURE 2
Risk-of-bias assessment graph: review authors’ judgements about each methodological quality item across all included studies – review of pharmacological thromboprophylaxis for preventing VTE.
TABLE 2
Risk-of-bias assessment summary: review authors’ judgements about each methodological quality item for each included study – review of pharmacological thromboprophylaxis for preventing VTE
Quantitative data synthesis
Details of the results of the primary studies are provided in Appendix 3. All 13 studies reported outcomes for any VTE, PE and major bleeding. The rate of any VTE in the control group ranged from 1.8% to 40.4% with a median of 12.2%. The rate of PE in the control group was zero in eight studies and ranged from 0.7% to 2.1% in the other four. There was only one bleeding event across all the control groups. Clinically relevant (proximal or symptomatic) DVTs were reported in 10 out of 13 studies, with control event rates ranging from 0.0% to 6.4% (median 1.5%). Clinically detected (symptomatic) DVTs were reported in all 13 studies, with control event rates ranging from 0.0% to 5.5% (median 0.7%). Any proximal or distal asymptomatic DVTs were reported in 10 out of 13 studies, with control event rates ranging from 1.6% to 25.7% (median 6.9%). Asymptomatic proximal DVTs were reported in 8 out of 13 studies, with control event rates ranging from 0.0% to 6.4% (median 0.7%). Asymptomatic distal DVTs were reported in 8 out of 13 studies, with control event rates ranging from 0.8% to 16.0% (median 3.0%).
A NMA was undertaken to compare the effectiveness of two alternative forms of thromboprophylaxis (i.e. LMWH or fondaparinux) with no thromboprophylaxis (i.e. aspirin, placebo or no treatment). Figure 3 presents the network of evidence. All 13 studies were included in the analysis and provided information on at least one of the outcomes being analysed. Eleven of the studies compared LMWH thromboprophylaxis with no thromboprophylaxis, one three-arm study compared LMWH with fondaparinux with no thromboprophylaxis, and one study compared LMWH with fondaparinux. A summary of the key results of fixed-effect and random-effects NMA are provided in Table 3.
TABLE 3
Results of fixed-effect and random-effects NMAs of different pharmacological thromboprophylaxis interventions vs. no thromboprophylaxis
Clinically detected deep-vein thrombosis (symptomatic)
Data were available from all 13 studies.23,49–60 The risk of clinically detected DVT (symptomatic) was lower in adult outpatients with lower-limb immobilisation who received LMWH (OR 0.40, 95% CrI 0.12 to 0.99) and fondaparinux (OR 0.10, 95% CrI 0.01 to 0.94) than those in the control group. Fondaparinux is likely to be the most effective treatment (probability of being the most effective = 0.91). However, the heterogeneity in treatment effects between studies suggests that the true effects may vary depending on study characteristics (between-study SD 0.55, 95% CrI 0.03 to 1.59).
Asymptomatic deep-vein thrombosis (proximal segment)
Data were available from eight studies.23,50–52,55,57,58,60 The risk of asymptomatic DVT (proximal segment) was lower in adult outpatients with lower-limb immobilisation who received LMWH (OR 0.21, 95% CrI 0.04 to 0.82) than those in adults in the control group. A similar effect was found for fondaparinux, although the results were inconclusive (OR 0.28, 95% CrI 0.02 to 3.42). The heterogeneity in treatment effects between studies suggests that the true effects may vary depending on study characteristics (between-study SD 0.42, 95% CrI 0.02 to 1.44).
Asymptomatic deep-vein thrombosis (distal)
Data were available from eight studies.23,50–52,56–58,60 The risk of asymptomatic DVT (distal) was lower in adult outpatients with lower-limb immobilisation who received fondaparinux (OR 0.11, 95% CrI 0.03 to 0.35) than in those in the control group; fondaparinux is likely to be the most effective treatment (probability of being the most effective = 1.00). There was insufficient evidence of an effect of LMWH (OR 0.69, 95% CrI 0.43 to 1.12) compared with control, although the effect favoured treatment with LMWH. There was evidence of mild to moderate heterogeneity between studies, suggesting that the true effects may vary depending on study characteristics (between-study SD 0.20, 95% CrI 0.01 to 0.83).
Asymptomatic deep-vein thrombosis (all)
Data were available from 10 studies.23,49–52,54,56–58,60 The risk of asymptomatic DVT (all) was lower in adult outpatients with lower-limb immobilisation who received LMWH (OR 0.57, 95% CrI 0.39 to 0.82) and fondaparinux (OR 0.14, 95% CrI 0.05 to 0.31) than in those in the control group. Fondaparinux is likely to be the most effective treatment (probability of being the most effective = 1.00). There was evidence of mild to moderate heterogeneity in treatment effects between studies, suggesting that the true effects may vary depending on study characteristics (between-study SD 0.17, 95% CrI 0.01 to 0.70).
Pulmonary embolism
Data were available from all 13 studies.23,49–60 The risk of PE was lower in adult outpatients with lower-limb immobilisation who received LMWH (OR 0.17, 95% CrI 0.01 to 0.88) than in those in the control group. A reduction in risk was also found for fondaparinux, although the results were inconclusive (OR 0.47, 95% CrI 0.01 to 9.54). The heterogeneity in treatment effects between studies suggests that the true effects may vary depending on study characteristics (between-study SD 0.81, 95% CrI 0.05 to 2.04).
Major bleeding
Data were available from all 13 studies,23,49–60 but, with only four events across all the studies, estimates of the effects of LMWH (OR 1.45, 95% CrI 0.08 to 32.17) and fondaparinux on the risk of major bleeding were inconclusive. Control had the highest probability of being the best treatment (probability of being the best = 0.59). The between-study SD was 0.50 (95% CrI 0.02 to 1.64).
Clinically relevant deep-vein thrombosis
Data were available from 10 studies.23,50–52,55,57,58,60 The risk of clinically relevant DVT was lower in adult outpatients with lower-limb immobilisation who received LMWH (OR 0.40, 95% CrI 0.16 to 0.85) than in those in the control group. The risk was also lower in patients treated with fondaparinux, although the results were inconclusive (OR 0.23, 95% CrI 0.03 to 1.36). The heterogeneity in treatment effects between studies suggests that the true effects may vary depending on study characteristics (between-study SD 0.45, 95% CrI 0.02 to 1.39).
Any venous thromboembolism
Data were available from all 13 studies.23,49–60 The risk of any VTE was lower in adult outpatients with lower-limb immobilisation who received LMWH (OR 0.52, 95% CrI 0.37 to 0.71) and fondaparinux (OR 0.13, 95% CrI 0.05 to 0.30) than in those not receiving thromboprophylaxis. Fondaparinux is likely to be the most effective treatment (probability of being the most effective = 1.00). There was mild to moderate heterogeneity in treatment effects between studies. Although the results suggest that the true effects may vary depending on study characteristics, the predictive distribution still favoured fondaparinux relative to control (between-study SD 0.23, 95% CrI 0.01 to 0.75).
There were few reported adverse effects in the treated patients. Minor bleeding event rates varied from 0.0% to 10.5% in the LMWH intervention groups, 0.0% to 1.5% in the fondaparinux intervention groups and 0.0% to 6.8% in the control groups. In the largest RCT to date,59 the most common adverse event (of infection) occurred at a similar rate in the intervention and control groups (1.6% vs. 2.0%, respectively). When assessed in the trials, compliance appeared good, with only a single open-label study49 recording pain on injection, which was seen in 1.4% of participants in the intervention group. In studies monitoring for the incidence of heparin-induced thrombocytopaenia, no cases were found.58 No deaths in any study were deemed attributable to either VTE or the use of an intervention.
The results of the network meta-regressions are detailed in Appendix 4. A network meta-regression of population characteristics (e.g. proportion of males, baseline risk of VTE), type of injury (i.e. fractures, Achilles tendon rupture, other soft-tissue injury), treatment of injury (surgical vs. conservative, above- vs. below-knee immobilisation) and the duration of thromboprophylaxis was undertaken for each available outcome. This showed that no covariate improved model fitted and, therefore, explained the variation in treatment effects.
The effect of the type of thromboprophylactic agent used (i.e. dalteparin, tinzaparin, certoparin, nadroparin, reviparin) was assessed using a separate NMA. This suggested that there were differences in the effects of the type of thromboprophylactic agent used, including between the different types of LMWH, with certoparin having the highest probability of having the greatest effect on any VTE. However, this finding was based on the effect of certoparin being used in one study,52 so it is not possible to draw any reliable conclusions.
Summary of key findings
- Thromboprophylaxis with LMWH approximately halves the risk of any VTE. The effects on different types of VTE are variable and uncertain (in accordance with random error), but all are consistent with a halving of risk.
- Thromboprophylaxis with fondaparinux appears to have a greater effect on the risk of VTE and a greater probability than LMWH of being the more clinically effective, but estimates for fondaparinux are based on only two trials.
- Major bleeding is very uncommon; therefore, the effect of thromboprophylaxis on major bleeding in this group is uncertain.
- Meta-regression did not identify any reliable evidence of effect modification by key covariates.
Review of individual risk factors associated with venous thromboembolic risk
Objectives
To identify individual, patient identifiable risk factors associated with VTE risk in patients with temporary lower-limb immobilisation due to injury.
Methods of reviewing effectiveness
Identification of studies
Studies were identified by searching the following electronic databases and research registers:
- Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, MEDLINE and Versions(R) (via OvidSP), 1946 to May 2017.
- EMBASE (via OvidSP), 1974 to May 2017.
- Cochrane Database of Systematic Reviews (via Wiley Online Library), 1996 to May 2017.
- Database of Abstracts of Review of Effects (via Wiley Online Library), 1995 to March 2015.
- Cochrane Central Register of Controlled Trials (via Wiley Online Library), 1898 to May 2017.
- HTA database (via Wiley Online Library), 1995 to May 2017.
- NHS EED (via Wiley Online Library), 1995 to March 2015.
- Science Citation Index Expanded (via Web of Science), 1900 to May 2017.
- ClinicalTrials.gov (via US National Institutes of Health), 2000 to May 2017.
- International Clinical Trials Registry Platform (via World Health Organization), 1990 to May 2017.
The search strategy used free-text and thesaurus terms and combined synonyms relating to the condition (i.e. VTE in people with lower-limb immobilisation) with risk factor assessment or risk prediction modelling terms (used in the searches of MEDLINE, The Cochrane Library and EMBASE only). No language or date restrictions were used on any database. Further details on the search strategy can be found in Appendix 5. Searches were supplemented by hand-searching the reference lists of all relevant studies (including existing systematic reviews), performing a citation search of relevant articles, contacting key experts in the field and undertaking systematic keyword searches of the internet using the Google search engine.
All identified citations from the electronic searches and other resources were imported into and managed using EndNote bibliographic software.
Inclusion and exclusion criteria
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer (AP) and any citations that clearly did not meet the inclusion criteria (e.g. non-human, unrelated to VTE) were excluded. Second, all abstracts and full-text articles were then examined independently by two reviewers (AP and DH). When necessary, non-English-language studies were translated using Google Translate to facilitate study selection and subsequent data extraction. Any disagreements in the selection process were resolved through discussion or, if necessary, arbitration by a third reviewer (SG), and articles were included by consensus.
Studies were considered eligible for inclusion if they met the following criteria: (1) any study design that included a measurement of VTE patient outcome, (2) a study population of adults (aged > 16 years) requiring temporary immobilisation (e.g. leg cast or brace in an ambulatory setting) for an isolated lower-limb injury, (3) any studies that reported and analysed data on individual risk factors associated with DVT or PE. Exclusion criteria for the selection included studies that involved hospital inpatient care or any patient requiring hospital admission for > 5 days, or studies that involved patients undergoing ambulant orthopaedic surgery (e.g. arthroscopy, arthroscopic surgery).
Data abstraction and quality assessment strategy
Data relating to study design, methodological quality and outcomes were extracted by one reviewer (AP) into a standardised data extraction form and independently checked for accuracy by a second reviewer (DH). Any discrepancies were resolved through discussion to achieve agreement. When differences were unresolved, the opinion of a third reviewer (SG) was sought. When multiple publications of the same study were identified, data were extracted and reported as a single study.
The methodological quality of each included study was assessed using the Risk Of Bias In Non-randomized Studies – of Interventions tool (ROBINS-I) [formerly called A Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI)].61 The tool is based on the original Cochrane Risk of Bias tool for randomised studies40 and also builds on related tools, such as the quality assessment of diagnostic accuracy studies (QUADAS-2).62
The ROBINS-I61 tool provides a detailed framework for assessment and judgement of risk-of-bias domains that may arise in three phases: (1) at pre-intervention, bias arising from confounding and selection of participants into the study; (2) at intervention, bias in measurement of interventions; and (3) at post-intervention, bias due to deviations from intended interventions, missing data, measurement of outcomes and selection of reported results. Each domain is rated as being at low, moderate, serious or critical risk of bias. A low risk of bias indicates that the study is comparable to a well-performed randomised trial in the domain being evaluated. A moderate risk of bias indicates that the study is sound for a non-randomised study but is not comparable to a well-performed randomised trial. A serious risk of bias indicates the presence of important problems in the domain and a critical risk of bias indicates that the study is too problematic to provide any useful evidence on the intervention effects. If insufficient information is provided to determine the risk of bias of a certain domain, the domain is marked as having no information. In general, the overall risk of bias of each study was determined to be equal to that of the most severe level of bias found in any domain.
All studies were analysed using ROBINS-I,61 regardless of whether or not the original study design included randomisation to other exposures, thus ensuring that risk of bias was assessed specifically for the comparisons of interest to this review. It is important to note that the quality assessment reflects how well a specific result evaluated the association of interest to this review, regardless of the objectives of the original study.
Methods of data synthesis and analysis
Venous thromboembolism was considered to comprise any subsequent recorded diagnosis of DVT or PE, or death attributable to either pathology. No attempt was made to distinguish between anatomical location, thrombus burden or clinical sequelae of VTE for this project, in accordance with the definitions of hospital-acquired thrombosis produced by NHS England.63 Individual risk factors highlighted through regression, OR analysis or parametric testing as significantly associated with an increased or decreased likelihood of subsequent VTE were extracted. In particular, each paper was scrutinised for evidence of individual risk factors, especially those highlighted within current risk stratification tools,17,31–33 and their predictive performance was recorded, when available. Other risk factors demonstrating an association with VTE in the context of individual studies were also reported. A meta-analysis was not possible owing to significant levels of heterogeneity between studies, variable reporting items and the high risk of attributable bias. Descriptive statistics and thematic analysis were used to synthesise risk factors acting in a reproducible fashion across studies. Thematic analysis took an inductive/semantic form, using familiarisation and coding directed by data content. Consistent risk factor themes were then highlighted in ordinal fashion. All analyses were conducted using Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Results
Quantity and quality of research available
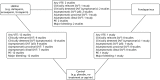
The literature searches identified 4771 citations. Of these, 15 studies9,11,23,50,52,53,60,64–71 met the inclusion criteria. Figure 4 presents a flow chart describing the process of identifying relevant literature. Sixty full-text articles were excluded as they did not meet all the prespecified inclusion criteria. The majority of the articles were excluded primarily on the basis of an inappropriate target population (not isolated lower-limb injury requiring temporary immobilisation), no data or analysis of risk factors associated with VTE, or an unsuitable publication type (i.e. reviews, commentaries, editorials or abstracts of excluded/included full-text papers). More specifically, two potentially relevant papers72,73 were excluded as they included a specific elective surgical population who were not considered to meet the inclusion criterion of lower-limb injury. A potentially relevant prospective observational cohort study74 was excluded, based on the authors’ conclusion of a low event rate precluding any subsequent analysis for predictors of VTE. Finally, a case–control study32 specifically seeking to derive a decision rule for the cohort of interest was excluded, based on the creation of this rule from a generic thrombosis cohort rather than a subgroup of patients with temporary lower-limb immobilisation. A full list of excluded studies with reasons for exclusion is presented in Appendix 6.
Description of included studies (design and patient characteristics)
The design and patient characteristics of the 15 included studies9,11,23,50,52,53,60,64–71 that provided data on individual patient identifiable risk factors associated with VTE risk in ambulatory trauma patients with temporary immobilisation following lower-limb injury is summarised in Table 4.
TABLE 4
Summary of design and patient characteristics: review of individual risk factors associated with VTE risk
All studies were published between 1993 and 2017 (five were RCTs with conservative arms,23,50,52,53,60 three were prospective observational cohort or cross-sectional studies,65,67,69 one was a case–control study70 and six were retrospective cohort studies)9,11,64,66,68,71 and conducted in 10 countries (Australia,11,64,65 Canada,9,50 China,60 Denmark,71 France,69 Germany,23,52,53 Iran,67 the Netherlands,70 the UK66 and the USA).68 Most of the studies (n = 11) were entirely outpatient based,11,23,50,52,53,60,64–67,69 whereas the remaining studies9,68,70,71 included patients with a short-duration inpatient stay to facilitate day-case surgery. In total, data were collated on 80,678 patients with a subsequent reported outcome of VTE positive or negative following temporary lower-limb immobilisation. The incidence of VTE across the studies with interpretable outcome data (79,202 patients) ranged from 0.22%66 to 23.5%9 (median 4.8%), mean age ranged from 33.8 years52 to 52.6 years71 and the proportion of male patients ranged from 45.8%65 to 86.1%,9 with a median across those studies with reported data of 56.3%.
The duration of follow-up varied between studies. Ten studies reported follow-up over a period of at least 3 months9,50,60,64–66,68–71 and one study followed up patients for up to 14 days.67 Although four studies failed to record the duration of follow-up,11,23,52,53 two of these appeared to report follow-up only for the duration of the plaster cast, which averaged at 15.7 days53 and 17 days.52 Eight studies collected data on risk factors prospectively via physician assessment or questionnaire,23,50,52,53,60,65,69,70 whereas six studies collected these data through clinical records, electronic patient notes or registries.9,11,64,66,68,71 One study did not report the methodology for this aspect of data collection.67 Analysis and methodology of VTE diagnosis subsequent to immobilisation varied markedly across studies and included prospective screening in all patients following plaster cast removal (seven studies),23,50,52,53,60,65,67 adjudicated diagnostic evaluation in those with symptoms (two studies)69,70 and retrospective identification of VTE through the interrogation of clinical records/health databases (six studies).9,11,64,66,68,71 A single study66 looked only at the subsequent diagnosis of PE as an outcome, with reduced prevalence as expected. The association of individual risk factors with subsequent VTE was highlighted through regression analyses (nine studies),11,50,60,64,66,68–71 non-parametric tests (two studies)9,65 and descriptive statistics (four studies).23,52,53,67
Quality characteristics
The overall methodological quality of the 15 included studies is summarised in Figure 5 and Table 5.
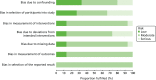
FIGURE 5
ROBINS-I risk-of-bias assessment graph: review authors’ judgements about each methodological quality item across all included studies – review of individual risk factors associated with VTE risk.
TABLE 5
ROBINS-I risk-of-bias assessment summary: review authors’ judgements about each methodological quality item for each included study – review of individual risk factors associated with VTE risk
All studies were deemed to be at overall moderate (seven studies)23,50,52,53,60,68,71 or serious (eight studies)9,11,64–67,69,70 risk of bias, using the ROBINS-I61 framework for assessment and judgement. Studies scoring a serious risk of bias did so predominantly on the selection of participants into the study, perhaps highlighting the issue with retrospective observational work into VTE outcomes; patients deemed to be at high risk in these cohorts are often treated with thromboprophylaxis (as highlighted in Table 4), or managed in a different manner from other patients. If this is not highlighted within a prospective analysis plan, a false low event rate is seen and risk is marginalised.
Narrative data synthesis
Age was the most consistent individual risk prediction factor, highlighted across 11 studies.9,11,50,52,53,60,65,68–71 ORs reported for age varied from 1.0560 to 3.48,11 with limited estimates of precision. Meta-analysis was not undertaken owing to limitations in the data reported and concerns about the heterogeneous nature of the study populations. Injury type was the second most highlighted risk factor across six studies,11,50,52,53,69,70 all using multivariate logistic regression to suggest that severe traumatic injuries and fractures (when compared with soft-tissue injuries) were independently associated with VTE. Body mass index (BMI) was the third most consistent individual risk highlighted, noted as independently predictive of VTE across four studies,53,60,70,71 with ORs ranging from 1.20160 to 17.2.70 Other risk factors were highlighted in fewer than four studies. Despite being present within several currently used risk stratification tools, pregnancy, recent hospital admission and preceding immobility prior to injury failed to demonstrate an independent association with VTE in any of the selected studies. Individual risk factors currently used within risk stratification tools and their association with VTE across all studies are shown in Table 6.
TABLE 6
Individual risk factors and their reported strength of association with developing VTE
Other potential risk factors associated with subsequent development of VTE after lower-limb immobilisation included recent air travel (one study),64 coagulopathy and peripheral arterial disease (one study).71 A single paper67 looked at the cumulative incidence of risk factors per patient and reported the presence of three or more factors to be significantly associated with the development of VTE. Methodology of reporting individual variables to have no association with subsequent VTE was inconsistent and heterogeneous. Six studies reported no association between sex and VTE in this cohort,11,50,52,65,66,70 five studies reported no association between exogenous oestrogen use and VTE9,50,52,53,65 and six studies reported no association between smoking and subsequent VTE.9,50,52,65,69,71 Several papers produced conflicting results; six studies reported no association between raised BMI and subsequent risk of VTE9,50,52,65,68,69 and one study reported no association of VTE with increasing age.66 These other identified risk factors and all negative associations are reported in Table 7.
TABLE 7
Other identified individual risk factors and their association with developing VTE
Summary of key findings
- Increasing age and injury severity were the most consistent risk factors associated with the development of VTE in patients with lower-limb injury and temporary immobilisation.
- Many clinical features considered to be risk factors for VTE were not examined or associated with VTE in the studies.
- All studies included in the review were deemed to be at moderate or serious risk of bias.
- The evidence base for tailored risk prediction in people with lower-limb immobilisation due to injury is very weak.
Review of risk assessment models for predicting venous thromboembolic risk
Objective
The objective was to identify RAMs that predict the risk of VTE in ambulatory trauma patients with temporary immobilisation following lower-limb injury.
Methods of reviewing effectiveness
Identification of studies
Studies were identified by searching the following electronic databases and research registers:
- Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, MEDLINE and Versions(R) (via OvidSP), 1946 to May 2017.
- EMBASE (via OvidSP), 1974 to May 2017.
- Cochrane Database of Systematic Reviews (via Wiley Online Library), 1996 to May 2017.
- Database of Abstracts of Review of Effects (via Wiley Online Library), 1995 to March 2015.
- Cochrane Central Register of Controlled Trials (via Wiley Online Library), 1898 to May 2017.
- HTA database (via Wiley Online Library), 1995 to May 2017.
- NHS EED (via Wiley Online Library), 1995 to March 2015.
- Science Citation Index Expanded (via Web of Science), 1900 to May 2017.
- ClinicalTrials.gov (via US National Institutes of Health), 2000 to May 2017.
- International Clinical Trials Registry Platform (via Word Health Organization), 1990 to May 2017.
The search strategy used free text and thesaurus terms and combined synonyms relating to the condition (i.e. VTE in people with lower-limb immobilisation) with risk factor assessment or risk prediction modelling terms (used in the searches of MEDLINE, The Cochrane Library and EMBASE only). No language or date restrictions were used on any database. Further details on the search strategy can be found in Appendix 5. Searches were supplemented by hand-searching the reference lists of all relevant studies (including existing systematic reviews), performing a citation search of relevant articles, contacting key experts in the field and undertaking systematic keyword searches of the World Wide Web using the Google search engine.
All identified citations from the electronic searches and other resources were imported into and managed using EndNote bibliographic software.
Inclusion and exclusion criteria
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer (AP) and any citations that clearly did not meet the inclusion criteria (e.g. non-human, unrelated to VTE) were excluded. Second, all abstracts and full-text articles were then examined independently by two reviewers (AP and DH). When necessary, non-English-language studies were translated using Google Translate to facilitate study selection and subsequent data extraction. Any disagreements in the selection process were resolved through discussion or, if necessary, arbitration by a third reviewer (SG), and articles were included by consensus.
Studies were considered eligible for inclusion if they met the following criteria: (1) any study design that included a measurement of VTE patient outcome, (2) studies that recruited adults (aged > 16 years) who required temporary immobilisation (e.g. leg cast or brace in an ambulatory setting) for an isolated lower-limb injury and (3) any studies that reported any validation, or estimates of utility and performance of VTE risk assessment models for people with lower-limb cast immobilisation.
Data abstraction and quality assessment strategy
Data relating to study design, methodological quality and outcomes were extracted by one reviewer (AP) into a standardised data extraction form and independently checked for accuracy by a second reviewer (DH). Any discrepancies were resolved through discussion or, if necessary, arbitration by a third reviewer (SG) and included by consensus. When multiple publications of the same study were identified, data were extracted and reported as a single study.
There are no validated (or widely agreed on) tools for the assessment of prognosis research studies and there is little empirical evidence to support the importance of particular study features affecting the reliability of findings, including the avoidance of bias.75 For this review, a generic list of important methodological features recommended by Altman76 and Moons et al.77 for prediction modelling studies was deemed to be the most appropriate (i.e. useful) to assess the internal validity of the included studies. In general, five domains were considered important for assessing biases sufficiently large to distort the findings of prognosis research. These included (1) participant selection, (2) predictor assessment, (3) outcome assessment, (4) sample size and missing data and (5) statistical analysis. An overall judgement of bias was assigned as ‘low risk’ if all domains were judged as low risk, as ‘high risk’ if at least one domain was judged to be at high risk and as ‘unclear risk’ if an unclear risk of bias was noted in at least one domain and it was ‘low risk’ for all other domains. The methodological quality of each included study was independently evaluated by two reviewers (AP and DH). Any discrepancies were resolved through discussion or, if necessary, through the involvement of a third reviewer (SG). Blinding of the quality assessor to author, institution or journal was not considered necessary.
Methods of data synthesis and analysis
Venous thromboembolism was considered to comprise any subsequent recorded diagnosis of DVT, PE or death attributable to either pathology. No attempt was made to distinguish between anatomical location, thrombus burden or clinical sequelae of VTE for this project, in accordance with the definitions of hospital-acquired thrombosis produced by NHS England.63 A narrative review of all identified scoring systems was performed, to compare design characteristics with thresholds for prophylaxis. Estimates of sensitivity, specificity, predictive values or likelihood ratios were directly extracted from validation studies or retrospectively calculated using available baseline data when applicable. All analyses were conducted using Microsoft Excel 2010.
Results
Quantity and quality of research available
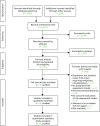
The literature searches identified 4771 citations. In total, only six studies32,78–82 met the inclusion criteria. Of these, one paper32 focused on prediction model development with external validation in independent data. The remaining papers focused on external model validation without model updating.78–82 A flow chart describing the process of identifying relevant literature is given in Figure 6. A total of 69 full-text articles were excluded as they did not meet all of the prespecified inclusion criteria. The majority of the articles were excluded on the basis of inappropriate target population (i.e. not isolated lower-limb injury requiring temporary immobilisation), having no relevant RAMs or outcome evaluations and being an unsuitable publication type (i.e. reviews, commentaries, editorial or abstracts of included full-text papers). A full list of excluded studies with reasons for exclusion is presented in Appendix 7.
Description of included studies (design and patient characteristics)
Study characteristics are described in Table 8. Two case–control studies32,82 and four observational studies78–81 that described the evaluation of seven RAMs were found. A single study32 presented data on derivation and validation; this study used a generic thrombosis database (including all acute thrombosis patients seen in an outpatient clinic matched with partner controls) to derive a new rule, rather than a relevant cohort of patients with temporary lower-limb immobilisation. All other papers looked to provide measures of external validation or implementation metrics regarding previously derived scores, with no description of the initial derivation methodology.
TABLE 8
Summary of key characteristics of RAM studies that predict the risk of VTE
The seven identified RAMs varied in design, structure, output and threshold for prophylaxis. Several scores were dichotomous, with others providing ordinal measure of risk. Design characteristics and threshold levels for each RAM are presented for comparison in Table 9. The majority of RAMs focused solely on the estimate of thromboembolic risk; a single method featured characteristics designed to balance the risk of bleeding with thromboprophylaxis.17 The individual predictors and their weighting varied markedly between RAMs. Variables (and their definitions) for each RAM are presented for comparison in Table 10.
TABLE 9
Summary of design characteristics and threshold levels of identified RAMs
TABLE 10
Summary of predictor variables included in studies of RAMs that predict the risk of VTE
Quality characteristics
The overall methodological quality of the six included studies is summarised in Figure 7 and Table 11. The majority of studies were deemed to be at high risk of bias,32,78,79,81,82 based primarily on participant selection; all reviewed studies attempted validation of proposed decision rules in heterogeneous cohorts, with pragmatic observational follow-up only. Outcomes were also non-standardised and varied by site in terms of description and inclusion.

FIGURE 7
Risk-of-bias assessment graph:, review authors’ judgements about each methodological quality item across all included studies in the review of RAMs that predict the risk of VTE.
TABLE 11
Risk-of-bias assessment summary:, review authors’ judgements about each methodological quality item for each included study in the review of RAMs that predict the risk of VTE
Both case–control studies32,82 had specific and notable limitations in methodology. The larger study, by Nemeth et al.,32 used three distinct generic thrombosis data sets to derive and validate the proposed RAM. As such, the tool is derived from a group of patients with unprovoked or hospital-acquired VTE and has limited potential generalisability to the cohort of interest in this study. The authors looked at a subgroup of patients with temporary lower-limb immobilisation within the study data, finding only a small proportion in whom to attempt validation (230 patients, 2% of the original derivation data set). The smaller study, by Watson et al.,82 derived case–control data using an equal measure of appropriate patients with thrombosis following cast immobilisation, alongside a separate cohort of those without. As such, the prevalence of thrombosis within this study cohort was 50%. This is 20 times the estimated prevalence within the literature and, therefore, renders their estimates of predictive value at high risk of error.
Narrative data synthesis
The study by Nemeth et al.32 derived and validated a clinical risk score for plaster cast patients: the Leiden thrombosis risk in plaster (cast) [L-TRiP(cast)] score. In this study,32 data from a large population-based case–control study of approximately 10,000 patients (4446 consecutive patients with a first episode of venous thrombosis and 6118 controls) were used in developing the model (included in patients with disease and many other confounding factors). After minimising the variables in an attempt to produce a clinical (14 environmental predictor variables) and pragmatic rule (11 predictor variables), the authors validated this rule in two subsequent VTE case–control data sets.
Watson et al.82 assessed the diagnostic accuracy of five RAMs (only two of these, the GEMNet model17 and the Plymouth model,31 were specific to patients with lower-limb trauma and cast immobilisation) in a case–control study of 42 patients with lower-limb immobilisation following injury (21 cases and 21 controls). The reported sensitivity and specificity of the GEMNet model (85.7% and 47.6%, respectively) did not seem to be compatible with the numbers of cases and controls, so contact was made with the authors for clarification. They identified an error in the sensitivity, which should have been reported as 4.76%, and provided the raw numbers for both RAMs, which were used to calculate all diagnostic parameters and confidence intervals (CIs).
Prognostic accuracy measures for the three scores evaluated in these two studies are presented in Table 12. Sensitivity ranged from 57.1% to 92.6% across the RAMs and specificity ranged from 4.76% to 60.8%. The L-TRiP(cast) data are displayed in this table using thresholds denoting optimal performance and to allow direct comparison with other validated scores. The estimates of positive and negative predictive value for the L-TRiP(cast) score were modelled using an appropriately low prevalence of VTE, whereas the estimates for the GEMNet and Plymouth models used the artificial 50% prevalence from the case–control study. This explains the relatively high positive predictive values and the relatively low negative predictive values for these scores.
TABLE 12
Diagnostic performance of the L-TRiP(cast), GEMNet and Plymouth RAMs
The area under the curve (AUC) for the L-TRiP(cast) score ranged from 0.77 (95% CI 0.66 to 0.87) in the derivation cohort to 0.77 (95% CI 0.58 to 0.96) and 0.95 (95% CI 0.91 to 0.99) in the two subsequent validation cohorts.
In addition to these rules, four additional models were identified (Saragas et al.,81 Haque et al.,80 Giannadakis et al.79 and Eingartner et al.78). No measures of external validation of these RAMs were found. All were found as single-centre small-scale implementation studies, revealing no further additional information on performance, utility or reliability.
Summary of key findings
- A number of RAMs have been developed using a variety of methods and based on a variety of predictor variables.
- External validation studies have weak designs and limited generalisability, so estimates of prognostic accuracy are very uncertain.
- The limited data available suggest that the L-TRiP(cast) score with a cut-off point of 8 can achieve reasonable sensitivity for predicting VTE without an excessive loss of specificity.
Identifying key variables to assess thromboembolic risk: a Delphi consensus exercise
Objectives
The systematic reviews revealed a lack of evidence relating to individual risk predictors and RAMs for VTE in lower-limb immobilisation due to trauma. Therefore, expert consensus methods were used to identify potential risk factors for VTE and expert consensus was sought on which were considered to be the most useful predictors. The aim was to bring together topic experts from haematology, orthopaedics and emergency medicine and achieve consensus through serial rounds and facilitated discussion. The results of this Delphi exercise would then be compared with current risk prediction models and consensus opinion on clinical engagement, utility and acceptability to patients would be gauged. Delphi methodology has been previously described85–87 and used throughout health services research for similar indications.88,89
Methods
Delphi methodology
A three-round Delphi study was conducted between August 2017 and April 2018 using a panel of international topic experts, identified from the published literature and national clinical research network (injuries and emergency theme).
Expertise was ascribed using two criteria: (1) evidence of experience in relevant guideline or risk assessment tool design and (2) routine clinical experience with the relevant patient cohort and topic of interest. These criteria were selected to allow disclosure of unpublished methodology deriving existing rules, confer practicality of any rule and to ensure broad dissemination and uptake of consensus findings. Experts were identified through national bodies, relevant literature and local thrombosis committee groups. National bodies included The British Orthopaedic Association, The Royal College of Emergency Medicine and The Clinical Research Network Injuries and Emergencies Specialty group. Additional national or international societies were not approached for independent representation owing to time constraints and workload. All individuals approached agreed to participate and provided written informed consent. Experts were entirely independent of the core review team (n = 11), local colleagues independent of the review work (n = 3) and members of the core review team (n = 6).
The open first round of the classical Delphi approach was replaced with a systematic literature review to identify possible VTE predictors, as previously described. Round 1 of the Delphi study was then delivered via a web-based platform using SmartSurvey Ltd (Tewkesbury, UK)90 through a subscribed account. All potential individual predictor variables identified through the previous systematic review of existing decision rules and the wider literature on risk prediction in the relevant cohort were presented to participants as potential candidate predictors. Participants were then asked to rate the strength of the VTE prediction risk for each variable using a four-point Likert scale, with the option to record uncertainty. Their replies were collated into quantitative and qualitative output for each individual question. An opportunity to identify new relevant predictor variables was provided and participants were encouraged to identify missing themes.
New candidate variables were proposed, and those failing to achieve consensus in round 1 of the Delphi exercise were carried forward to a second round. Participants were presented with these variables together with a summary of the panel results from round 1, when applicable. Participants were asked to complete the same Likert scale as before, with the advantage of having additional insight into comments and quantitative results revealed by the rest of the group.
At the end of round 2 of the Delphi exercise, all variables were carried forward to a facilitated round-table discussion where consensus results and comments were provided to all participants. Data were collated and analysed to calculate frequencies, mean and range of scores.
Data synthesis and analysis
Criteria for inclusion in the decision rule were defined by a variable identified as a moderate or strong predictor by consensus between two, three or more respondents. Variables identified as uncertain or not or weakly predicting VTE risk by consensus between two, three or more respondents were likewise excluded from the exercise. Quantitative data from rounds 1 and 2 were presented to participants as bar charts with percentages. All analyses were conducted using Microsoft Excel 2010.
Results
Expert engagement
Twenty participants were identified to participate in the study. All (100%) completed round 1; 19 participants (95.0%) completed round 2. Ten participants (50.0%) contributed to the final facilitated round-table discussion. A list of participants and the clinical scope of the Delphi panel is provided in Appendix 8.
Systematic review and identification of candidate predictors
Trial flow results from the relevant systematic reviews prior to the Delphi exercise have been previously described (see Review of individual risk factors associated with venous thromboembolic risk and Review of risk assessment models for predicting venous thromboembolic risk). Thirty-five individual candidate predictors were identified and included for dissemination in round 1. Predictors were subdivided into risks related to injury/immobilisation and generic thrombosis risks. Initial proposed candidate predictors are shown in Table 13, which also shows the results of subsequent Delphi rounds.
TABLE 13
Summary of results from rounds 1 and 2 of the Delphi consensus exercise
Round 1 results
Seven variables were identified as predictive of VTE risk by consensus criteria during round 1. Thirteen variables were identified as not predictive of VTE by consensus criteria and excluded from the exercise. No consensus was achieved on 15 variables; 14 predictors were carried forward to the second round, with moderated peer feedback and tabular display. A single variable was excluded from the exercise after collated comments, group discussion and feedback regarding the lack of primary suitability for inclusion. Four new variables were suggested during the round 1 exercise and these were also carried forward. All candidate predictors and their round 1 results are presented in Table 13.
Round 2 results
In the second round, consensus was achieved on six further variables as predictive of VTE risk. Five variables were identified on reflection as not predictive of VTE risk by consensus criteria and these were excluded from the exercise. No consensus remained for 7 of the 17 variables carried forward from round 1. No further risk predictors were suggested by participants during round 2. Candidate variables taken forward and their round 2 results are presented in Table 13.
Variables failing to achieve consensus
Of the seven variables failing to achieve consensus, two failed because of a dichotomous split with clear unresolvable disagreement by experts (intravenous drug use and comminuted fracture). The other five variables appeared to be categorised as weakly to moderately predictive by all, but fell short of agreed criteria for inclusion. Further rounds were deemed unlikely to generate further consensus at this stage and the trial team proposed a move to the facilitated round-table discussion to achieve consensus.
Facilitated round-table discussion
There was general round-table agreement about all variables for which consensus had been achieved by the Delphi exercise. A specific point was made by the group regarding the inclusion of several variables depicting the degree of immobilisation of the calf pump, and whether or not this should become a single ordinal variable. A single variable (active intravenous drug use) on which the group did not reach consensus was discussed in further detail, with the majority of the round table proposing that the variable be refined or this entire cohort of patients be excluded based on safety concerns. Several discussion points followed regarding the need for strict inclusion criteria when applying any decision rule, with particular regard to the type of immobilisation in this group. The final agreed variables considered to be predictors of VTE risk via the Delphi expert consensus were eight generic VTE risk predictors (i.e. thrombophilia, pregnancy/puerperium, active cancer, surgery in the preceding 3 months, prior VTE, exogenous oestrogen/hormone therapy, lower-limb paralysis and superficial thrombophlebitis), two patient demographics (i.e. age and BMI) and three variables specific to lower-limb immobilisation or injury (i.e. Achilles tendon rupture, rigid immobilisation and above-knee cast).
Table 14 compares the expert consensus variables to those included in the RAMs. Most of the expert consensus variables were included in one or more of the RAMs but the RAMs also included many variables that were not supported by expert consensus.
TABLE 14
Summary of predictor variables included in studies of RAMs, with comparison with the Delphi consensus exercise
Originally, it was planned to use the expert consensus methods to refine existing RAMs or construct up to five new RAMs from the selected risk factors, and then produce a consensus estimate of sensitivity and specificity for each RAM that would allow the exploration of the trade-off between sensitivity and specificity in decision-analytic modelling. It was decided not to proceed with this for the following reasons:
- The difficulty of achieving consensus on individual risk factors (which was felt to be unsurprising given the limited available evidence) suggested that it would not be possible to achieve the necessary consensus on the content and structure required to refine an existing RAM or construct a new RAM.
- The receiver operating characteristic (ROC) analysis of the L-TRiP(cast) score published by Nemeth et al.32 (identified in the systematic review of RAMs) provided an estimate of the trade-off between sensitivity and specificity that could be used in decision-analytic modelling, and would be more credible and usable than expert-derived estimates of sensitivity and specificity for expert-derived RAMs.
Summary of key findings
- Expert consensus on 13 variables most likely to predict VTE risk for outpatients with lower-limb injury and temporary immobilisation has been established: eight generic VTE risk predictors (i.e. thrombophilia, pregnancy/puerperium, active cancer, surgery in the preceding 3 months, prior VTE, exogenous oestrogen/hormone therapy, lower-limb paralysis and superficial thrombophlebitis), two patient demographics (i.e. age and BMI) and three variables specific to lower-limb immobilisation or injury (i.e. Achilles tendon rupture, rigid immobilisation and above-knee cast).
- It was not possible to achieve expert consensus on the following seven variables: intravenous drug use, significant injury in the preceding 3 months, significant medical comorbidity, preceding immobility, comminuted injury, non-weight-bearing status and family history of VTE.
- Injury- and plaster-associated risk was proposed as a single ordinal variable based primarily on the degree of calf pump immobilisation.
- Review of pharmacological thromboprophylaxis for preventing venous thromboembolism
- Review of individual risk factors associated with venous thromboembolic risk
- Review of risk assessment models for predicting venous thromboembolic risk
- Identifying key variables to assess thromboembolic risk: a Delphi consensus exercise
- Assessment of clinical effectiveness - Different strategies for pharmacological ...Assessment of clinical effectiveness - Different strategies for pharmacological thromboprophylaxis for lower-limb immobilisation after injury: systematic review and economic evaluation
- Introduction - Duration of intravenous antibiotic therapy for children with acut...Introduction - Duration of intravenous antibiotic therapy for children with acute osteomyelitis or septic arthritis: a feasibility study
- List of abbreviations - An Occupational Therapy intervention for residents with ...List of abbreviations - An Occupational Therapy intervention for residents with stroke-related disabilities in UK Care Homes (OTCH): cluster randomised controlled trial with economic evaluation
- List of abbreviations - Clinical effectiveness and cost-effectiveness of a multi...List of abbreviations - Clinical effectiveness and cost-effectiveness of a multifaceted podiatry intervention for falls prevention in older people: a multicentre cohort randomised controlled trial (the REducing Falls with ORthoses and a Multifaceted podiatry intervention trial)
- Microbiology substudy - Duration of intravenous antibiotic therapy for children ...Microbiology substudy - Duration of intravenous antibiotic therapy for children with acute osteomyelitis or septic arthritis: a feasibility study
Your browsing activity is empty.
Activity recording is turned off.
See more...