NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coomarasamy A, Harb HM, Devall AJ, et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Southampton (UK): NIHR Journals Library; 2020 Jun. (Health Technology Assessment, No. 24.33.)

Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT.
Show detailsIntroduction
The economic evaluation conducted alongside the PRISM trial is reported in this chapter. The primary objective of the trial was to investigate whether or not progesterone used by women with bleeding in early pregnancy (up to 16 weeks of gestation) can prevent miscarriage and lead to live births at ≥ 34 weeks of pregnancy. The overall aim of the economic evaluation was to assess the relative cost-effectiveness of progesterone compared with placebo in these women.
Methods
A within-trial incremental cost-effectiveness analysis (CEA) was performed from the perspective of the NHS and the NHS/PSS.44 The CEA results are expressed in terms of cost per additional live birth at ≥ 34 completed weeks of gestation. Given that the duration of the trial was less than 1 year, neither costs nor outcomes were discounted. The health economic analysis was reported following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS).45
Outcomes
The primary outcome of the CEA was live births at ≥ 34 completed weeks of gestation based on the principal outcome of the clinical trial. Gestational age was estimated based on participants’ ultrasonography result and 11–14 weeks, when available, or otherwise based on the ultrasonography at randomisation. An additional outcome of the PRISM trial was neonatal survival at 28 days post partum, and this was explored as a secondary outcome in the CEA.
Data
Resource use and costs
Data on all resources consumed in the hospital setting by each woman from randomisation to hospital discharge were collected prospectively using researcher-recorded trial collection forms. The use of other services provided by the community during the same period was captured retrospectively via health services self-completed questionnaires. Unit costs for each resource item (Table 14) were identified from established national sources.47,50 All costs were expressed in 2017–18 Great British pounds. Cost estimates from earlier years were inflated to 2017–18 prices using the Hospital and Community Health Services (HCHS) pay and prices index.50 Hospital-related unit costs values were obtained from the NHS Reference Costs 2016/1747 where available. Otherwise, these costs were obtained from reference costs for earlier years or from other sources, such as the Personal Social Services Research Unit (PSSRU) costs. In cases where there are different categories associated with a resource use, weighted averages were used (see Table 14).
TABLE 14
Unit costs of resource items (2017–18 prices)
Resource use data within the hospital setting (inpatient and outpatient) focused on the:
- quantity of progesterone administered
- antenatal period
- intrapartum period
- postnatal period (maternal and neonatal).
Data were collected for primary care resources such as:
- contacts with GPs
- contacts with community midwives.
Progesterone
The quantity of progesterone vaginal pessaries was calculated based on the number of days they were used from randomisation until the end of 16 weeks of gestation (or earlier if miscarriage occurred before 16 weeks). The cost of progesterone was identified from the BNF46 as £21 for a 21-pessary pack. In the trial, each woman used four pessaries daily, which is equivalent to a cost of £4 per day (see Table 14).
Antenatal period
For the antenatal period, resource use data were collected on the number of hospital, day assessment unit (DAU) and emergency visits as well as the number of inpatient day admissions (for a stay of < 24 hours) and nights of inpatient admissions. Based on the descriptions in the trial literature, the costs of antenatal hospital (routine observation) and DAU (antenatal specialised non-routine ultrasound scan) visits were obtained from the NHS Reference Costs 2016/17.47
For inpatient day admission (< 24 hours), this was described in the trial as a day-case management of an antenatal disorder.47 Emergency visit (antenatal diagnostic procedures) costs were provided from the NHS Reference Costs 2013 to 2014,48 whereas inpatient night admissions costs were obtained from an earlier PSSRU cost.49 Because all participants in the trial underwent routine ultrasonography at specified times in the study, the cost of an ultrasound was not included in the analysis.
Intrapartum period
The resource use collected at the end of pregnancy varied depending on whether or not the baby was born alive. Where live births occurred, the onset of labour was recorded as spontaneous, augmented, pre-labour C-section or induced. Information on the mode of delivery included unassisted vaginal deliveries, instrumental vaginal deliveries, elective C-sections, emergency C-sections or vaginal breech deliveries with or without intrapartum complications. Deliveries such as water births and home deliveries were captured as ‘others’.
The Healthcare Resource Group (HRG) unit costs47 for delivery mode are categorised as normal vaginal delivery, assisted vaginal delivery, planned C-section and emergency C-section, corresponding to unassisted vaginal delivery, instrumental vaginal delivery, elective C-section and emergency C-section on the case report form (CRF), respectively. Each category is grouped further as with or without complications. Weighted averages of the unit costs for the different levels of complications were calculated. As there were no HRG unit costs available for breech delivery or ‘other’, in consultation with the clinical team, we assumed that the costs were the same as those for a normal vaginal delivery. For labour onset, we assumed that these costs were already accounted for by the delivery mode and, hence, these were not costed separately.
Where babies were not born alive, the outcome was recorded as miscarriage, ectopic pregnancy, termination or stillbirth. The management of such outcomes was recorded as spontaneous resolution, surgical management or medical management. The costs of management were provided in the NHS Reference Costs as threatened or spontaneous miscarriage with intervention (medical or surgical management) and without intervention (spontaneous resolution).47
Postnatal period
During the postnatal period, data were collected for both maternal and neonatal resource use, from pregnancy end to 28 days post discharge.
Maternal resource use
The unit costs for hospital visits, DAU visits, emergency visits and others that applied to the antenatal period were also relevant to the postnatal period resource use; relevant unit costs are presented in Table 14. For postnatal DAU visits and inpatient admissions, the corresponding costs for the antenatal indices were used. The cost of a postnatal emergency visit was obtained using a weighted average of emergency medicine for patients requiring category 0–2 treatment and category 0–1 investigation.47 Postnatal hospital visit costs were obtained from the PSSRU.49
Data collected for immediate maternal postnatal care resource use included admissions to a HDU (level 2 care) or an ITU (level 3 care). Using the UK definitions for level 2 care (patient receiving a single organ support) and level 3 care (patient receiving at least two organ supports),53 the unit costs for these admissions were obtained from the NHS Reference Costs 2016/17.47 For level 2 care (HDU admissions), ‘adult critical care – one organ supported’ was used; for level 3 care (ITU admissions), a weighted average was taken across five HRGs [adult critical care (two organs supported) to adult critical care (six or more organs supported)].
Neonatal resource use
The immediate neonatal care resource use included the number of nights receiving intensive care, high-dependency care and special care. Costs for these resources were obtained from the costs schedule.47
Serious adverse events
Information on SAEs was collected using SAE forms. In this study, a SAE was defined as an untoward event resulting in maternal death, stillbirth, hospitalisation, persistent or significant disability, congenital anomaly or birth defect. Only clinically specified SAEs deemed to have arisen from the trial intervention were considered to be relevant to the economic analysis. Because there were no SAEs that were clearly related to the use of progesterone, we did not include such costs in the analysis.
Primary care services
Service use questionnaires, completed by a subsample of women, captured data on primary care service resource use in the trial period. These included the number of visits to the GP, practice/community midwife, practice nurse, psychologist (or counsellor), health visitor, social worker and other community services. However, these services were recorded as the number of visits without a specified duration.
The costs for primary care services were obtained from the Unit Costs of Health and Social Care 2017.50 To cost each primary care resource use, we used the recommended average duration of 9.22 minutes for a GP face-to-face visit, 30 minutes for a midwife visit, 15.5 minutes for a GP nurse visit and 20 minutes for the remaining variables. Because no telephone contacts with the GP or practice nurse were recorded, we did not include these costs.
Primary economic analysis
A within-trial incremental CEA was conducted to estimate the relative costs and benefits of progesterone compared with placebo. The cost-effectiveness of progesterone was expressed in terms of the cost per additional live birth after ≥ 34 complete weeks of gestation. The base-case primary analysis focused on the hospital-related (inpatient and outpatient) costs for the participants incurred in the trial period.
Using study-specific resource use and costs, the total cost over the trial period was calculated by multiplying the resource items used by the corresponding unit cost and adding up all items. The mean total costs and mean total resource use for participants across the trial arms were calculated. Given the skewness inherent in most cost data and the concern of economic analyses with mean costs, we calculated 95% CIs around mean differences through the analyses of 1000 resamples using the bias-corrected and accelerated (BCa) bootstrap method.54
To explore heterogeneity in the trial population, multivariate cost analyses were performed using seemingly unrelated regressions.55,56 Seemingly unrelated regression has been shown to be robust to skewed data and allows for a correlation in the error terms between costs and outcomes.57 Model covariates included baseline data on age, BMI, the quantity of bleeding and the number of previous miscarriages. The selection of covariates was informed by the prognostic variables used by the clinical team. All results were presented as mean values with SD and, where applicable, as mean differences in costs and effects with 95% CIs.
Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the difference in mean total cost between the trial arms by the difference in the number of live births at ≥ 34 weeks. The ICER is a measure that depicts the additional cost ascribed to an additional effect. To calculate ICERs, the formula below was used, with C representing cost and E representing effects:
To quantify the uncertainty that typically occurs as a result of variations in sampling, we used non-parametric bootstrapping to resample the joint distribution in the mean cost and outcome difference.58 This generated 5000 paired estimates of incremental costs and outcomes, which were plotted in a cost-effectiveness plane as a scatterplot.59 A cost-effectiveness plane is a four-quadrant plane depicting bootstrap estimates. Based on the location of the scatterplot dots on the quadrant, an intervention may be deemed more effective and less costly (south-east), more effective and more costly (north-east), less effective and more costly (north-west) or less effective and less costly (south-west) than the alternative intervention.
A cost-effectiveness acceptability curve (CEAC) was constructed to show the probability that progesterone is a cost-effective intervention compared with placebo across a range of values, representing the decision-maker’s willingness to pay (WTP) for an additional benefit.44 Currently, there is no standard valuation for an additional live birth.2,60 In the UK, NICE typically uses a WTP threshold of £20,000–30,000 per quality-adjusted life-year (QALY) in approving a health-care intervention.44
Secondary economic analyses
Secondary analyses involving ICER calculations on the primary outcome are based on the following costs.
- Hospital-related costs for resource use for women only: the hospital-related costs for women included antenatal, intrapartum and postnatal care costs. We excluded the neonatal care costs accrued by infants from the total cost for this analysis to examine which aspect of cost had the largest effect on the result – the women’s cost or the neonatal care cost.
- Hospital and primary care costs: here we added primary care costs such as GP and practice nurse visits to the hospital costs. This analysis was conducted to explore the perspective of the NHS/PSS.
- Adjusting for missing data: the main base-case analysis was carried out on only individuals for whom there were outcome data. For this analysis, we included participants who were lost to follow-up. We assumed that all women lost to follow-up had a miscarriage and we imputed the costs for this subgroup. Missing costs were imputed using multiple imputations61 by applying chained equations with predictive mean matching across 60 imputations.62
- Hospital-related costs for women with three or more previous miscarriages: preliminary results suggested that there was clinical effectiveness for women with three or more previous miscarriages. Therefore, we conducted a CEA for this subgroup.
We also carried out a secondary incremental CEA based on the final end point using hospital-related costs for participants with complete data. We reported the analysis in terms of cost per additional baby who survived beyond 28 days after birth for each woman.
Sensitivity analysis
Deterministic sensitivity analyses were conducted to explore the inherent uncertainty in key assumptions and variations in the analytical methods used, and to consider the broader issue of the generalisability of the results. This involved varying some of the parameters while leaving others at their baseline value. A number of sensitivity analyses were conducted, as detailed below.
- Fixed cost of treatment until 16 weeks of gestation. In the PRISM trial, women in early pregnancy started treatment from randomisation until 16 weeks’ gestation. If it is assumed that all women started treatment at approximately 7.4 weeks (52 days) with no miscarriage and continued treatment until 16 weeks (112 days) of gestation, this would translate to 60 days of treatment. Based on the progesterone vaginal pessary cost of £4 per day (as described above for four pessaries) and assuming that on day 1 participants used two pessaries, the expected cost of progesterone is £238 [2 + (59 × 4)]. In a real-life scenario, the intervention would be provided for the expected treatment period (from 6 to 8 weeks) until 16 weeks, and hence we explored the impact of a fixed cost of progesterone until 16 weeks of gestation.
- Unit costs. We explored the impact of alternative cost estimates. For inpatient night of admission for both the antenatal and postnatal periods, we replaced the cost used in the primary analysis with the cost of excess bed-days (£311).47 The cost of management of miscarriage used in the main analyses was obtained from the NHS Reference Costs.47 However, the NICE guideline on miscarriage management3,63 provides an estimate of £1522 for medical management and £1827 for surgical management. These values have been used by other studies.13 For the sensitivity analysis, we replaced the costs of management with these costs.
Model-based analysis
Preliminary results showed that there was no clinically detectable effect on neonatal outcomes as a result of the PRISM trial that was a result of progesterone. This finding is in keeping with an earlier study in which women were given progesterone.2,64 In view of this result, modelling costs and outcomes beyond the trial period was not deemed necessary.
Results
A total of 4153 women were recruited to the PRISM trial and randomised to either the progesterone (n = 2079) or the placebo (n = 2074) arm. Among the 4153 women recruited, 30 women withdrew from the trial and 85 women were lost to follow-up. Hence, the base-case primary analysis was conducted for 4038 participants: 2025 in the progesterone arm and 2013 in the placebo arm.
Outcomes
The details of the major outcomes of the trial are presented in Table 15. At the end of pregnancy, 1513 (74.72%) and 1459 (72.48%) women in the progesterone and placebo arms, respectively, had live births after 34 completed weeks of pregnancy. This translates to an effect difference of approximately 2.2% (0.022, 95% CI –0.004 to 0.050). Among women who had live births during the trial period, babies born to 1538 out of 2025 (75.95%) women in the progesterone arm and 1487 out of 2013 (73.87%) women in the placebo arm survived beyond 28 days of birth.
TABLE 15
Outcomes across treatment groups
Resource use and costs
A breakdown of the resource use data by trial arm is presented in Table 16. Mean health-care costs per participant by trial arm are presented in Table 17. During the trial, 2023 women in the intervention arm received progesterone and 2009 women in the non-intervention arm received placebo. Based on the mean number of days that participants utilised progesterone pessaries, the average cost of the intervention was calculated to be £204 (95% CI £200 to £207) per woman (see Table 17). The most substantial costs accrued during the trial by participants were from antenatal hospital visits, with a mean cost of £2339 (SD £2672) per woman for the progesterone arm and £2334 (SD £2665) per woman for the placebo arm.
TABLE 16
Mean resource use across treatment groups
TABLE 17
Disaggregated costs by trial arms (2017–18 prices)
During the antenatal period, women allocated to the progesterone arm had, on average, a higher frequency of antenatal and DAU visits but a smaller number of emergency room visits and hospital admissions than women in the placebo arm. During the postnatal period, women in the progesterone arm utilised similar services more than those in the placebo arm except for emergency hospital visits, which were the same for both arms. However, women in the placebo arm had more admissions to the HDU [mean 0.06 (SD 0.46) for placebo vs. mean 0.05 (SD 0.30) for the progesterone arm]. Similarly, babies born to women in the placebo arm had, on average, a greater number of admissions to the HDU [mean 0.52 (SD 3.90) for placebo vs. mean 0.42 (SD 4.02) for the progesterone arm] and neonatal special care [mean 1.16 (SD 4.95) for placebo vs. mean 1.02 (SD 5.07) for the progesterone arm].
For delivery mode, women in the placebo arm had, on average, more emergency C-sections than women in the intervention arm. On average, women in the placebo arm utilised more neonatal care services than those in the intervention arm.
In keeping with the mean resource use, antenatal care costs for DAU and hospital visits were higher in the intervention arm, whereas emergency visits and hospital admissions costs were higher in the placebo arm.
In terms of cost differences, the greatest mean value for the participants was for emergency C-section with complications, which was greater in the placebo group than in the treatment group (–£137, 95% CI –£246 to –£28). The highest cost difference as a result of the intervention was for elective C-section without complication with a mean difference of £48 (95% CI –£11 to £108) per participant. Neonatal care variables were consistently lower in the progesterone group [ITU, –£10 (95% CI –£442 to £421); HDU, –£93 (95% CI –£344 to £159) and special care unit, –£76 (95% CI –£260 to £109)].
Mean total costs
The mean total costs by trial group for different variables are presented in Table 18. The average hospital-related service costs per woman for the trial period was £7452 in the progesterone group and £7572 in the placebo group, generating a mean cost difference of –£127 (BCa mean –£127, 95% CI –£759 to £505). The inclusion of the intervention cost (£204) generated a mean difference of £83 (adjusted mean £76, 95% CI –£559 to £711) per woman, which increased slightly to £78 (95% CI –£563). The differences in cost were not statistically significant.
TABLE 18
Mean costs per woman (2017–18 prices)
Primary analysis
The primary (base-case) cost-effectiveness outcome of the PRISM trial was the cost for an additional live birth after ≥ 34 completed weeks of pregnancy. The progesterone intervention appeared to be slightly more effective than placebo, resulting in an additional two live births per 100 women (an effect difference of 0.022, 95% CI –0.004 to 0.050) at ≥ 34 weeks’ gestation. The ICER, which combines the differences in costs in both groups, is presented in Table 19. The administration of progesterone resulted in an estimated additional cost of £83 per woman (adjusted mean £76, 95% CI –£559 to £711).
TABLE 19
Point estimate ICER for primary analysis using hospital-related costs
Given the differences in costs and effects, the point ICER estimate for progesterone compared with placebo was calculated at £3305 per additional live birth.
Figure 9 shows the results of 5000 bootstrap replications plotted on the cost-effectiveness plane for the primary analysis. Each point on the plane depicts a pair of incremental cost and incremental effectiveness estimates for the comparison between progesterone and placebo. This suggests that progesterone is likely to be more effective, given that the majority of the scatterplots are in the south-east and north-east quadrants. However, it is uncertain whether the progesterone intervention is likely to be more costly (north-east) or less costly (south-east) than no intervention.
The CEAC for the primary analysis (Figure 10) shows the probability of progesterone being cost-effective at various values of decision-makers’ WTP per additional live birth. Figure 10 indicates that, for thresholds of WTP per additional live birth of > £15,000, there is > 80% probability that progesterone is cost-effective. For WTP thresholds of > £30,000, the probability of cost-effectiveness exceeds 90%.
Secondary analyses
A series of secondary analyses were conducted to explore the impact of varying costs on the primary outcome.
Secondary analysis I (hospital-related costs for participants minus neonatal care costs)
For the first secondary analysis, we removed neonatal care costs from the hospital costs. This resulted in an adjusted mean cost difference of £170 (95% CI –£113 to £453) (Table 20).
TABLE 20
Point estimate ICER for secondary analysis I (maternal hospital costs)
The ICER was calculated as £7370 per additional live birth beyond 34 weeks of gestation. The increased ICER value for this analysis is probably because, on average, women in the placebo arm utilised more neonatal hospital resources than women in the progesterone arm. Hence, the removal of neonatal care costs resulted in a higher cost difference.
The cost-effectiveness plane (Figure 11) shows that progesterone is the more effective and more costly intervention, with the majority of the bootstrap replications in the south-east quadrant. The CEAC (Figure 12) suggests that, for WTP thresholds of > £12,000 per additional live birth, there is > 95% probability that progesterone is a cost-effective intervention.

FIGURE 11
Cost-effectiveness plane for secondary analysis I (maternal hospital costs).

FIGURE 12
The CEAC for secondary analysis I (maternal hospital costs).
Secondary analysis II (hospital and primary care costs for participants)
In another secondary analysis, we included hospital-related costs and primary care costs for the participants. First, we explored the total primary care cost for women with complete primary care service use data; complete data were available for 272 participants. In this subgroup, the mean total cost was £120 for women in the progesterone arm and £98 for women in the placebo arm. For each woman in the progesterone arm, we added £120 to the total hospital-related cost; for each woman in the placebo arm, we added £98 to the total hospital-related cost (Table 21). We calculated an additional cost of £106 (adjusted mean £98, 95% CI –£537 to £733) and an ICER of £4264 per additional live birth.
TABLE 21
Point estimate ICER for secondary analysis II (hospital and primary care costs)
Again, the cost-effectiveness plane (Figure 13) clearly suggests that progesterone is more effective. Figure 14 depicts the CEAC for this analysis. For WTP thresholds of > £15,000, per additional live birth beyond 34 weeks’ gestation, the probability of progesterone being more effective than placebo is over 80%. The probability of cost-effectiveness exceeds 90% for WTP thresholds of > £30,000.

FIGURE 13
Cost-effectiveness plane for secondary analysis II (hospital and primary care costs).

FIGURE 14
The CEAC for secondary analysis II (hospital and primary care costs).
Secondary analysis III (hospital costs for participants including those lost to follow-up)
For this analysis, we included both the women used for the primary base-case analysis and those who were lost to follow-up. We explored a worst-case scenario and assumed that all women lost to follow-up had a miscarriage. We used multiple imputations to impute missing costs and re-ran the primary analysis. This included 2069 participants in the progesterone arm and 2054 participants in the placebo arm.
The results (Table 22) showed that progesterone was slightly more costly (cost difference £29, 95% CI –£593 to £651) and more effective than no progesterone for this subgroup, with an ICER of £1378 per additional live birth beyond 34 weeks of gestation.
TABLE 22
Point estimate ICER for secondary analysis III (hospital costs for participants including those lost to follow-up)
From the cost-effectiveness plane (Figure 15), it is evident that progesterone is more effective; however, it is uncertain which intervention is more costly. The CEAC (Figure 16) shows that, for WTP thresholds of > £15,000 per additional live birth beyond 34 weeks of gestation, the probability of progesterone being cost-effective is approximately 85%, and the probability exceeds 90% for WTP thresholds of > £30,000.

FIGURE 15
Cost-effectiveness plane for secondary analysis III (hospital costs for participants including those lost to follow-up).

FIGURE 16
The CEAC for secondary analysis III (hospital costs for participants including those lost to follow-up).
Secondary analysis IV (hospital costs for participants with three or more previous miscarriages)
We conducted a subgroup analysis of women with three or more previous miscarriages. This included 137 women in the intervention arm and 148 women in the placebo arm. The intervention was more effective, with an additional gain of 15 live births per 100 women, beyond ≥ 34 weeks' gestation (Table 23). An ICER of £11,606 per additional live birth beyond 34 weeks’ gestation was calculated for this subgroup.
TABLE 23
Point estimate ICER for participants with three or more previous miscarriages
Similarly, the cost-effective plane (Figure 17) depicts that progesterone is more effective and more costly with the majority of the bootstrap replications in the south-east quadrant. The CEAC (Figure 18) shows over > 90% probability of the intervention being cost-effective for WTP thresholds of > £15,000.
Analyses using secondary outcomes
Incremental CEAs were conducted for the final end point of the PRISM trial (Table 24). The analysis was based on the incremental cost of the intervention for an additional baby survival for each woman at 28 days post partum. The effect difference was 0.021 (95% CI –0.005 to 0.048). The ICER for this analysis was £3037 per additional baby who survived beyond 28 days post birth.
TABLE 24
Point ICERs for secondary outcomes
Sensitivity analyses
We conducted sensitivity analyses in which we explored different scenarios (Table 25). Using alternative cost estimates for nights of hospital admissions and miscarriage management appeared to have a limited effect on the resulting ICER (see Table 25).
TABLE 25
Point estimate ICER for the sensitivity analyses
However, using a fixed cost of progesterone until 16 weeks increased the ICER to £4977 per additional live birth beyond 34 weeks’ gestation (see Table 25). The cost-effectiveness plane (Figure 19) showed dominance in the north-east and south-east quadrants, which indicated that progesterone was more effective and either less costly or more costly. The CEAC (Figure 20) shows over 90% probability of the intervention being cost-effective for WTP thresholds of > £25,000. Likewise, the removal of the cost of delivery increased the ICER to £3743 per additional live birth beyond 34 weeks (see Table 25).
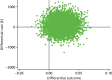
FIGURE 19
Cost-effectiveness plane for a fixed cost of progesterone until 16 weeks’ gestation.
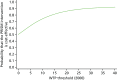
FIGURE 20
The CEAC for a fixed cost of progesterone until 16 weeks’ gestation.
In secondary analysis II, we added mean totals of the primary care costs to the total costs for each woman depending on the trial arm. From this, we calculated an ICER of £4264 per additional live birth beyond 34 weeks of gestation (see Table 21). The sensitivity analysis in which we imputed costs via multiple imputations did not have much effect on the ICER, with a slight increase to £4321 per additional live birth beyond 34 weeks (see Table 25).
Discussion of the health economic findings
Principal findings
We evaluated the cost-effectiveness of progesterone in preventing miscarriage and leading to a live birth at ≥ 34 weeks of pregnancy in women who presented with bleeding in early pregnancy. Our results suggest that progesterone is more effective and slightly more costly than placebo. More specifically, progesterone resulted in an additional two live births per 100 women (0.022, 95% CI –0.004 to 0.050) at ≥ 34 weeks of gestation relative to placebo, with an additional cost of £83 (adjusted mean difference £76, 95% CI –£559 to £711) per woman. The additional cost was mainly attributable to the cost of progesterone administration (mean cost £204). The ICER was estimated at £3305 per live birth at ≥ 34 weeks. A conclusion on the cost-effectiveness of the PRISM trial would depend on the amount that society is willing to pay to increase the chances of an additional live birth at ≥ 34 weeks of pregnancy. For potentially acceptable WTP threshold values for an additional live birth,44 the probability of progesterone being cost-effective for this population group exceeds 90%.
The National Institute for Health and Care Excellence attached a value to an averted stillbirth of 25 QALYs.65 This assumes that life lost has a typical life expectancy in good health, but when discounting is applied to the expected years in full health, it yields 25 discounted QALYs. To interpret the primary CEA results in relation to QALYs, we used the NICE value (25 QALYs) as a proxy. If we assume that babies born alive at ≥ 34 weeks live in full health and divide the ICER (£3305 per additional live birth) by 25, then the cost per QALY is likely to be £132. If a baby did live in full health for the anticipated life expectancy, then on the basis of this ICER (£132) the intervention is cost-effective.44 Furthermore, evidence from the NHS Reference Costs schedule47 indicates that the upper cost quartile of the most expensive delivery is about £15,000, which could go much higher if we allow for the cost of excess bed-days. This further suggests that progesterone intervention is cost-effective.
The ICER for the final end point (secondary outcome) of the trial was £3037 per additional baby surviving beyond 28 days after birth. The intervention was more effective, with a gain of three neonates per 100 women surviving beyond 28 days post partum. A subgroup analysis of women with three or more previous miscarriages led to an increase of 15 live births per 100 women in the intervention group.
Strengths and limitations of the economic analyses
The strength of the CEA is that it was based on a large, robust, multicentre randomised controlled trial involving over 4000 participants, making this the largest study to explore whether or not progesterone provides value for the public health-care resources. The outcome and resource use data were prospectively collected at different points in the trial using CRFs. Unit costs were obtained from established national sources. In cases where HRGs did not clearly depict our variables, we liaised with the clinical team to decide on the most appropriate HRG. The CEA also benefited from the robustness of the main analyses and the sensitivity analyses. However, data on primary care services were available for < 10% of the participants: we accounted for this by imputing missing costs in our analyses. A limitation of this analysis was the failure to explore the wider societal costs to the participants. However, this was beyond the scope of the study and beyond the requested resource.
Comparison with the literature
To our knowledge, this is the first UK study to investigate the cost-effectiveness of progesterone in preventing miscarriage and achieving a live birth beyond 34 weeks of gestation. A similar study investigated the cost-effectiveness of progesterone in preventing miscarriages in women with a history of recurrent miscarriages and leading to a live birth beyond 24 weeks of gestation.2 The authors reported that the total mean cost of the intervention was £332.17 higher in the progesterone arm than in the placebo arm and an ICER of £18,053 per additional live birth beyond 24 weeks for the base-case analysis, with a cost-effectiveness probability of 50% at this value.
Implications for policy
The results of the CEA suggest that progesterone is likely to be considered a cost-effective intervention by decision-makers for women presenting with early pregnancy bleeding (threatened miscarriage) within 12 weeks of gestation.
Summary of health economic findings
- For the primary analysis, the mean total cost was higher in the progesterone group (£7655) than in the placebo group (£7572), with an additional cost of £83 (1% higher cost than usual care).
- The additional difference in the mean probability of a live birth beyond ≥ 34 completed weeks of gestation was 0.022 (95% CI –0.004 to 0.050), indicating that the progesterone intervention resulted in an additional two live births per 100 women at ≥ 34 weeks.
- For the primary analysis, the ICER per additional live birth beyond 34 weeks of gestation was calculated as £3305.
- There is > 80% confidence that progesterone is cost-effective if decision-makers are prepared to pay £15,000 per additional live birth and > 90% if the WTP threshold is £30,000.
- Currently, in the UK, progesterone is not routinely given to women who are at high risk of miscarriage. The results of the CEA suggest that progesterone is likely to be considered cost-effective, particularly for women (with one or more miscarriages) who present with bleeding in early pregnancy.
- Health economic analysis - Progesterone to prevent miscarriage in women with ear...Health economic analysis - Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT
- Consensus meeting to assess the feasibility of a future randomised clinical tria...Consensus meeting to assess the feasibility of a future randomised clinical trial in children with bone and joint infections - Duration of intravenous antibiotic therapy for children with acute osteomyelitis or septic arthritis: a feasibility study
- Glossary - Enzyme-linked immunosorbent assays for monitoring TNF-alpha inhibitor...Glossary - Enzyme-linked immunosorbent assays for monitoring TNF-alpha inhibitors and antibody levels in people with rheumatoid arthritis: a systematic review and economic evaluation
- lens intrinsic membrane protein 2, partial [Homo sapiens]lens intrinsic membrane protein 2, partial [Homo sapiens]gi|992391488|gb|AMH87787.1|Protein
- Sisymbrium yunnanense isolate TB13627_syun_2 CER1 protein (CER1) gene, partial c...Sisymbrium yunnanense isolate TB13627_syun_2 CER1 protein (CER1) gene, partial cdsgi|2022635263|gb|MW319187.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...



