NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Worthington J, Lane JA, Taylor H, et al. Thulium laser transurethral vaporesection versus transurethral resection of the prostate for benign prostatic obstruction: the UNBLOCS RCT. Southampton (UK): NIHR Journals Library; 2020 Sep. (Health Technology Assessment, No. 24.41.)

Thulium laser transurethral vaporesection versus transurethral resection of the prostate for benign prostatic obstruction: the UNBLOCS RCT.
Show detailsParticipant flow
Figure 1 shows the layout of the trial and the different levels of dropout and analysis. Overall, 16 randomised patients withdrew from the study before their 12-month end point (with three requesting complete data withdrawal), with a median withdrawal time of 3.8 months (of the 13 recorded). The IPSS was able to be analysed for 74% and 78% at 12 months in the ThuVARP and TURP arms, respectively. Qmax was able to be analysed for 82% and 86% of participants, respectively.
Recruitment
Overall, 410 patients were randomised to receive either ThuVARP or TURP, 205 in each arm (Figure 2). The first patient to receive surgery was randomised in July 2014 and the final randomised patient had surgery in December 2016.

FIGURE 2
Recruitment.
Baseline data
Baseline comparisons for the UNBLOCS trial are presented in Tables 5–7. Baseline information on flow rates and urinary/sexual symptoms and quality of life was not available for those patients with an indwelling catheter; therefore, the figures are rather low for some baseline variables.
TABLE 5
Baseline sociodemographic characteristics of eligible patients who underwent randomisation
TABLE 7
Baseline patient-reported outcome measures of eligible patients who underwent randomisation
Overall, participants in the TURP arm appeared to be very slightly worse off in terms of patient-reported measures of symptom burden. However, no large differences were seen between the arms at baseline in terms of sociodemographic (see Table 5) or clinical characteristics (see Table 6). Painful ejaculation was the only patient-reported outcome measure that differed by more than an absolute difference of 10% (see Table 7).
TABLE 6
Baseline clinical characteristics of eligible patients who underwent randomisation
Numbers analysed
When comparing our analysable sample with those patients who withdrew or were lost to follow-up (i.e. those patients who did not complete the 12-month Qmax or the IPSS), we can see differences that met the criteria used to look for imbalance between the arms (> 10% or 0.5 SDs). These differences were seen in centre, daytime urinary frequency and the effect on participants’ sex lives (Table 8). A difference was also seen in comorbidities, but this did not reach the 10% absolute difference. All variables not presented in Table 8 were balanced between those patients who were and those patients who were not analysed at 12 months. However, in general, those patients who withdrew or were lost to follow-up had slightly worse symptoms at baseline.
TABLE 8
Baseline characteristics of those patients assessed in the primary analysis and those patients who withdrew or were lost to follow-up
The numbers of patients who had outcome data were also relatively balanced between the arms, as are the numbers of withdrawals. The numbers of patients undergoing their assigned treatment differed between the arms, with only 75% of those patients randomised to ThuVARP actually receiving their treatment (p < 0.001) (Table 9).
TABLE 9
Data quality in the UNBLOCS trial
Reasons for withdrawal were relatively similar between the arms (Table 10). A large proportion of changes in treatment were because of equipment failure in the ThuVARP arm, with 18 participants being changed to TURP straight away or converting mid-procedure (Table 11). Prostate size also resulted in nine conversions to TURP. The proportion of participants receiving conversions compared with receiving ThuVARP was relatively balanced across the centres and over time. Between 6% and 25% of participants in each centre received a conversion from ThuVARP to TURP. When breaking the recruitment period per surgeon into halves, the conversion rate in the first half was 11%, whereas it increased to 28% in the second half.
TABLE 10
Reasons for withdrawal
TABLE 11
Reasons for change in treatment
Statistical outcomes and estimation
International Prostate Symptom Score
The first of two primary outcomes was the IPSS at 12 months. The null hypothesis was that the two surgical procedures differed by at least 2.5 points, while the alternative hypothesis was that the two procedures were equivalent. At the 12-month point from surgery, IPSS (overall median 4.0 points) was much lower than that recorded in baseline questionnaires (overall median 23.0 points). However, it was possible to obtain IPSS at baseline only for those patients who did not have an indwelling catheter (n = 175). The distribution of scores at 12 months is presented in Figure 3.

FIGURE 3
Distribution of IPSS at 12 months for (a) ThuVARP; and (b) TURP.
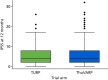
The box plot in Figure 4 shows the distribution of IPSS for each arm. They are very similar, both sharing a median score of 4 and an IQR of 2–8 points.
Given the skewed nature of the data, as well as the prespecified linear regression model, a bootstrap regression model was calculated as a post hoc sensitivity analysis.
Maximum urine flow rate values
The second of the two primary outcomes was the maximum urine flow rate at 12 months. The null hypothesis was that the two surgical procedures differed by at least 4 ml/second, while the alternative hypothesis was that the two procedures were equivalent. As with IPSS, 12 months after surgery, Qmax values were much improved from those values recorded at baseline (Figure 5). However, baseline Qmax values were possible to collect from only patients without an indwelling catheter.

FIGURE 5
Distribution of Qmax values at 12 months for (a) ThuVARP; and (b) TURP.
The box plot in Figure 6 shows the maximum urine flow rates by trial arm. The results show that those patients in the ThuVARP arm had a median 12-month Qmax of 17 ml/second (IQR 11–27 ml/second), whereas those in the TURP arm had a median Qmax of 20 ml/second (IQR 13–32 ml/second).
As with the IPSS, given the slightly skewed nature of the data, a bootstrap regression model was calculated as a post hoc sensitivity analysis.
Primary analysis results
The multiple imputation model included all randomised patients, apart from those patients who withdrew all data (n = 3). It imputed the catheter status, IPSS and Qmax at baseline and 12 months where data were incomplete. It employed a conditional imputation, whereby initially catheter status was imputed followed by IPSS/Qmax values only for those patients without a catheter. For participants who died during the trial period, we did not impute any data after their time of death (Table 12).
TABLE 12
Primary analysis results
The equivalence margin for IPSS was prespecified as 2.5 points. The difference between the arms, using the imputed model, was 0.28 points. The two procedures appear to be equivalent for the IPSS as the CIs are within the range –2.5 to 2.5 points; therefore, this blinded trial has demonstrated that a patient’s perception of urinary tract symptoms after treatment is equivalent for the ThuVARP and TURP procedures.
The equivalence margin for the Qmax levels was prespecified as 4 ml/second. The ThuVARP procedure gives a lower maximum urine flow rate at 12 months than TURP (just over 3 ml/second). The CIs are outside the range –4 to 4 ml/second, with the lower reaching almost 6 ml/second, deeming the treatments non-equivalent with respect to Qmax. Changing the test to superiority, which does not carry a statistical penalty after a non-inferiority or equivalence trial, did lead us to conclude that TURP is superior to ThuVARP in terms of maximum urine flow rate for both the complete-case and the imputation analyses.28
Success of blinding
In their 12-month questionnaire, participants were asked if they thought they knew which type of surgery they had undergone. Overall, 70% (238/339) of patients said that they did not know which operation they received. When asked to predict their surgery, 40% (138/346) of patients did so. Of those who predicted ThuVARP, 54% were correct; of those who predicted TURP, 82% were correct. However, of those who were correct, 80% went on to say that they did not actually know/they had guessed. Based on this, the team felt that blinding had been successful. Unfortunately, a research nurse unblinded nine participants at their 12-month clinic appointment (before they had completed their 12-month questionnaire). In a sensitivity analysis, these participants were removed to avoid any potential bias from the IPSS results.
Secondary outcomes: surgical complications
Surgical complications were recorded during surgery, postoperatively and at the 3-month and 12-month clinics. Where participants did not attend follow-up clinic, details of complications were extracted from the participants’ medical notes.
Perioperative complications
Overall, there were 28 complications in theatre or during the recovery period: 17 in the ThuVARP arm and 11 in the TURP arm (Table 13).
TABLE 13
Perioperative surgical outcomes
Although there appeared to be more events in the ThuVARP arm, when looking at treatment received we could establish that only 8 of those 17 complications were experienced during ThuVARP, whereas 2 were from TURP and 7 were during conversions from ThuVARP to TURP. ‘Other’ complications were not presented as these were reported differently across sites and did not always reflect true complications (e.g. broken laser fibre).
Postoperative complications
Data on surgical outcomes (Table 14) and postoperative complications (Table 15) were collected from postoperative, 3-month and 12-month CRFs completed by a clinician. The average length of stay was 48 hours in both arms of the trial. Transfusion and catheter requirement rates were low and similar. There was no evidence to suggest that one arm was better than the other for surgical outcomes. There was some evidence to suggest that there might have been a trend towards higher post-void residuals in the ThuVARP arm; however, when comparing the number of men with zero post-void residual volume, there did not appear to be a difference.
TABLE 14
Additional surgical outcomes
TABLE 15
Postoperative surgical complications (Clavien–Dindo scores per patient)
The numbers of events in each arm were extremely similar (see Table 15). Rows have been omitted when certain grades were not experienced by a single participant; for example, the odds ratio for urinary tract infections suggested that those patients in the ThuVARP arm were at a 2% higher odds of being in a higher Clavien–Dindo grade (0–5) than those patients in the TURP arm (p = 0.938). The highest Clavien–Dindo score recorded was IVb (a life-threatening complication requiring intensive care – multiorgan dysfunction). This was experienced twice by the same participant during a urinary tract infection and sepsis episode. This participant died 1 month later, but this was deemed unrelated (see Serious adverse events).
If we look at the total number of events experienced per participant, we see that the majority do not experience an event: 56% in the ThuVARP arm and 53% in the TURP arm (Table 16). There was no evidence to suggest that there are more events in one arm of the trial.
TABLE 16
Total number of complications during the 12-month period
Secondary outcomes: patient-reported outcomes
Overall patient-reported outcomes were similar in the two arms. Although urinary symptoms were generally worse in the ThuVARP arm, all differences could be explained by chance (Table 17). For nocturia (getting up to urinate more than once per night), there was some evidence to suggest that TURP was more effective in reducing the proportion of men reporting this outcome, which was strengthened when looking at this on an ordinal scale (p = 0.031). However, given the large number of secondary outcomes, we cannot rule out that this may have been a chance finding. Sexual symptoms were similar but with reduction in painful ejaculation still slightly in favour of ThuVARP; however, this difference had been already present at baseline.
TABLE 17
Secondary outcome: urinary and sexual symptoms
Participants in both arms seemed generally satisfied with their treatment, with 207 out of 340 (61%) of all patients giving the maximum score of 10 (Table 18). Participants in the ThuVARP arm were at a lower odds of saying that they would have the same treatment again if they had the same problem in the future, but there was only very weak statistical evidence to support this finding.
TABLE 18
Secondary outcome: satisfaction with treatment
Overall, quality of life was very high in both arms at 12 months (Table 19). Fifty-eight per cent (193/335) of all participants answered ‘not at all’ to the question ‘Overall, how much do urinary symptoms interfere with your everyday life?’ on the ICIQ-LUTSqol. This was reflected in the IPSS quality of life question, to which 170 (50%) participants responded that they would be delighted if they were to spend the rest of their lives with their current urinary condition.
TABLE 19
Secondary outcome: quality-of-life questionnaire
Looking at specific scores, we see weak evidence to suggest that those in the ThuVARP arm are at a lower odds of both getting embarrassed by their urinary problem and scoring at least 1 on the King’s Health Questionnaire severity measures scale (which includes wearing pads, changing underclothes because of leakage, worrying in case of smell and being careful of fluid intake).
Statistical ancillary analyses
Subgroup analyses
Formal tests of interaction were employed to explore potential effect modifiers. Looking at the interaction tests for the prespecified subgroups (Tables 20 and 21), we can see that those participants diagnosed with LUTS benefited from a greater increase in Qmax if they were in the TURP arm, whereas there was little difference between the arms in those patients diagnosed with urinary retention. Younger men were also more likely to benefit from TURP than from ThuVARP in terms of Qmax. However, all 95% CIs were consistent with no interaction effects, although these analyses are likely to be underpowered.
TABLE 20
Subgroup analyses: IPSS at 12 months
TABLE 21
Subgroup analyses: Qmax at 12 months
Sensitivity analyses
Several sensitivity analyses were conducted to test the robustness of the primary outcome results. The per-protocol and CACE analyses (although prone to bias) strengthened the intention-to-treat results. In the imputation primary analysis, the IPSS differed by 0.28, whereas in the per-protocol and CACE analyses the differences were –0.04 and 0.05, respectively, with CIs indicating equivalence (Figure 7 and Table 22).

FIGURE 7
Testing equivalence for IPSS.
TABLE 22
Sensitivity analyses: IPSS and Qmax – difference between arms
In the imputation primary analysis, Qmax levels differed between the arms by –3.12 in favour of TURP. For the per-protocol and CACE analyses, the differences were –4.61 and –4.67, respectively, indicating a larger improvement for the TURP arm (Figure 8 and see Table 22). The multiple imputation, complete-case, per-protocol and CACE analyses all demonstrated non-equivalence. Changing the hypothesis to superiority, not penalised after non-inferiority or equivalence, the results demonstrated that TURP was superior to ThuVARP.28
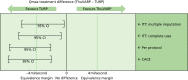
FIGURE 8
Testing equivalence for Qmax levels.
All sensitivity analyses were in agreement with the results from the main analyses. Adjusting for imbalance (painful ejaculation) reduced the sample size substantially as those patients with a catheter had not answered a questionnaire at baseline. The post hoc bootstrap analysis, and log-transformed analyses, conducted to ensure that the slightly skewed distribution did not affect the outcome, were also in agreement.
The type of TURP procedure was included as a prespecified subgroup analysis in the trial protocol paper;1 however, given that an interaction term was not possible, the team chose to use it in an exploratory analysis instead. Figure 9 shows how each of the centres differed in their use of monopolar and bipolar TURP.
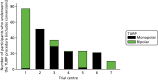
FIGURE 9
Monopolar and bipolar TURP use across the trial centres.
The IPSS results were equivalent when comparing ThuVARP separately with monopolar TURP and bipolar TURP. The monopolar technique showed the greatest improvement when looking at Qmax, resulting in non-equivalence (Table 23).
TABLE 23
Sensitivity analyses: ThuVARP compared with monopolar and bipolar TURP
Looking at the Qmax results, we can see that the results from bipolar TURP versus ThuVARP comparison included the zero difference in its CI. When comparing monopolar with bipolar directly (Table 24), we can see that IPSS scores are slightly reduced in patients receiving the bipolar TURP procedure than in those patients receiving the monopolar procedure, whereas the monopolar technique fared better than the bipolar technique in terms of Qmax levels. However, the CIs suggested that these observations could have been a result of chance. These analyses were exploratory and were hypothesis generating rather than confirmatory.
TABLE 24
Sensitivity analyses: monopolar vs. bipolar TURP
Serious adverse events
Pathological findings
Pathology findings (including the weight of the resected prostate) were collected from 3-month CRFs and were available for 386 (94%) of the randomised patients. Cases of high-grade prostatic intraepithelial neoplasia (PIN) were excluded from analyses comparing benign and prostate cancer diagnoses. Although exploratory, our results suggest that participants in the ThuVARP arm were at a 65% lower odds of finding prostate cancer than those patients in the TURP arm (p = 0.007) (Table 25). This is probably due to the prostate weight available after resection, which was, on average, 15 ml higher in the TURP arm than in the ThuVARP arm (p < 0.001).
TABLE 25
Pathological findings (intention to treat)
Looking at this on a treatment-received basis, we can see that the gap between ThuVARP and TURP becomes wider (Table 26). Conversions from ThuVARP to TURP gave results that were very similar to those for TURP alone.
TABLE 26
Pathological findings (treatment received)
Serious adverse events
There were a total of 53 serious adverse events across 41 participants in the ThuVARP arm and 53 adverse events across 45 participants in the TURP arm.
Overall, 20% and 22% of patients in ThuVARP and TURP arms, respectively, suffered from a serious adverse event (Table 27). Two unrelated deaths occurred during the follow-up period, one in each arm. In the ThuVARP arm, this occurred 34 days after the operation (bowel ischaemia and subsequent organ failure) and the post-mortem results confirmed that it was unrelated to treatment. In the TURP arm, this occurred 298 days after the operation, when the participant died from an acute myocardial infarction.
TABLE 27
Serious adverse events by randomisation arm (intention to treat)
There is a slightly larger difference between the arms when we look at the actual treatment received, with 19% of participants receiving ThuVARP suffering a serious adverse event, compared with 23% of participants receiving TURP (Table 28). Conversions to TURP resulted in fewer serious adverse events, with only 14% of participants suffering from an event; however, there were very few participants to base this on and even fewer in the alternative treatment group.
TABLE 28
Serious adverse events by treatment received
Of the 106 events in total, 39 occurred in participants receiving ThuVARP surgery, 57 occurred in participants receiving TURP, six occurred in participants receiving a conversion from ThuVARP to TURP and four occurred in participants receiving an alternative treatment (see Table 28).
The majority of events were unrelated to treatment (Table 29), with 25 events listed as ‘probably’ related. Most of these were haematuria (ThuVARP, n = 2; TURP, n = 5), urinary retention (ThuVARP, n = 3; TURP, n = 4) and infections (ThuVARP, n = 3; TURP, n = 6). An additional six serious adverse events (including four deaths) were collected after 365 days of follow-up. These have not been included here to avoid potential bias from differential reporting across sites. All six were confirmed as unrelated or unlikely to be related to treatment.
TABLE 29
Serious adverse event relationship to treatment by treatment received
Statistical results summary
- Primary analysis results demonstrate that TURP and ThuVARP are equivalent in terms of IPSS at 12 months following treatment, but not in terms of Qmax, with TURP showing additional benefit over ThuVARP.
- Sensitivity analyses, including a complete-case, a per-protocol and a CACE analysis, demonstrated that these findings were robust to the statistical assumptions made.
- No differences were observed between TURP and ThuVARP in terms of complications.
- Other surgical outcomes, such as length of hospital stay, transfusion rates and blood levels, were also very similar between the arms.
- Patient-reported outcomes for urinary symptoms showed no differences between the arms at 12 months.
- Patient-reported outcomes for sexual symptoms showed no differences between the arms at 12 months.
- Quality of life and satisfaction with treatment were high in both arms of the trial, with no evidence to suggest that one arm was better.
- There was no evidence to suggest any subgroup effects, although these analyses are likely to be underpowered.
- The number of participants undergoing their randomised procedure differed between the two groups, with fewer participants randomised to ThuVARP receiving their allocated treatment.
- A post hoc exploratory analysis of the pathology data suggested that those patients in the TURP arm were more likely to be diagnosed with prostate cancer than those patients in the ThuVARP arm.
- Results - Thulium laser transurethral vaporesection versus transurethral resecti...Results - Thulium laser transurethral vaporesection versus transurethral resection of the prostate for benign prostatic obstruction: the UNBLOCS RCT
- Introduction - Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, p...Introduction - Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation
- Substudy of human immunodeficiency virus-positive participants - Interferon gamm...Substudy of human immunodeficiency virus-positive participants - Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis (IDEA): test accuracy study and economic evaluation
- Background - The effectiveness and cost-effectiveness of erythropoiesis-stimulat...Background - The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of technology appraisal no. 142): a systematic review and economic model
- Trial results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hyper...Trial results - Saline in Acute Bronchiolitis RCT and Economic evaluation: hypertonic saline in acute bronchiolitis – randomised controlled trial and systematic review
Your browsing activity is empty.
Activity recording is turned off.
See more...