NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walton M, Wade R, Claxton L, et al. Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation. Southampton (UK): NIHR Journals Library; 2020 Sep. (Health Technology Assessment, No. 24.48.)

Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation.
Show detailsOverview
Studies assessing the clinical effectiveness of SIRT for patients with unresectable HCC have been discussed and summarised in Chapter 3. The PRISMA flow diagram describing the selection process is shown in Figure 1. Treatment options vary greatly for patients with unresectable HCC according to the stage and severity of cancer and liver disease, as described in Chapter 1, Current service provision. Therefore, three NMA models were produced to represent the different populations of unresectable HCC patients. The 26 comparative studies and RCTs included in the systematic review of clinical effectiveness (see Table 3) and the 11 RCTs of CTTs (see Table 4) were screened for inclusion in each of the three NMA models. Alongside this, two studies of systemic therapies were identified from recent NICE single technology appraisals of sorafenib and lenvatinib: Llovet et al.80 and Kudo et al.81 Therefore, 39 studies were screened for inclusion in each of the three NMAs.
Network meta-analysis of adults with unresectable hepatocellular carcinoma who are eligible for transplant and of those eligible for conventional transarterial therapies
Meta-analysis using mixed treatment comparisons enables the estimation of different parameters when direct evidence on comparisons of interest is absent or sparse. The statistical synthesis method of NMA enables the comparison of multiple treatment options using both direct comparisons of interventions from RCTs and indirect comparisons across trials based on a common comparator.82 As suggested by the term, NMA needs a ‘network of evidence’ to be established between all the interventions of interest.
Network 1: adults with unresectable hepatocellular carcinoma who are eligible for transplant
The first model (network 1) included patients with early/intermediate-stage unresectable HCC who were eligible for transplant. SIRT could potentially be used as a bridging treatment for patients awaiting transplant as described in Chapter 1, Description of the technology under assessment. These patients are generally classed as BCLC stage A patients, with preserved liver function and performance status 0–1. To ensure consistency in the compared studies, studies were included only if ≥ 70% of the recruited population had early-stage HCC or if results were split by disease stage. Only 2 out of 39 studies were selected for network 1. This included two small RCTs: PREMIERE25 and Kulik et al.28 The main reason for the exclusion of studies was patients having advanced-stage disease and, therefore, not being eligible for transplant. The reasons for including and excluding each study are reported in Table 7.
TABLE 7
Network 1: adults with unresectable HCC who are potentially eligible for transplant
However, clinical advice was that there are short transplant waiting times in the UK (< 2 months), whereas the two trials in the network had transplant waiting times of roughly 7–9 months (mean 7.8 months in Kulik et al.28 and median 8.8 months in Salem et al.25). Therefore, the network may not be generalisable to the UK and there may be limited opportunity for benefit in the UK given the short waiting times. Clinicians advised that, in the UK, bridging treatment is also used during the work-up phase, before the patient goes on to the waiting list. However, TACE rather than SIRT is more commonly used in this context. Furthermore, the two RCTs included in the network have very small sample sizes and, therefore, any efficacy estimates produced would be highly uncertain. Therefore, network 1, of patients with early/intermediate-stage HCC, was not conducted as it was deemed unsuitable for decision-making.
Network 2: adults with unresectable hepatocellular carcinoma who are eligible for conventional transarterial therapies
The second model was for patients with unresectable HCC who are eligible for CTTs. Patients in this population tend to have intermediate-stage HCC (BCLC B); however, patients with advanced-stage HCC (BCLC C) can also be eligible if they do not have PVT/PVI or extrahepatic spread. Studies in which the majority of patients had intermediate-stage HCC (BCLC B) and ≤ 30% of patients had advanced disease (BCLC C) were included. If studies reported results split by disease stage, they were included. A small proportion of patients in this population may also be eligible for downstaging to transplant; however, there was very little evidence to inform this. Furthermore, clinicians advised that the role of downstaging HCC for liver transplantation is currently under evaluation in the UK and SIRT is not specifically required for downstaging as this can be achieved using existing therapies, most commonly TACE.
After screening the 39 studies described in the previous section, seven studies were identified as relevant for the population of patients who are eligible for CTT: six RCTs and one retrospective comparative study. The reasons for inclusion and exclusion are listed in Table 8. The main reason for exclusion was the population being substantially mixed in terms of stage of HCC disease or patients having advanced-stage disease, which made them ineligible for CTT. SIRTACE,22 which is a RCT comparing SIR-Spheres and TACE described in Chapter 3, Efficacy and safety of SIR-Spheres, included a mixed population of patients with early-, intermediate- and advanced-stage HCC. The trial was funded by Sirtex; therefore, data split by disease stage were requested. However, Sirtex was unable to provide the data as it did not have access to them, so the trial could not be included in the NMA.
TABLE 8
Network 2: adults with unresectable HCC who are eligible for CTTs
The studies included in network 2 were a RCT directly comparing SIR-Spheres with DEB-TACE,23 five RCTs comparing different CTTs59,63–66 and one retrospective comparative study comparing SIR-Spheres with TheraSphere.40 The RCT that compared SIR-Spheres with DEB-TACE23 included only 24 patients (described in more detail in Chapter 3, Efficacy and safety of SIR-Spheres) and was the only direct evidence between SIR-Spheres and CTT. There were no studies comparing TheraSphere with CTT. The retrospective study comparing SIR-Spheres with TheraSphere40 was rated as being at a high risk of bias, as described in Chapter 3, Efficacy and safety of SIR-Spheres.
The five RCTs comparing different CTTs, which were deemed relevant for this population, were included to inform the network. This includes three RCTs comparing TACE and TAE.63–65 The risk-of-bias assessment reported some concerns regarding bias in the randomisation process for all three trials. The assessment also highlighted concerns regarding protocol deviations from the intended interventions for Chang et al.63 Both Yu et al.65 and Meyer et al.64 showed no significant differences in OS or PFS. Chang et al.63 reported only survival rates between groups but did not find any significant differences.
There was one RCT comparing DEB-TACE and TAE: Malagari et al.66 The risk-of-bias assessment reported some concerns with this study regarding bias in the randomisation process and in protocol deviations from the intended interventions. The trial was conducted in 95 patients and found that TTP was significantly longer in the DEB-TACE arm (42.4 ± 9.5 weeks) than in the TAE arm (36.2 ± 9.0 weeks). The remaining RCT compared DEB-TACE and TACE: Sacco et al.59 This trial was rated as being at a high overall risk of bias owing to an open randomisation process. The trial found no significant differences in survival rates or other relevant outcomes between the two groups. Full results of the risk-of-bias judgements are presented in Appendix 9 and the study details and results are presented in Appendix 10.
The network diagram representing the model is shown in Figure 2. There are missing direct comparisons and there is no common comparator in the evidence base for both OS and PFS outcomes in this population; therefore, it forms a ‘disconnected network’. Implementing a NMA in this population would produce very uncertain results as it relies on a single small trial by Pitton et al.23 to connect SIR-Spheres in the network. Furthermore, it would not provide reliable evidence on TheraSphere comparisons with CTT as there is only one small, retrospective, low-quality study connecting TheraSphere in the network. Therefore, network 2, of patients with unresectable HCC who are eligible for CTT, was not conducted as it was deemed unsuitable for decision-making.
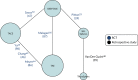
FIGURE 2
Network 2: patients eligible for CTTs.
Network 3: adults with unresectable hepatocellular carcinoma who are ineligible for conventional transarterial therapies
The third model was for patients with unresectable HCC who are ineligible for CTT. Patients in this population tend to have advanced-stage HCC (BCLC C) with or without PVT/PVI. This population may, however, include some patients with intermediate-stage disease (BCLC B) who are ineligible for CTT or who have previously failed CTT.
There were 26 comparative studies included in the systematic review of clinical effectiveness, which were identified as potentially eligible for the third network; the 11 RCTs comparing different CTTs were not screened as they are not relevant for this population. A further two studies of systemic therapies identified from previous technology appraisals were additionally screened for inclusion in this network. Out of 28 studies, three RCTs and five retrospective comparative studies were initially selected as relevant for this population. Twenty studies were excluded, mainly because of irrelevant comparisons or not reporting relevant outcomes. The NMA diagram is illustrated in Figure 3.
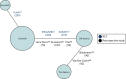
FIGURE 3
Network 3: adults with unresectable HCC who are ineligible for CTTs.
The network includes robust direct evidence between SIR-Spheres and sorafenib from the two large RCTs SARAH84 and SIRveNIB,21 which are described in more detail in Chapter 3, Efficacy and safety of SIR-Spheres. There are also three smaller retrospective comparative studies comparing SIR-Spheres and sorafenib.44,45,85 On closer examination, all three of these studies were rated as being at a high risk of bias owing to an imbalance in baseline characteristics, unclear reporting of missing data and unblinded outcome assessors (see Appendix 8). Therefore, owing to already having identified high-quality RCTs comparing SIR-Spheres and sorafenib, these three retrospective studies were removed. Including low-quality studies where there is already reliable evidence may invalidate the NMA and consequently the results. Furthermore, the two retrospective studies, Biederman et al.39 and Van Der Gucht et al.,40 were also considered to have a high risk of bias, as described in Chapter 3, Direct comparisons of different selective internal radiation therapies. However, these studies were included as a sensitivity analysis as they are the only studies with direct evidence between TheraSphere and SIR-Spheres.
The network was updated and the final NMA of patients ineligible for CTT includes two RCTs comparing SIR-Spheres and sorafenib,19,21 one RCT comparing lenvatinib and sorafenib81 and two retrospective comparative studies comparing SIR-Spheres and TheraSphere (included as a sensitivity analysis) (Figure 4).38,40 The decisions for including and excluding each study are detailed in Table 9. The study selection process for this NMA (updated network 3) is illustrated in Figure 5.
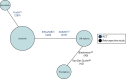
FIGURE 4
Updated network 3: adults with unresectable HCC who are ineligible for CTTs.
TABLE 9
Network 3: adults with unresectable HCC who are ineligible for CTTs
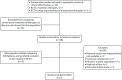
FIGURE 5
Flow diagram of the study selection process for the NMA of adults ineligible for CTTs.
Methods of data analysis
This section describes a NMA of all relevant RCTs (Table 10) and a NMA of RCTs that included only patients with Child–Pugh class A liver function. Currently, in the UK, systemic therapy, such as sorafenib and lenvatinib, is licensed for only Child–Pugh class A patients with unresectable HCC. However, results for all patients in the ITT population are reported in Appendix 12, Tables 39 and 40.
TABLE 10
Summary of studies included in the NMA
In the SARAH19 and SIRveNIB21 trials, 22.4% and 28.6% of patients allocated to SIR-Spheres did not receive SIRT. Patients who did not receive their allocated treatment were excluded from the per-protocol analysis. Therefore, the NMA of Child–Pugh class A patients with unresectable HCC who are ineligible for CTT in the per-protocol population is the base-case scenario. However, the ITT results are used for the REFLECT trial.12 Therefore, the results for the ITT population are also reported. Both OS and PFS were assessed as outcomes. However, PFS in Child–Pugh class A patients was not reported for the SIRveNIB study21 or for patients in the Biederman et al.39 study. Therefore, PFS could not be assessed in the base-case population or in the sensitivity analyses.
The NMA was estimated using Bayesian Markov chain Monte Carlo techniques in WinBUGS, using code obtained from the NICE Decision Support Unit (DSU)’s Technical Support Document.86 An initial burn-in of at least 50,000 simulations was used, and convergence was confirmed through visual inspection of the Brook–Gelman–Rubin diagnostic and history plots. This was followed by 100,000 simulations on three chains to estimate the sampled parameters. Where available, Kaplan–Meier (KM) data were extracted using methods reported by Guyot et al.87 When KM data were not available, HRs and their variance were extracted, and log-hazard ratios synthesised. To synthesise HRs across studies, it is required that the proportional hazards assumption holds. Therefore, the deviation from proportional hazards was tested and the Schoenfeld residuals, survival curves and piecewise hazards visually inspected. It was decided to conduct more complex time-varying models only if simple models were not a good fit to the data. A model was chosen by visually inspecting the development of the hazard over time for the different trials and then by comparing deviance information criterion (DIC) values for the competing models. It was decided that a hierarchical model with classes of treatments composed of individual treatments, which would allow each treatment effect to be estimated as well as the overall class mean, was not possible owing to the small number of studies in the NMA.86 Finally, both fixed- and random-effects models were evaluated and between-trial heterogeneity was assessed using the between-study standard deviation (SD). Inconsistency did not need to be examined, as there were no loops in the network.
Model selection
A Bayesian evidence synthesis approach was employed. With a Bayesian framework, prior belief about a treatment effect is combined with a likelihood distribution that summarises the data to obtain a posterior distribution reflecting the belief about the treatment effect after incorporating the evidence. Normal identity link models were used for this NMA.86 The Schoenfeld residuals were visually inspected and statistically tested for each survival curve except for the REFLECT study because only a subgroup of the data were used, for which there was no KM curve (see Appendix 11). Although the KM curves for each study cross over, which suggests that there are some concerns about the proportional hazards assumption, there is no clear statistical evidence that the assumption is violated for all of the included studies.12 The viability of the network depends on the proportional hazards assumption. Therefore, HRs were synthesised across studies. The choice of prior distributions for the between-study variance was explored. A half-normal (0, 0.192) prior was chosen as a uniform (0, 3) prior was too influential. The justification for the half-normal prior is that it expresses the prior belief that 95% of trials will give HRs within a factor of 2 from the estimated median HR. However, owing to the small number of studies, there was little evidence to inform the between-study heterogeneity. The half-normal prior was also influential, although less so than the uniform prior. According to DIC and total residual deviance statistics, the fixed-effects model provided a better fit to the data than did the random-effects counterpart. The fixed-effects model had both a lower DIC and fewer parameters. This is again because of the small number of studies and the influence of the prior on the between-study heterogeneity. Owing to both models having similar results, the fixed-effects model was chosen as it is a simpler model. Results from both are presented for comparison.
Scenario and subgroup analyses
Scenario analyses including the two low-quality retrospective studies, by Biederman et al.39 and Van Der Gucht et al.,40 were carried out, as discussed in Chapter 3, Network 3: adults with unresectable hepatocellular carcinoma who are ineligible for conventional transarterial therapies. For the first scenario, the Biederman et al.39 study was added to the base-case NMA: adults with unresectable HCC who are Child–Pugh class A and ineligible for CTT in both the per-protocol population and the ITT population. There were no available data on Child–Pugh class A patients in the Van Der Gucht et al.40 study; therefore, it was not included. For the second scenario, which is reported in Appendix 12, both the Biederman et al.39 and Van Der Gucht et al.40 studies were added to the NMA of all adults who are ineligible for CTT in the ITT population. Biederman et al.39 did not report PFS outcomes; therefore, the second scenario was used for the OS outcome only.
A sensitivity analysis that excluded the RCT SIRveNIB21 was conducted. Patients in the SIRveNIB trial are from the Asia-Pacific region and, thus, have different HCC disease aetiology and consequently differing treatments to those from Europe. This is discussed in more detail in Chapter 3, Efficacy and safety of SIR-Spheres. Therefore, a scenario was conducted in which SIRveNIB was excluded from the base-case NMA.
It was not possible to conduct a subgroup analysis in Child–Pugh class A patients with PVT or in patients with PVI. The only available data for this subgroup of patients were from the two RCTs comparing SIR-Spheres and sorafenib: SARAH19,20 and SIRveNIB.21 However, SIRveNIB reported results for only the subgroup of patients with PVT, and SARAH reported results for only patients with PVI.
Results
Results of the base-case network meta-analysis in the per-protocol population: adults with unresectable hepatocellular carcinoma who are Child–Pugh class A and ineligible for conventional transarterial therapy
Three studies were included in the base-case analysis: two RCTs comparing SIR-Spheres and sorafenib and one RCT comparing lenvatinib and sorafenib. The baseline characteristics of these studies are detailed in Table 10. The REFLECT trial,81 which compares lenvatinib and sorafenib, included patients with extrahepatic spread (61% in the lenvatinib arm and 62% in the sorafenib arm). All the other trials excluded patients with extrahepatic spread; therefore, the subgroup of patients without extrahepatic spread or PVI was used for the REFLECT trial. A more appropriate subgroup was not reported.
The results of both the fixed-effects analysis and the random-effects analysis are shown in Table 11.
TABLE 11
Overall survival results for the base-case NMA in the per-protocol population
The results provide no evidence that the random-effects model should be preferred. The DIC is marginally higher (–0.40 for the random-effects model, compared with –1.38 for the fixed-effects model; lower DIC values are preferred, with differences of 2–5 considered important).86 In addition, the high level of uncertainty around the random-effects CrI indicates that there is little information to inform the random-effects parameter. Therefore, the results of the fixed-effects model will be used for the base-case and all scenario analyses. Both fixed-effects and random-effects results are reported in Appendix 13, Tables 43–46, for comparison.
There were no meaningful differences in OS in the per-protocol population between any of the three treatments and all treatments appear to have a similar effect. SIR-Spheres shows a marginal improvement in OS when compared with sorafenib (HR 0.94, 95% CrI 0.77 to 1.14) and lenvatinib (HR 0.91, 95% CrI 0.63 to 1.26); however, the treatment effects are uncertain as the CrI crosses 1. Lenvatinib shows a marginal reduction in OS when compared with sorafenib (HR 1.06, 95% CrI 0.79 to 1.40), although again the CrI crosses 1 (Table 12). Figure 6 presents the cumulative ranking curves for each treatment, with rank 1 being the best and rank 3 being the worst. SIR-Spheres was ranked as the most efficacious therapy, with a probability of being the best of 0.61. Lenvatinib was ranked as the worst treatment, with a probability of being best of 0.22. Sorafenib was ranked as the second best, with a probability of being best of 0.16.
TABLE 12
Hazard ratio estimates (95% CrIs) for OS for each treatment comparison for the base-case NMA in the per-protocol population

FIGURE 6
Cumulative ranking probability plots for each treatment in the base-case NMA for the per-protocol population.
Results of the base-case network meta-analysis in the intention-to-treat population: adults with unresectable hepatocellular carcinoma who are Child–Pugh class A and ineligible for conventional transarterial therapy
Similar to the per-protocol population, there were no significant differences between treatments in the base-case NMA in the ITT population (Table 13).
TABLE 13
Overall survival results for the base-case NMA in the ITT population
SIR-Spheres appears to increase mortality when compared with sorafenib and lenvatinib (HR 1.13, 95% CrI 0.96 to 1.32 and 1.09, 95% CrI 0.77 to 1.48, respectively). However, the CrIs indicate that these results are uncertain. Lenvatinib also shows a reduction in OS when compared with sorafenib (1.06, 95% CrI 0.79 to 1.40); however, the 95% CrI crosses 1, indicating that there is not a significant treatment effect.
The HRs for all patients in the ITT population for OS and PFS are shown in Appendix 12, Tables 41 and 42, respectively.
Scenario 1: inclusion of Biederman et al. into the base-case network meta-analysis
The Biederman et al.39 study was added to the base-case NMA in a scenario analysis, which allowed for a comparison to be made against TheraSphere. Biederman et al.39 reports a very strong treatment effect on OS with TheraSphere compared with SIR-Spheres (HR 0.40, 95% CrI 0.20 to 0.78). However, as discussed earlier, Biederman et al.39 is a retrospective, poor-quality study; therefore, these results may either in part or in full reflect the impact of bias. Furthermore, all patients in the Biederman et al.39 study have PVT, which is much higher than the proportion of patients who have PVT/PVI in the other included studies. Adding this study has a substantial effect on the NMA results. In the per-protocol population, TheraSphere shows a substantial significant improvement in OS when compared with SIR-Spheres (HR 0.44, 95% CrI 0.20 to 0.84), sorafenib (HR 0.41, 95% CrI 0.20 to 0.77) and lenvatinib (HR 0.40, 95% CrI 0.18 to 0.78). There were no significant differences in OS between any of the other treatments (Table 14).
TABLE 14
Overall survival results adding Biederman et al. to the base-case NMA
Similarly, in the ITT population, there was a significant improvement in OS with TheraSphere compared with sorafenib (HR 0.47, 95% CrI 0.21 to 0.88), SIR-Spheres (HR 0.41, 95% CrI 0.20 to 0.77) and lenvatinib (HR 0.45, 95% CrI 0.20 to 0.89). There were no significant differences in OS between SIR-Spheres, sorafenib and lenvatinib (see Table 14).
Sensitivity analysis
Exclusion of the SIRveNIB study from the base-case network meta-analysis
The SIRveNIB trial,21 which compares SIR-Spheres and sorafenib, was conducted in the Asia-Pacific region. This has implications for the generalisability of the SIRveNIB trial results to the UK population. The aetiology of HCC and the consequent treatment in the Asia-Pacific region are different, as described in more detail in Chapter 3, Efficacy and safety of SIR-Spheres. A sensitivity analysis was therefore implemented, in which the SIRveNIB study was excluded from the base-case NMA. Excluding SIRveNIB had very little impact on the results for OS in the ITT population compared with the base-case NMA. All treatment effects for all comparisons were similar to the base-case NMA (Table 15). The OS results in the per-protocol population, however, showed a slight change after excluding SIRveNIB. The treatment effect estimate for SIR-Spheres versus sorafenib increased (1.02, 95% CrI 0.79 to 1.29) compared with the base-case NMA (0.94, 95% CrI 0.77 to 1.14). This showed a reduction in OS with SIR-Spheres rather than an improvement, as seen in the base-case per-protocol population, although neither were statistically significant.
TABLE 15
Results of the base-case NMA excluding the SIRveNIB study
Summary of findings of relative efficacy from network meta-analysis
Treatment options and outcomes vary greatly for patients with unresectable HCC according to the severity of cancer and liver disease. Therefore, three NMA models were produced to represent the different populations of unresectable HCC patients: patients eligible for transplant, patients ineligible for transplant but eligible for CTT and patients ineligible for CTT.
The NMA in patients eligible for transplant was not conducted. Clinical advice was that there are short transplant waiting times in the UK, whereas these were much longer in the trials in the NMA. Therefore, the network may not be generalisable to the UK and there may be limited opportunity for benefit, given the short waiting times. Furthermore, the two RCTs included in the network have very small sample sizes and, therefore, any efficacy estimates produced would be highly uncertain. The NMA of patients eligible for CTT was also not conducted because of the lack of good-quality evidence in this population. There was only one RCT of 24 patients directly comparing SIR-Spheres and the comparator therapies of interest. There were no studies comparing TheraSphere and CTT. Therefore, with missing direct comparisons and only one small study to connect the network, results produced would be very uncertain and unsuitable for decision-making.
Several NMAs of patients who are ineligible for CTT were conducted for both OS outcomes and PFS outcomes in the per-protocol and ITT populations.
The base-case NMA was in adults with unresectable HCC who have Child–Pugh class A liver disease and are ineligible for CTT in the per-protocol population. Three studies were included in the base-case analysis: two RCTs comparing SIR-Spheres and sorafenib and one RCT comparing lenvatinib and sorafenib. The results provided no evidence that the random-effects model should be preferred. In addition, the high level of uncertainty around the random-effects CrI indicated that there is little information to inform the random-effect parameter. Therefore, the results of the fixed-effects model were used for the base-case and scenario analyses.
There were no meaningful differences in OS between any of the three treatments in the per-protocol or ITT populations. All treatments appear to have a similar effect. In the per-protocol population, SIR-Spheres showed a non-significant marginal improvement in OS when compared with sorafenib (HR 0.94, 95% CrI 0.77 to 1.14), although the CrI indicates that this result is uncertain. SIR-Spheres was ranked as the most efficacious therapy, with a probability of being the best of 0.61. Lenvatinib was ranked as the worst treatment, with a probability of being best of 0.22. Sorafenib was ranked as the second best, with a probability of being best of 0.16.
To produce an efficacy estimate for TheraSphere, the only two studies that directly compared TheraSphere and SIR-Spheres for patients ineligible for CTT, Biederman et al.39 and Van Der Gucht et al.,40 were included as a sensitivity analysis. Both are low-quality retrospective studies, which reported strong treatment effects on OS with TheraSphere compared with SIR-Spheres (HR 0.40, 95% CrI 0.20 to 0.78, and HR 0.77, 95% CrI 0.27 to 2.18, respectively). Adding these studies had a substantial effect on the NMA results. In the per-protocol population, TheraSphere showed a substantial and statistically significant improvement in OS when compared with SIR-Spheres (HR 0.44, 95% CrI 0.20 to 0.84), sorafenib (HR 0.41, 95% CrI 0.20 to 0.77) and lenvatinib (HR 0.40, 95% CrI 0.18 to 0.78). In the ITT population, there was also a significant improvement in OS with TheraSphere when compared with sorafenib (HR 0.53, 95% CrI 0.31 to 0.84), SIR-Spheres (HR 0.46, 95% CrI 0.28 to 0.72) and lenvatinib (HR 0.51, 95% CrI 0.28 to 0.86). A sensitivity analysis, which excluded the SIRveNIB study from the base-case NMA was also conducted. The SIRveNIB trial, which compared SIR-Spheres and sorafenib, was conducted in the Asia-Pacific region. This has implications for the generalisability of the SIRveNIB trial results to the UK population. Excluding SIRveNIB, however, had very little impact on the results for OS and PFS in the per-protocol and ITT populations compared with the base-case NMA. There were no significant differences in treatment effects for any comparisons.
- Evidence synthesis to inform the relative efficacy of the interventions - Select...Evidence synthesis to inform the relative efficacy of the interventions - Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation
- Introduction - Use of drug therapy in the management of symptomatic ureteric sto...Introduction - Use of drug therapy in the management of symptomatic ureteric stones in hospitalised adults: a multicentre, placebo-controlled, randomised controlled trial and cost-effectiveness analysis of a calcium channel blocker (nifedipine) and an alpha-blocker (tamsulosin) (the SUSPEND trial)
- Background - The second Randomised Evaluation of the Effectiveness, cost-effecti...Background - The second Randomised Evaluation of the Effectiveness, cost-effectiveness and Acceptability of Computerised Therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? A randomised controlled trial
- Clinical effectiveness: overview of included studies - Treatments for hyperemesi...Clinical effectiveness: overview of included studies - Treatments for hyperemesis gravidarum and nausea and vomiting in pregnancy: a systematic review and economic assessment
- Clinical effectiveness: aromatherapy - Treatments for hyperemesis gravidarum and...Clinical effectiveness: aromatherapy - Treatments for hyperemesis gravidarum and nausea and vomiting in pregnancy: a systematic review and economic assessment
Your browsing activity is empty.
Activity recording is turned off.
See more...